Abstract
In the present work, capillary electrophoresis (CE) was used for the first time for the simultaneous analysis of azodicarbonamide (ADA) and semicarbazide (SEM), and the capillary electrophoresis separation conditions, extraction agents, and derivatization conditions were investigated. In 20 mmol L−1 sodium tetraborate, 30 mmol L−1 β-cyclodextrin (β-CD), 17 % isopropanol (v/v), and 25 mmol L−1 sodium dodecyl sulfate (SDS) running buffer, ADA and SEM previously derivatized with 9-fluorenylmethyl chloroformate (FMOC) were separated in less than 25 min with good sensitivity. The linear ranges were 8.3 × 10−4∼6.6 × 10−2 mmol L−1 and 1.9 × 10−3∼3.4 × 10−2 mmol L−1, and detection limits (S/N = 10) were 0.5 and 0.15 mg kg−1 for ADA and SEM, respectively. The proposed method was successfully applied for the simultaneous analysis of ADA and SEM in five flour samples with satisfactory recovery data from 88.0 to 93.0 % for ADA and 98.0 to 106.0 % for SEM, indicating the valuable potential application of this method for food analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Azodicarbonamide (ADA), which previously was used as a blowing agent in rubber products and foamed polyethylene, is also approved as a food additive in certain countries (Becalski et al. 2004). ADA itself has low acute toxicity, however chronic exposure to ADA will bring some adverse effects such as respiratory symptoms and skin sensitization reactions. There were no reports relating the potential of ADA to produce other systemic adverse effects. Flour is one of the main food in most countries. As a new flour gluten fortifier, ADA has been widely used in China, USA, and some other countries due to the advantage of low cost, dough-improving, and good test. The limit of ADA in flour issued by the US Food and Drug Administration (FDA) is 45 mg kg−1, while it had been banned in the European Union (Joiner et al. 1963; Ye et al. 2011). ADA is stable in dry flour and however often reacts with moist flour as an oxidizing agent. The main reaction product is biurea, an essentially nontoxic compound which is not stable during heating. It has been proved that acid hydrolysis of biurea can form semicarbazide (SEM). Earlier studies showed that SEM had mutagenic activity and latent carcinogenicity, while recent researches have further proved that SEM not only caused histological and morphological alternation in target organs but also disturbed the nervous and endocrine systems. Although ADA had been banned in the European Union, in October 2003, European Food Safety Authority (EFSA) reported that foamed polyvinylchloride (PVC) cap liners manufactured with ADA were the source of SEM contamination, and ADA was also found in a variety of jarred foods (Becalski et al. 2004; Ye et al. 2011). In recent years, ADA as the source of SEM contamination in flour and flour products has been reported by many researches (Becalski et al. 2004, 2006; Noonan et al. 2005, 2008; De la Calle and Anklam 2005; Chen et al. 2008; Ye et al. 2011). So, it is necessary to develop effective analytical methods for the monitoring of ADA and SEM.
To date, various detection techniques including high-performance liquid chromatography (HPLC) (Bechtold et al. 1988, 1989; Mulder et al. 2007), liquid chromatography-mass spectrometry (LC-MS) (Noonan et al. 2005), liquid chromatography-electrospray tandem mass spectrometry (LC-MS/MS) (Leitner et al. 2001; Ye et al. 2011), surface-enhanced Raman spectroscopy (SERS), and others have been applied in the determination of ADA and SEM. However, these methods are time-consuming, expensive, and labor-intensive, and above all, the flour sample pretreatment process was complicated and no researches were reported referring to the simultaneous detection of ADA and SEM. So, there is an urgent need to develop a rapid and simple method for the simultaneous monitoring of ADA and SEM in flour. In comparison, capillary electrophoresis (CE) is a simple, environmentally friendly, and less expensive method with comparable separation performance, which can be used as a good alternative to HPLC. There have been many reports describing the analysis of food components using CE (Pinero et al. 2011; Mu et al. 2012; Castro-Puyana et al. 2012; García-Cañas et al. 2014). Petruci et al. (2013) successfully determined 2-methylimidazole and 4-methylimidazole in caramel colors by CE method. Tezcan et al. (2013) successfully applied micellar electrokinetic chromatography-laser-induced fluorescence (MEKC-LIF) method for the determination of amino acids in pomegranate juices. An electrophoretic method based on MEKC used green reagents to determine all of the ten water-soluble vitamins in food supplements (Da Silva et al. 2013). Analysis of amino acids in the vinegars using CE was reported by Carlavilla et al. (2006).
