Abstract
The taste profile of the juice from four cultivars of pomegranate (PMG) (‘Shani’, ‘Acco’, ‘Emeq’, and ‘116’) was evaluated using an electronic tongue in comparison with commonly used methods such as chemical analysis and a sensory panel. Estimated intensity of taste (EIT) for sourness, astringency and bitterness (including the aftertaste) were calculated according to the linear range outputs of citric acid, tannic acid and iso-alpha acid solutions. The aftertaste EITast and EITbit values showed good correlations with the sensory panel scores, R 2 = 0.92 and R 2 = 0.78, respectively. Six PMG juices were discriminated based on the taste profile results from the e-tongue sensors and the sensory panel. Sourness and sweetness attributes were the main factors for the variance axis (F1 = 53.2 %) with the juice of ‘116’ being the most sour. Bitterness and astringency were plotted on the second axis, positioning the cvs. ‘Shani’ and ‘Organic Acco’ with positive correlations. The sensors for astringency and bitterness were significantly correlated with the polyphenol and hydrolysable tannin contents. The e-tongue could be used to monitor PMG juice quality in shelf life, mainly for changes in astringency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing popularity of pomegranate (Punica granatum L.) fruit and juice is related to the growing recognition by consumers that pomegranate (PMG) is a rich source of healthy ingredients. The fruit or juice has proven health benefits for prevention of cardiovascular diseases, diabetes, and cancer (Johanningsmeier and Harris 2011; Viuda-Martos et al. 2010). The rich content of phenolic compounds, including anthocyanin, lignans, and tannins, contributes to the high antioxidant activity of the fruit (Tezcan et al. 2009). The fruit has a wide growing diversity from tropical to subtropical countries with more than 500 known pomegranate varieties that comprise several cultivated species. Among them, ‘Acco’, ‘Emeq’, ‘Shani’, and ‘116’ were developed in Israel by conventional breeding aimed at early harvesting. The late-harvested ‘Wonderful’ cultivar was originally developed in the USA and further genetically improved in Israel.
The flavor profile of PMG fruit varies significantly between the cultivars as well as growth locations. The quality attributes differ in taste, aroma, seed size and texture, color and fruit size (Mayuoni-Kirshinbaum and Porat 2014). The PMG juice flavor can be described as sweet and sour, having fruity odors with some musty/earthy notes and an aftertaste feeling of astringency (Koppel and Chambers Iv 2010). The taste profile of most PMG juices can be categorized into three groups characterized by mean acidity: sweet (0.32 %), sweet–sour (0.79 %), and sour (2.72 %) (Mayuoni-Kirshinbaum and Porat 2014). The juice sweetness also derives from the relative proportions between glucose, fructose, and trace amounts of sucrose. The sourness is mainly related to the presence of malic and citric acids as well as other minor organic acids such as succinic, oxalic, tartaric, and ascorbic acids. The polyphenol compounds in the fruit are responsible for the slight bitterness. The PMG juice becomes more bitter when it is squeezed from the fruit and high levels of polyphenols are released from the peel and capillary membrane.
The taste profile of PMG juice has been studied among other sensory and analytical parameters to define the juice quality differences between varieties, postharvest conditions, climate effects, adulteration, and processing (Fawole and Opara 2013; Mayuoni-Kirshinbaum et al. 2013; Vázquez-Araújo et al. 2011). PMG juice taste is usually measured by a combination of analytical instruments (e.g., total soluble solids (TSS) for sweetness, titratable acidity, and pH for sourness and spectrophotometric analysis of polyphenols and tannins for bitterness and astringency) and a sensory panel. However, the current methods cannot fully mimic the actual human gustatory taste and usually provide only an indirect measurement. The prediction of taste intensity can be difficult as it depends not only on the concentration of taste compounds present, but is also affected by the ratio and interactions between molecules. The common method to evaluate taste intensity is by using a sensory panel that has been trained for long period until reaching sufficient reproducibility.