Although CE methods have been adopted well in many analyses, the concentration detection limit with a conventional UV detector is restricted. This is considered as a major disadvantage of CE. On the other hand, some analytes such as SEM and some amino acids lack UV absorbance. Thus, derivatization using a suitable chromophore becomes necessary for the sensitive detection of these analytes. Such a derivatization can lead to a considerable increase in sensitivity (Noonan et al. 2005). Up to now, many derivatization agents such as o-phthaldialdehyde (OPA) (Ro and Hahn 2005), naphthalene-2,3-dicarboxaldehyde (NDA) (Tseng et al. 2009), fluorescein isothiocyanate (FITC) (Carlavilla et al. 2006), dansyl chloride (DNS) (Giuffrida et al. 2009), and 9-fluorenylmethyl chloroformate (FMOC) (Han and Chen 2007) have been used for the derivatization. FMOC, a chloroformate type of fluorescent derivatization reagent, the reaction conditions of which are simple, could also provide excellent spectral properties. In addition, the advantages of FMOC, including low cost of chemicals, a short reaction time, highly stable derivatives, and high-yield derivatives, are also attracted. FMOC is often used as the derivatization reagent of amino acids (AAs). For example, Wan and Blomberg (2000) adopted MEKC to separate AAs derivatized with FMOC. Derivatization of AAs with FMOC and the analysis of FMOC-AAs by chiral MEKC have been reported by Fradi et al. (2012). Thus, FMOC was preferred to be applied to SEM derivatization in this work.
The detection of ADA and SEM in the flour by CE has not been reported. The aim of this study was to develop a simple sample pretreatment process and an effective method for simultaneous CE analysis of ADA and SEM in the flour samples. In this work, a method of sample pretreatment procedure for extracting ADA and SEM in flour adopted was simpler than ever before; then, the MEKC method had been firstly successfully applied for the simultaneous analysis of ADA and SEM in the flour.
Materials and Methods
Chemicals and Samples
ADA (99 %) and SEM (98 %) were purchased from Beijing Ouhe Technology Co., Ltd. β-Cyclodextrin (β-CD) was obtained from Solarbio (Beijing, China). FMOC was supplied from J&K Scientific. Acetonitrile, ethyl acetate, isopropanol, acetone, and sodium tetraborate were purchased from Beijing Chemical Works. Other chemicals were of analytical grade or above.
Five different brands of flour were studied in this work. All of the samples were bought from local supermarket. Stock solutions of SEM were prepared in deionized water. ADA was dissolved in 20 mmol L−1 sodium tetraborate. FMOC was dissolved in acetonitrile at a concentration of 10 mmol L−1. Standard solutions were stored in the dark at 4 °C.
Instrumentation and Conditions
CE separations were performed on a P/ACE MDQ capillary electrophoresis system equipped with DAD detector (Beckman, USA). The CE instrument was controlled by a PC running the System Karat 32 software from Beckman. Fused-silica capillaries of 75 μm i.d. × 375 μm o.d., 62 cm total length, and 50 cm effective length (Hebei Yongnian Optical Fiber Factory, China) were used for CE separation under 25 °C. The CE procedure used 20 mmol L−1 sodium tetraborate at pH 9.2, 30 mmol L−1 β-CD, 17 % isopropanol (v/v), and 25 mmol L−1 sodium dodecyl sulfate (SDS) as the background electrolyte (BGE). The detection wavelength was set at 203 nm, and the operating voltage was 20 kV. The capillary was rinsed with acetonitrile for 1 min, followed by a 1-min flush with 1 mol L−1 sodium hydroxide (NaOH) solution, a 1-min flush with deionized water, and a 2-min flush with electrolyte solution before each run. All solutions were filtered through 0.45-μm membranes prior to use.
Sample Preparation
Flour samples (respectively, 1 g) were homogenized with 10 mL of 20 mmol L−1 sodium tetraborate (pH 9.2) in 50-mL centrifuge tubes. The samples were first shaken on a rotary shaker at room temperature for 15 min. After the mixtures were centrifuged at 10,000 rpm for 5 min, 8 mL of supernatant on upper was extracted and 1 mL of acetonitrile and 300 μL of 10 mmol L−1 FMOC (dissolved in acetonitrile) were then added for reaction. The mixtures were heated at 37 °C for 2 h. After derivatization with FMOC, the pH was adjusted to between 7 and 7.5 with 1 mol L−1 HCl and 1 mol L−1 NaOH, and the derivatives were extracted (phase separation assisted by centrifugation) with 10 mL of ethyl acetate for three times. The ethyl acetate was removed by reduced pressure distillation at 25 °C, and the residues were dissolved in 25 % acetonitrile in water, filtered through a 0.22-μm filter prior to CE analysis.
Results and Discussion
Optimization of Derivatization Procedure
In this study, the experiment results showed that FMOC can rapidly react with SEM to generate highly stable derivatives, and the MEKC method which was successfully applied to determine FMOC-labeled SEM can also detect FMOC-labeled ADA. After derivatization, the analytical sensitivity of ADA and SEM was increased. The derivatization procedure including reaction time and pH of sodium borate was studied in order to achieve the maximum signal. Figure 1 shows derivatization reaction of ADA was slow when the sodium borate was neutral (pH 7.0), but very propitious at pH 9.2. Therefore, sodium borate of pH 9.2 was selected in the derivatization reaction. For the trace analysis of SEM and ADA, the reaction could be completely finished within 2 h. At last, the derivatization procedure was mixing 1 mL of acetonitrile and 300 μL of 10 mmol L−1 FMOC (dissolved in acetonitrile) with 8 mL of supernatant extracted on upper from flour samples; then, the reaction mixtures were heated on a rotary shaker at 37 °C for 2 h.
Optimization of Electrophoretic Conditions
As mentioned above, CE was used for the simultaneous analysis of ADA and SEM in the flour products. In the separation, the BGE is an important factor. According to the characteristics of these analytes, different BGEs would have different separation effects. Because the derivatization with FMOC takes place at the amino group of SEM, the acid-base properties of SEM derivatives will not have obvious differences. So, capillary zone electrophoresis (CZE) based only on the pH-sensitive properties cannot separate derivatives. The peaks of FMOC and SEM derivatives are nearly at the same time in the BGE containing 15 mmol L−1 borate buffer (pH 9.2). However, when a BGE containing 25 mmol L−1 SDS and 20 mmol L−1 borate buffer (pH 9.2) was used, a better separation between FMOC and the two derivatives was achieved as shown in Fig. 2. Thus, MEKC method is a better choice for the separation of FMOC and the derivatives.
Electropherograms of FMOC, FMOC-labeled SEM, and FMOC-labeled ADA using a 20 mmol L−1 SDS and 20 mmol L−1 sodium tetraborate buffer. Electrophoretic conditions were as for Fig. 1. Asterisk indicates solvent peak
The separation conditions were investigated, and the optimal separation conditions were selected as 20 mmol L−1 sodium tetraborate, 30 mmol L−1 β-CD, 17 % isopropanol (v/v), and 25 mmol L−1 SDS. According to the UV absorption spectrum, the absorption peak of analytes in 203 nm was significant. So, the detection wavelength was set at 203 nm. The temperature and voltage of the separation were established at 25 °C and 20 kV since a good resolution of the analytes was obtained in less time and with an acceptable current (below 40 μA). As shown in Fig. 3, FMOC, FMOC-labeled SEM, and FMOC-labeled ADA obtained a good separation at the optimal conditions.