In order to obtain a more objective, accurate and sensitive taste intensity evaluation, an advanced instrument called the electronic tongue (e-tongue) was developed (Tahara and Toko 2013). The mechanism behind the e-tongue is based on potentiometric chemical sensors that respond to interactions with many taste-charged soluble substances by producing specific, non-specific, and overlapping signals. The e-tongue is not able to detect neither neutral-charged nor volatile compounds. The majority of the e-tongues comprise an array of sensors with a low selectivity for specific taste type compounds, but providing instead a global overall taste profile, like a digital fingerprint. In order to discriminate between the samples, further multivariate statistical analysis needs to be engaged, such as principal component analysis (Escuder-Gilabert and Peris 2010). Tahara and Toko (2013) describe the e-tongue that they developed in Japan in comparison with other types of e-tongues: (1) high global selectivity for taste like the human tongue; specific sensors were designed to attract certain taste compounds, (2) similar threshold sensitivity as the human tongue, (3) providing an analytical unit sense for taste, and (4) taking into account interactions between taste substances in taste evaluation. The sensor material made of a lipid membrane and plasticizers of different types and concentrations provide the high selectivity, sensitivity, and reproducibility of results. The e-tongue has been used in many studies in food and pharmaceutical applications (Uyen Tran et al. 2004; Woertz et al. 2011).
The correlation between human taste evaluation and the e-tongue taste intensity was tested in a previous work on green tea (Hayashi et al. 2013). The authors developed an algorithm to define the estimated intensity of taste (EIT) value based on a pure taste compound response by the corresponding sensor (e.g., ethyl gallate for bitterness or epigallocatechin-3-O-gallate for astringency). There was a similar tendency between the perceived sensorial feelings of panelists to the e-tongue EIT values. The study demonstrated the ability of the e-tongue to discriminate between different types of tea according to specific taste intestines, resembling a sensory panel outcome.
The aim of this study was to evaluate the use of the e-tongue as an objective method to measure the intensity scales of the PMG juice taste profile compared to sensory panel and commonly used analytical methods. This study examines the validation of EIT procedure on a complex taste profile PMG juice in correlation between the e-tongue results and human taste. The PMG juice may act as a model for other food products in the future to be evaluated with the e-tongue. Several common Israeli PMG varieties were chosen for the analysis, including a determination of shelf life duration, using the e-tongue.
Materials and Methods
Samples and Chemical Materials
Pomegranate fruits from four different Israeli-bred cultivars (‘Acco’ or ‘Organic Acco’, ‘Shani’,‘116’ and ‘Emeq’) and the ‘Wonderful’ cultivar that was grown under the same conditions as the Israeli-bred cultivars were used in this study. All fruit cultivars were kindly donated by the Har-Kor fruit storage company. The fruits were harvested in the late summer–early autumn of 2014 when the fruits were fully ripe. The fruit of each cultivar were chosen for uniform size and appearance.
All the chemicals used in the research were of analytical grade (>95 % purity). The chemicals, except iso-alpha acid that was provided by Insent (Kanagawa, Japan), were purchased from Sigma-Aldrich (Rehovot, Israel).
Pomegranate Juice Preparation
The fruits were freshly cut in half and hand-pressed with a kitchen juice press. Fifteen fruits from each cultivar were divided randomly into three replicates of five fruits each. The juices were immediately stored in the dark at −20 °C until chemical or sensory analysis was performed. The juices prepared for the e-tongue measurement were further filtered with Whatman paper #1 to obtain a clear juice without pulp or seeds. No additive or preservative was added to the juice.
Determination of pH, Titratable Acidity, Soluble Solids, and Color
pH was measured with a Cyberscan 500 pH meter (Eutech Instruments Ltd, Landsmeer, NL). Titratable acidity was measured using an automatic titrator TA10plus (Schott instruments, Switzerland) and calculated as percentage of citric acid, the prevalent acid in PMG juice, by titrating 0.5 mL juice in 20 mL water with 0.1 M NaOH till pH 8.2. The amount of soluble solids in Brix degrees was obtained using a refractometer (Reichert Depew, NY, USA). The PMG juice color was determined using the Minolta colorimeter (CR400 Chroma meter set, Minolta, Japan) according to the Commission International de l’Eclairage (CIE) and expressed as L*, a*, and b* values. The peel color was measured on three sides of each fruit, while juice color was measured on 15 mL placed in a 50 mL glass beaker, from the flat bottom,
Juice Total Polyphenols and Tannin Concentration
Total polyphenol content was determined by using the Folin–Ciocalteu method (Ben Nasr et al. 1996). 100 μL PMG juice diluted 1:20 with distilled water was mixed with 5 mL of diluted Folin–Ciocalteu reagent (1:10 v/v with water) and alkalinized with 4 mL 5 % sodium carbonate. The mixture was incubated for 5 min at 50 °C and absorbance was measured with a UV–VIS spectrophotometer at 760 nm. The total polyphenol amount was calculated a from calibration curve with gallic acid (mg/L).