Electropherogram of standard FMOC-labeled SEM and FMOC-labeled ADA in the buffer solution of 20 mmol L−1 sodium tetraborate at pH 9.2, 30 mmol L−1 β-CD, 17 % isopropanol, and 25 mmol L−1 SDS; other conditions were as for Fig. 1. Asterisk indicates solvent peak
Optimization of Sample Pretreatment
The presence of interferences is one of the main difficulties in the development of an analytical method for a complex matrix. In the previous research, the pretreatment of the flour sample for the analysis of ADA and SEM usually only focused on either of them, and the reported HPLC methods only included individual extraction of ADA. As CE has high separation efficiency, we can simply extract derivatives by ethyl acetate without solid-phase extraction device which was used in the previous method. What is more, the derivatization time was shorter than before. Different sample pretreatments would have been done in the previous method for ADA and SEM, respectively (Noonan et al. 2005; 2008), for an example of SEM extraction, complex C18 solid-phase extraction technology was used in both the sample extraction and after derivatization in the previous method. So far, there were no papers reporting simultaneous extraction of ADA and SEM. A suitable extraction solvent should be chosen appropriately for the simultaneous extraction of ADA and SEM. ADA which has strong polarity could be dissolved in dimethyl sulfoxide or N,N-dimethylformamide, and it is slightly soluble in water, acetone, alcohol, etc. (Chen et al. 2008). However, as an extraction solvent, dimethyl sulfoxide and N,N-dimethylformamide are uneasy to evaporate and remove. In this work, for SEM, it could be dissolved in water and hardly dissolved in acetone and acetonitrile. Water has been selected as an extraction solvent, since it can meet the demand of trace extraction of SEM and ADA. Although using water as an extraction solvent cannot avoid the introduction of the water-soluble impurities, the impurities were removed by the following derivative extraction procedure and separated by optimized MEKC method.
After extraction with water, 1 mL of acetonitrile and 300 μL of 10 mmol L−1 FMOC (dissolved in acetonitrile) were added to 8 mL of supernatant extracted on upper, as described in the derivatization procedure. The extraction of derivatives with ethyl acetate was further optimized in this study for removing the impurities in the aqueous phase. In order to make sure the derivative extraction was complete, we detected the residual derivatives in the aqueous phase. By comparison, extraction with 10 mL of ethyl acetate for three times can achieve efficient extraction, as shown in Fig. 4. After removing the ethyl acetate by reduced pressure distillation at 25 °C, residues were redissolved in 25 % acetonitrile in water, filtered through a 0.22-μm filter prior to use. No more additional treatments of the samples were performed for MEKC analysis.
Effect of the amount of ethyl acetate. Electropherograms of a residual in water phase after extraction with ethyl acetate (3 × 10 mL), b blank flour sample, c spiked flour sample after extraction with ethyl acetate (3 × 5 mL), and d spiked flour sample after extraction with ethyl acetate (3 × 10 mL). Electrophoretic conditions were as for Fig. 1
Quantification of ADA and SEM
The present method exhibited good stability and repeatability for analysis of FMOC-labeled SEM and FMOC-labeled ADA in less than 25 min. The relative standard deviations (RSDs) of peak area and migration time of analytes were evaluated by five consecutive analyses. As expected, the RSDs were less than 2.00 % for peak area and 0.90 % for migration time. Analytical curves were constructed on the basis of peak area versus concentration. As shown in Fig. 5, the MEKC method had a good linearity in the range of 8.3 × 10−4∼6.6 × 10−2 mmol L−1 (r 2 = 0.998) for analysis of ADA and had a good linearity in the range of 1.9 × 10−3∼3.4 × 10−2 mmol L−1 (r 2 = 0.998) for analysis of SEM, respectively. The limit of quantification (S/N = 10, actual samples) was 0.50 and 0.15 mg kg−1 for ADA and SEM, respectively. In order to evaluate possible interferences, blank samples were submitted to the proposed method and no peaks were found comigrating with the analytes. When increasing the concentration of the analytes spiked, the peaks also increased. The recoveries of ADA and SEM from flour spiked at three levels of 5.0, 10.0, and 20.0 mg kg−1 were in the range of 88.0∼93.0 % (RSD = 3.0 %) and 98.0∼106.0 % (RSD = 9.1 %), respectively. (The results obtained for the accuracy of the method are summarized in Table 1.)