Tannin Content
The tannin content was determined by the precipitation method using bovine serum albumin (BSA) protein and ferric chloride (Hagerman and Butler 1978). PMG juice (1 mL) was mixed with 5 mL methanol. 1 mL from the upper phase was added to 2 mL of reagent A (acetate buffer (pH 5.0) + 0.1 % (w/v) BSA + 0.99 % (w/v) NaCl). The solution was centrifuged at 3000 rpm for 15 min. The precipitated tannins were dissolved in 4 mL reagent B (1 % SDS + 5 % triethanolamine) for 1 h at 37 °C. Addition of 1 mL reagent C (0.27 % FeCl36H2O + 0.1 N HCl) was added to solution with final shaking for 30 min before reading at 510 nm.
Sensory Evaluations
A sensory panel of 13 trained panelists (four male and nine female: age ranged 30 to 60) recruited from Tel Hai College evaluated PMG juice taste. The panel was trained for ten sessions of 1 h each prior to the study, and became familiar with the taste lexicon and sensory tests of PMG juice. The panel was trained with standard taste solutions to evaluate EIT correlation with the PMG juice taste attributes using unstructured 150 mm: sweetness (0 and 100 mm defined by 110 and 380 mM sucrose, respectively), sourness (0 and 100 mm defined by 0.39 and 1.3 mM citric acid, respectively), bitterness by caffeine (15 mM) referred as 100 mm and astringency by tannic acid (0.11 mM) referred as 100 mm in the intensity scale.
The sample evaluation procedure included a pre-evaluation session, testing a reference PMG juice and reaching a consensus agreement of the taste profile. The study consisted of three time sessions of 15 min, where the panelists had to evaluate five different random coded samples of PMG juice (30 mL). A sensory acceptance test was also performed using 60 non-trained volunteers from Tel Hai College in a 9-point hedonic scale ranging from very strong dislike to very strong like. The panelists were requested to eat an unsalted cracker and rinse the mouth between samples. The evaluation was conducted in sensory booths under controlled environment (21 ± 1 °C and RH 55 ± 5 %) illuminated by a red light to eliminate the effect of juice color for all trials.
E-tongue Measurement
The electrical taste profile of PMG juice was determined by the Taste-Sensing System SA-402B (Intelligent Sensor Technology Co., Kanagawa., Japan), referred to in this study as the e-tongue. The system is composed of six sensors: three positively charged lipid membranes for sweetness (GL1), acidic bitterness (C00) and astringency (AE1); three negatively charged lipid membranes for saltiness (CT0), sourness (CA0) and umami (AAE). The measurement and cleaning procedure are well described in previous studies (Nobuyuki Hayashi, et al. 2013; Laureati et al. 2010). Before performing a measurement, the sensors were checked for stability and accuracy using reference solutions (30 mM KCl and 0.3 mM tartaric acid) and a mixture solution of taste compounds (300 mM KCl, 3 mM tartaric acid, 11 mM monosodium glutamate, 0.3 mM iso-α acid, 0.29 mM tannic acid, and 500 mM sucrose). The taste intensity signal of PMG juices (70 mL) was measured as the potential difference between the sample and the reference solution. Astringency and bitter aftertaste were achieved by measuring the signal after 3 s rinsing in alcohol solution and comparing it with the initial sample signal. The signals are referred to as change of membrane potential (CPA) and found to have a strong correlation with adsorption of bitter and astringent compounds on the sensor membranes (Kobayashi et al. 2010). A mean of the last three measurements out of four was calculated for each sample for more stable results.
Statistical Analysis
The data were analyzed using the statistical software MS Excel XlSTAT (version 2015.1.02, Addinsoft, New York, USA). Each measurement was expressed as mean ± standard deviation (SD) of triplicate samples. Different tests were performed including one-way analysis of variance (ANOVA, P < 0.05), Tukey honest significant difference (HSD) post hoc test, principle component analysis (PCA, P < 0.05), linear regression and Pearson’s correlation matrix. The confidence level was 95 %.
Results and Discussion
Chemical Properties
The pomegranate juice chemical properties of four cultivars (‘116’, ‘Shani’, ‘Acco’ and ‘Emeq’) were analyzed (Table 1). The pH ranged from 3.2 to 3.6 with the corresponding titratable acidity (TA) between 0.9 and 1.6. The total soluble solids (TSS) can serve as a good indicator for the total sugar content and sweetness. There was no significant difference between the juice cvs. for TSS. Cv.‘116’ juice was the most acidic, resulting in a significantly lower TSS/TA value, compared to the other cvs. The sweetness and sourness of the pomegranate fruit are dominated mainly by the mixture of fructose to glucose and citric acid to malic acid, respectively (Dafny-Yalin et al. 2010). The total polyphenol content (TPC) and hydrolysable tannins showed significant difference between cvs. ‘Emeq’, for example, had the lowest values for both (452 mg/L and 811 mg/L, respectively). ‘Emeq’ juice red color, represented by ‘a*’, did not show any significant differences between the cvs. Cv. ‘Wonderful’ was not measured for all chemical properties as being not included in the four cvs. for full comparison. ‘Wonderful’ pH and TSS were 3.08 and 16.4, respectively (data not shown). In general the chemical properties of PMG juices were in the acceptable range according to previous data of the same PMG species (Mayuoni-Kirshenbaum et al. 2013; Tzulker et al. 2007).
Taste Intensity Estimation Using the E-tongue
The e-tongue’s ability to selectively respond to known PMG juice taste compounds with specific taste attributes was evaluated using standard solutions of single compounds (Qu et al. 2012). Table 2 shows the corresponding signals from each of the six food taste sensors including two CPA (change of membrane potential) signals from sensors AE1 and C00 after treating with alcohol. For most solutions, there are one or two dominant signals from the sensors with matched sensorial attributes. For example, fructose had a no meaning signal from the mentioned sensors except for the sweetness sensor GL1 with −69 mV. The phenolic acids (e.g., gallic acid and tannic acid) that have astringent and bitter notes showed clear signals from AE1 and C00 sensors and the corresponding CPA values. The differences in the CPA signal intensity for the same concentration of phenolic compounds (1 mM) indicate different levels of adsorption affinity for to the sensors. The aftertaste perception of astringency and bitterness is related to the remaining adsorbed tannins and bitter substances on the tongue surface (N. Hayashi et al. 2006). In some cases, other sensors, such as CT0, AAE, and CA0 also showed signals where they were not expected. The reason is related to an indirect effect causing certain taste compounds to influence the sensors’ potential difference. Hayashi et al. (2008) found that the bitter catechin compound had a negative potential of −30 mV with the AAE umami sensor. These indirect signals are less important in terms of CT0 (saltiness) and AAE (umami), as both taste perceptions are not typical of PMG juice. The sensor global selectivity and sensitivity for specific taste compounds can be explained by the manufacture design for specific lipid membrane composition to modulate the charge density and hydrophobicity (Kobayashi et al. 2010).
The estimated taste intensity (EIT) of the major taste and aftertaste attributes of PMG juice (sourness, bitterness, and astringency) were calculated according to a conversion method previously described (N. Hayashi et al. 2008; Ujihara et al. 2013). The authors found positive correlations between human gustatory senses and the calculated EIT values for astringency and bitterness perception using the same taste sensor system as in the current study. The color of PMG juice did not affect the taste evaluation of the sensory panel due to the masking affect by the red light in the booths. In order to calculate the EIT values, standard solutions at different concentrations were prepared on the base of a reference solution (30 mM KCl and 0.3 mM tartaric acid) with tannic acid for astringency (0.01–5 mM), iso-alpha acid for bitterness (0.03–15 mM) and citric acid for sourness (0.01–500 mM). The concentration range for each solution was chosen in correlation with the human range of gustatory taste sensing. The relationship between AE1 values and the concentration of tannic acid solution is shown in Fig. 1. The EITast for astringency was calculated based on the AE1 sensor output difference of two points within the linear range (shown by squares and the line in Fig. 1). The EIT calculation takes into consideration Weber–Fechner’s law for the change in taste perception according to a logarithmic relationship between taste substance concentrations. In other words, one unit on the EIT scale is defined as the minimum requirement for noticeable taste perception based on a 20 % increase in concentration of the standard solution (Schutz and Pilgrim 1957). In this study, the EIT values were positively normalized using subtraction against the lowest sensor value. The linear EIT value range and the linear correlation coefficient for the taste attributes were as follows: astringency +2 to+20, r 2 = 0.93; aftertaste +1.5 to +20; r 2 = 0.96; bitterness +2 to +12, r 2 = 0.93; aftertaste +1.5 to +12, r 2 = 0.95; sourness +0.5 to +20; r 2 = 0.90. A conversion factor was calculated from each EIT linear curve relevant to the taste attribute based on the results from the currently used sensors and applied research. The EIT values from standard solutions were validated against a human sensory panel. Throughout the linear concentration range of tannic acid (0.05–5 mM) for EITast, a positive significant correlation was found with the sensory panel (Fig. 2.) The panel scored the samples with corresponding EITast values as such: from 0 to 5 for low astringency, 10–20 for some astringency, and above 20 for excessively high astringency. Converted values for sweetness from the GL1 sensor were discarded because the calculated values for PMG juice were outside the linear range. The difference in pH between the acidic PMG juice and the neutral pH standard solution for sweetness can cause a shift in the sensitive GL1 sensor output (according to the manufacturer’s manual).