Calibration curves obtained for ADA and SEM (S/N = 10). Electrophoretic conditions were as for Fig. 1
Analysis of ADA and SEM in Commercial Flours
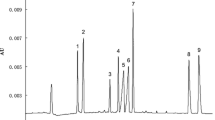
This new method shows good stability, sensitivity, and satisfactory recoveries for the studied chemicals. These characteristics mentioned above indicated that the proposed method could be suitable for the monitoring of ADA and SEM in flour in the laboratory. Five bands of flour (F1, F2, F3, F4, and F5) were studied in this work. The results are summarized in Table 2, and the corresponding electropherograms of samples are shown in Fig. 6. As seen from the electropherograms, ADA and SEM existed in all flour samples, and these results showed that the concentration of ADA in the flour samples was higher than the concentration of SEM. The above conclusion was suitable for all studied samples.
Electropherograms of ADA and SEM in five commercial flour samples. Electrophoretic conditions were as for Fig. 1
Conclusion
In summary, a simple and reliable MEKC method was established for simultaneous quantifying of ADA and SEM in the flour samples. A simple method of sample pretreatment was provided before MEKC analysis. The MEKC method is simple, rapid, and less expensive in comparison with previous methods. The method firstly realized the simultaneous detection of ADA and SEM and was successfully applied to the analysis of commercial samples. The performance characteristics indicated that the proposed methodology should be suitable for the routine analysis of ADA and SEM in the flour samples.
References
Becalski A, Lau BPY, Lewis D, Seaman SW (2004) Semicarbazide formation in azodicarbonamide-treated flour: a model study. J Agric Food Chem 52:5730–5734
Becalski A, Lau BPY, Lewis D, Seaman SW (2006) Semicarbazide in Canadian bakery products. Food Addit Contam 23:107–109
Bechtold WE, Shopp GM, Cheng YS (1988) The determination of biurea in the presence of azodicarbonamide by HPLC. J Anal Toxicol 12:89–93
Bechtold WE, Medinsky MA, Cheng YS, Hobbs CH (1989) Azodicarbonamide: methods for the analysis in tissues of rats and inhalation disposition. Xenobiotica 19:1003–1012
Carlavilla D, Moreno-Arribas MV, Fanali S, Cifuentes A (2006) Chiral MEKC-LIF of amino acids in foods: analysis of vinegars. Electrophoresis 27:2551–2557
Castro-Puyana M, García-Cañas V, Simó C, Cifuentes A (2012) Recent advances in the application of capillary electromigration methods for food analysis and Foodomics. Electrophoresis 33:147–167
Chen B, Jin BH, Lin YK, Xie LQ (2008) Determination of azodicarbonamide in flour by high-performance liquid chromatography. J Instrum Anal 27:1002–1004
Da Silva DC, Visentainer JV, De Souza NE, Oli-veira CC (2013) Micellar electrokinetic chromatography method for determination of the ten water-soluble vitamins in food supplements. Food Anal Methods 6:1592–1606
De la Calle MB, Anklam E (2005) Semicarbazide: occurrence in food products and state-of-the-art in analytical methods used for its determination. Anal Bioanal Chem 382:968–977
Fradi I, Servais AC, Lamalle C, Kallel M, Abidi M, Crommen J, Fillet M (2012) Chemo- and enantio-selective method for the analysis of amino acids by capillary electrophoresis with in-capillary derivatization. J Chromatogr A 1267:121–126
García-Cañas V, Simó C, Castro-Puyana M, Cifuentes A (2014) Recent advances in the application of capillary electromigration methods for food analysis and Foodomics. Electrophoresis 35:147–169
Giuffrida A, Contino A, Maccarrone G, Messina M, Cucinotta V (2009) The 3-amino-derivative of γ-cyclodextrin as chiral selector of Dns-amino acids in electrokinetic chromatography. J Chromatogr A 1216:3678–3686