Estimated Taste Intensity Correlation to Sensory Panel Evaluation
The taste intensity profile of PMG juice can be determined by the same method used for standard solutions. In order to use the EIT value for a food matrix like PMG juice, the corresponding sensor output should lie within the linear range defined by the standard solutions. The AE1 sensor signals related to astringency for all PMG juices were between −15 to −40 mV (shaded zone in Fig. 1.). In case the sensor values are slightly outside the linear range, the true calculated EIT values should be corrected using an alternative line fixed to the outer range. Such a case occurred for the acidic bitterness sensor C00 and aftertaste CPA. The values were slightly below the linear range (C00 < −11 mV, CPA < −5.5 mV) and therefore the real EIT values should be slightly higher. In many cases, the taste attribute for bitterness and astringency is related to aftertaste measurement CPA of AE1 and C00. The measurement provides a valid indication for astringent and bitter compound adsorption on the sensor membrane despite the short washing step. Hayashi et al. (2006) found the CPA converted value EITast suitable to predict the astringency in green tea infusion. In the comparison between EIT values for astringency and bitterness (CPA) and sensory panel scores, high correlations were observed (r 2 > 0.92 and r 2 > 0.78 respectively, Fig. 3.). Previous studies using the EIT conversion values showed a similar tendency with sensory panel results: bitter and astringent taste for oolong tea (Nobuyuki Hayashi et al. 2013), umami and astringent taste for green tea (N. Hayashi et al. 2008; N. Hayashi et al. 2006), and bitterness for cooked meat (Nodake et al. 2013). Another type of e-tongue successfully managed to predict human sensory perception for saltiness and bitterness of Korean rice wine (Kang et al. 2014). The low values for EIT (0.9 to 1.5) indicate a weak intensity of bitterness and astringency for the PMG juices, as indicated by the panel. Even so, the slight differences between the cvs. could be detected and appreciated. ‘Organic Acco’ was found to have the highest score for both bitterness and astringency, while ‘Acco’ and ‘Emeq’ were the lowest (Fig. 3). The lower linear coefficient for bitterness (Fig. 3b) can be explained by the relatively large standard deviation of the sensory panel scores (RSD 20–30 %), the less accurate EIT converted values due to the sensor output outside the linear range and the fact that not all the bitter compounds in PMG juice were detectable by the C00 sensor. The positively charged membrane of the C00 sensor is designated to attract negatively charged bitter molecules such as iso-alpha acid. Some of the bitter substances in PMG juice are negatively charged, such as ellagic acid and epigallate, but there are other substances that are neutral and not detectable by the sensor. One of the most dominant flavonoids in PMG fruit is naringin that is perceived as bitter but cannot be detected by the e-tongue (Mphahlele et al. 2014).
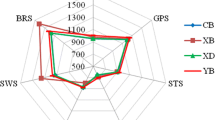
Multivariate analysis is a statistical tool used in many studies with the e-tongue to discriminate between samples into categories (Asikin et al. 2014; Qiu et al. 2015). In Fig. 4a, principle component analysis (PCA) is plotted for six different PMG cvs. against the e-tongue sensor outputs and the sensory panel scores. A total variation of 91.8 % between the cvs. is accounted for by 53.2 % for the first principle component (F1) and 38.6 % for the second component (F2). The separation of the different species according to taste can be described by the most prominent vectors on the first component axis, sweetness and sourness. The latter has a calculated squared cosine of 0.88 for CA0 (sensor representing sourness) and 0.82 for sourness perception by the panel. A closer value of the cosine to 1.0 indicates a greater link with the corresponding axis. The ‘116’ PMG cv. is positioned in the left positive square for the sourest juice, whereas ‘Acco’ and ‘Shani’ cvs. are on the opposite side, with the least sourness but the most sweetness. The F2 component expresses the variation with the bitterness and astringency attributes that are more pronounced on the positive side. Therefore, ‘Organic Acco’ and ‘Shani’ are both positioned as more bitter and astringent than the other cvs. The loading for the e-tongue sensors appears to be well-associated with the loading of the sensory panel score, according to the positive correlation between the methods. A previous study on PMG juice taste characteristics using another type of e-tongue showed less ability to discriminate between the different brands compared to the more distinctive distribution in the PCA plot for the sensory analysis (Bett-Garber et al. 2014). The use of cross-selective sensors in the current e-tongue provided a more reliable outcome in terms of sensory perception. A hedonic test was performed for the appreciation of the different PMG juices. The size of the circle indicates the degree of preference (Fig. 4). ‘Wonderful’ received the highest rank, while ‘Shani’ and ‘Organic Acco’ were the least liked. According to the PCA plot, it may be assumed that the preferred PMG juice is the one with some mild sourness, slightly astringent and bitter. Juices with a high degree of sweetness and lacking in sourness were less appreciated. These conclusions for the taste preference of PMG species might be somewhat speculative due to the relatively small differences in the EITast/bit values. A noticeable change in taste perception is considered when EIT > 1, whereas the EIT values were at the most 0.6 (Kobayashi et al. 2010).
Correlation Between E-tongue Analysis and Chemical Properties
In order to validate the ability of the e-tongue to evaluate the taste profile of PMG juice, a correlation matrix was performed against the chemical properties (Table 3). The coefficients of correlation with significant difference (p < 0.05) are presented in bold. A good inverse correlation (r 2 > 0.95) was found between the polyphenol and tannin contents and the signal readings from AE1 and CO0, the astringent and bitter sensors. The signal from the aftertaste CPA of AE1 also showed a negative correlation, although it was not as significant (p = 0.1). These correlations provide evidence that the e-tongue can detect the presence of bitter and astringent compounds in the juice, and reflect their taste intensity level. Newman et al. (2014) showed the capability of an e-tongue to evaluate bitterness by positive correlation with quantitative changes in dairy protein hydrolysates. Another significant correlation was found between sensor CA0 for sourness to pH but not to acidity. The acidity in PMG juice comes from the presence of both citric and malic acids that act differently as titratable acids than the direct measurement of the pH acid protons. The CA0 sensor is based on a more direct measurement, like pH, which explains this correlation.
Shelf Life Effect on Taste Profile
The use of e-tongue to predict changes in taste with time was demonstrated in a study about curing meat (Nodake et al. 2013). The authors emphasize the advantage of using the sensitive e-tongue to distinguish changes in taste that the sensory panel may find difficult to identify. Shelf life evaluation based on taste profile was performed for four PMG juices (from cvs. ‘Acco’, ‘Shani’, ‘Emeq’ and ‘116’) after a month’s storage at 4 °C. The shelf life of pasteurized PMG fruit juice is about 2–3 months in a sealed glass bottle. In the current study, the juices were not pasteurized and it was therefore assumed that 1 month should be sufficient to observe changes in taste. Apparently, the overall change in taste for all the cvs. during this period was less than expected. The juices maintained CA0 values at similar levels that correlated with a stable pH and as a consequence, there was no change in sourness. Whereas the sweetness sensor GL1 indicated a slight decrease (from 15.1 to 13.6 mV for ‘Acco’), together with a 10–15 % decrease in TSS, the results may suggest a growing population of microorganisms in the juice that could affect the sugar content. The more interesting result was a steady rise in EITast in ‘Acco’ juice that occurred during storage (Fig. 5). The increased astringency showed a similar trend with a more positive signal from the AAE sensor. The same behavior occurred for the other PMG cvs. but to a lesser extent. Polyphenols in fruit juices (e.g., wine, PMG) have the tendency to polymerize and cause a higher degree of astringency (Noble 2009). Peleg et al. (1999) describe the phenomenon of polymerization of flavan-3-ol molecules as a reason for increase in astringency. Such an increase in the sensor AE1 signal might have occurred due to the formation of larger complexes of phenol molecules during shelf life.