Han Y, Chen Y (2007) On-column labeling technique and chiral CE of amino acids with mixed chiral selectors and UV detection. Electrophoresis 28:2765–2770
Joiner RR, Vidal FD, Marks HC (1963) A new powdered agent for flour maturing. Cereal Chem 40:539–553
Leitner A, Zollner P, Lindner W (2001) Determination of the metabolites of nitrofuran antibiotics in animal tissue by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 939:49–58
Mu GF, Liu HT, Gao Y, Luan F (2012) Determination of benzoyl peroxide, as benzoic acid, in wheat flour by capillary electrophoresis compared with HPLC. J Sci Food Agric 92:960–964
Mulder PPJ, Beumer B, Van Rhijn JA (2007) The determination of biurea: a novel method to discriminate between nitrofurazone and azodicarbonamide use in food products. Anal Chim Acta 586:366–373
Noonan GO, Warner CR, Hsu W, Begley TH, Perfetti GA, Diachenko GW (2005) The determination of semicarbazide (Naminourea) in commercial bread products by liquid chromatography-mass spectrometry. J Agric Food Chem 53:4680–4685
Noonan GO, Begley TH, Diachenko GW (2008) Semicarbazide formation in flour and bread. J Agric Food Chem 56:2064–2067
Petruci JFD, Pereira EA, Cardoso AA (2013) Determination of 2-methylimidazole and 4-methylimidazole in caramel colors by capillary electrophoresis. J Agric Food Chem 61:2263–2267
Pinero MY, Bauza R, Arce L (2011) Thirty years of capillary electrophoresis in food analysis laboratories: potential applications. Electrophoresis 32:1379–1393
Ro KW, Hahn JH (2005) Precolumn diastereomerization and micellar electrokinetic chromatography on a plastic microchip: rapid chiral analysis of amino acids. Electrophoresis 26:4767–4773
Tezcan F, Uzasci S, Uyar G, Oztekin N, Erim FB (2013) Determination of amino acids in pomegranate juices and fingerprint for adulteration with apple juices. Food Chem 141:1187–1191
Tseng WL, Hsu CY, Wu TH, Huang SW, Hsieh MM (2009) Highly sensitive detection of chiral amino acids by CE based on on-line stacking techniques. Electrophoresis 30:2558–2564
Wan H, Blomberg LG (2000) Chiral separation of amino acids and peptides by capillary electrophoresis. J Chromatogr A 875:43–88
Ye J, Wang XH, Sang YX, Liu Q (2011) Assessment of the determination of azodicarbonamide and its decomposition product semicarbazide: investigation of variation in flour and flour products. J Agric Food Chem 59:9313–9318
Compliance with Ethical Standards
Funding
This study was funded by the State Key Laboratory of Environment Chemistry and Ecotoxicology, Research Center for Eco-environmental Sciences, Chinese Academy of Sciences (KF2014-06); the Special Fund for Strategic Pilot Technology Chinese Academy of Sciences (XDB14030203); the National Natural Science Foundation of China (21405159); and the China Postdoctoral Science Foundation (No. 2014M550800).
Conflict of Interest
Li Chen declares that she has no conflict of interest. Hong Cui declares that she has no conflict of interest. Yalei Dong declares that she has no conflict of interest. Dongqiang Guo declares that he has no conflict of interest. Yujian He declares that he has no conflict of interest. Xiangjun Li declares that he has no conflict of interest. Zhuobin Yuan declares that he has no conflict of interest. Hong Zou declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Li Chen and Hong Cui contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, L., Cui, H., Dong, Y. et al. Simultaneous Detection of Azodicarbonamide and the Metabolic Product Semicarbazide in Flour by Capillary Electrophoresis. Food Anal. Methods 9, 1106–1111 (2016). https://doi.org/10.1007/s12161-015-0276-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0276-6