Conclusions
This research shows the ability of an electronic tongue to distinguish between juices of PMG cultivars according to their taste profiles. The results indicate good correlations between the sensor output and both PMG juice chemical composition and sensory panel evaluation. The converted e-tongue sensor values can be used as an accurate method to assess the intensity of the dominant taste attributes of the juice (bitterness, astringency, and sourness). Multivariate analysis mapped the differences between the juice types according to sensor outputs and sensory scores. The results add validation to previous studies using the e-tongue as an objective tool to evaluate the taste profile of food products. Applications using this method can be expected in places where sensory panel evaluation is not sufficient and the cause for taste impairment is not clear between samples.
References
Asikin Y, Fukunaga H, Yamano Y, Hou DX, Maeda G, Wada K (2014) Effect of cultivation line and peeling on food composition, taste characteristic, aroma profile, and antioxidant activity of Shiikuwasha (Citrus depressa Hayata) juice. J Sci Food Agric 94(12):2384–2392
Ben Nasr C, Ayed N, Metche M (1996) Quantitative determination of the polyphenolic content of pomegranate peel. Eur Food Res Technol 203(4):374–378
Bett-Garber KL, Watson MA, Lea JM, Bai J, Baldwin E, Raithore S (2014) Efficacy of monitoring the sensory taste characteristics in pomegranate juice with electronic tongue and chemical measurements. J Food Qual 37(6):383–394
Dafny-Yalin M, Glazer I, Bar-Ilan I, Kerem Z, Holland D, Amir R (2010) Color, sugars and organic acids composition in aril juices and peel homogenates prepared from different pomegranate accessions. J Agric Food Chem 58(7):4342–4352
Escuder-Gilabert L, Peris M (2010) Review: highlights in recent applications of electronic tongues in food analysis. Anal Chim Acta 665(1):15–25
Fawole OA, Opara UL (2013) Harvest discrimination of pomegranate fruit: postharvest quality changes and relationships between instrumental and sensory attributes during shelf life. J Food Sci 78(8):S1264–S1272
Hagerman AE, Butler LG (1978) Protein precipitation method for the quantitative determination of tannins. J Agric Food Chem 26(4):809–812
Hayashi N, Chen R, Ikezaki H, Yamaguchi S, Maruyama D, Yamaguchi Y, Ujihara T, Kohata K (2006) Techniques for universal evaluation of astringency of green tea infusion by the use of a taste sensor system. Biosci Biotechnol Biochem 70(3):626–631
Hayashi N, Chen R, Ikezaki H, Ujihara T (2008) Evaluation of the umami taste intensity of green tea by a taste sensor. J Agric Food Chem 56(16):7384–7387
Hayashi N, Ujihara T, Chen R, Irie K, Ikezaki H (2013) Objective evaluation methods for the bitter and astringent taste intensities of black and oolong teas by a taste sensor. Food Res Int 53(2):816–821
Johanningsmeier SD, Harris GK (2011) Pomegranate as a functional food and nutraceutical source. Ann Rev Food Sci Technol 2:181–201
Kang BS, Lee JE, Park HJ (2014) Electronic tongue-based discrimination of Korean rice wines (makgeolli) including prediction of sensory evaluation and instrumental measurements. Food Chem 151:317–323
Kobayashi Y, Habara M, Ikezazki H, Chen R, Naito Y, Toko K (2010) Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors 10(4):3411–3443
Koppel K, Chambers Iv E (2010) Development and application of a lexicon to describe the flavor of pomegranate juice. J Sens Stud 25(6):819–837
Laureati M, Buratti S, Bassoli A, Borgonovo G, Pagliarini E (2010) Discrimination and characterisation of three cultivars of Perilla frutescens by means of sensory descriptors and electronic nose and tongue analysis. Food Res Int 43(4):959–964
Mayuoni-Kirshenbaum L, Bar-Ya’Akov I, Hatib K, Holland D, Porat R (2013) Genetic diversity and sensory preference in pomegranate fruits. Fruits 68(6):517–524
Mayuoni-Kirshinbaum L, Porat R (2014) The flavor of pomegranate fruit: a review. J Sci Food Agric 94(1):21–27
Mayuoni-Kirshinbaum L, Daus A, Porat R (2013) Changes in sensory quality and aroma volatile composition during prolonged storage of ‘Wonderful’ pomegranate fruit. Int J Food Sci Technol 48(8):1569–1578
Mphahlele RR, Stander MA, Fawole OA, Opara UL (2014) Effect of fruit maturity and growing location on the postharvest contents of flavonoids, phenolic acids, vitamin C and antioxidant activity of pomegranate juice (cv. Wonderful). Sci Hortic 179:36–45
Newman J, Egan T, Harbourne N, O’Riordan D, Jacquier JC, O’Sullivan M (2014) Correlation of sensory bitterness in dairy protein hydrolysates: comparison of prediction models built using sensory, chromatographic and electronic tongue data. Talanta 126:46–53
Noble C (2009) Why do wines taste bitter and feel astringent? in Chemistry of Wine Flavor (Vol. 714): ACS Symposium Series
Nodake K, Numata M, Kosai K, Kim YJ, Nishiumi T (2013) Evaluation of changes in the taste of cooked meat products during curing using an artificial taste sensor. Anim Sci J Nihon chikusan Gakkaihō 84(8):613–621
Peleg H, Gacon K, Schlich P, Noble AC (1999) Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J Sci Food Agric 79(8):1123–1128
Qiu S, Wang J, Gao L (2015) Qualification and quantisation of processed strawberry juice based on electronic nose and tongue. LWT Food Sci Technol 60(1):115–123
Qu W, Breksa Iii AP, Pan Z, Ma H (2012) Quantitative determination of major polyphenol constituents in pomegranate products. Food Chem 132(3):1585–1591
Schutz HG, Pilgrim FJ (1957) Differential sensitivity in gustation. J Exp Psychol 54(1):41–48
Tahara Y, Toko K (2013) Electronic tongues–a review. IEEE Sensors J 13(8):3001–3011
Tezcan F, Gültekin-Özgüven M, Diken T, Özçelik B, Erim FB (2009) Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem 115(3):873–877
Tzulker R, Glazer I, Bar-Ilan I, Holland D, Aviram M, Amir R (2007) Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J Agric Food Chem 55(23):9559–9570
Ujihara T, Hayashi N, Ikezaki H (2013) Objective evaluation of astringent and umami taste intensities of matcha using a taste sensor system. Food Sci Technol Res 19(6):1099–1105
Uyen Tran T, Suzuki K, Okadome H, Homma S, Ohtsubo K (2004) Analysis of the tastes of brown rice and milled rice with different milling yields using a taste sensing system. Food Chem 88(4):557–566
Vázquez-Araújo L, Koppel K, Chambers Iv E, Adhikari K, Carbonell-Barrachina AA (2011) Instrumental and sensory aroma profile of pomegranate juices from the USA: differences between fresh and commercial juice. Flavour Fragr J 26(2):129–138
Viuda-Martos M, Fernández-Lóaez J, Pérez-álvarez JA (2010) Pomegranate and its many functional components as related to human health: a review. Compr Rev Food Sci Food Saf 9(6):635–654
Woertz K, Tissen C, Kleinebudde P, Breitkreutz J (2011) Taste sensing systems (electronic tongues) for pharmaceutical applications. Int J Pharm 417(1–2):256–271
Acknowledgments
The authors want to thank Prof. Rachel Amir from the MIGAL plant metabolism laboratory for the support with the PMG taste compounds, Tel Hai chemistry technician Natalie Laversovitc and the Fruit Storage Research Laboratory staff for the assistance in the biochemistry analysis
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Tel Hai College research authority.
Conflict of Interest
Author Ofir Benjamin declares that he has no conflict of interest. Author Danny Gamrasni declares that he has no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Benjamin, O., Gamrasni, D. Electronic Tongue as an Objective Evaluation Method for Taste Profile of Pomegranate Juice in Comparison with Sensory Panel and Chemical Analysis. Food Anal. Methods 9, 1726–1735 (2016). https://doi.org/10.1007/s12161-015-0350-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0350-0