Abstract
In this study, manganese(IV) oxide (MnO2) nanoparticle (np)-modified glassy carbon paste electrode is used for ascorbic acid (AA) detection in fruit juice samples. The experimental parameters like MnO2 np amount and pH were optimized by using modified full factorial design model. By means of this model, the number of experiments has been reduced. Under optimal conditions, the linear range for AA was obtained between 2.64 × 10−6 and −1.5 × 10−3 M. Limit of detection (LOD) (3 s/m) and relative standard deviation (RSD) were calculated as 8 × 10−7 M and 4.56 %, respectively. Developed sensor was applied to AA detection in fruit juice samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of metal/metal oxide nanoparticle superstructures for the organization of electrochemical sensing devices is an extremely promising prospect. In electroanalytical applications, metal nanoparticles provide some important functions including the roughening of the conductive sensing interface and some catalytic properties that result in amplification of electrochemical signal (Wang 2005). Chemical properties of some nanoparticles could be different from those of the bulk materials since nanostructures have higher surface energy than bulk counterparts. Due to this property, usually nanoparticles are chemically more active than the related bulk materials.
Manganese(IV) oxides (MnO2) are a kind of inorganic materials that show catalytic activity towards some reagents. Also, it is known that bulk MnO2 catalyzes the decomposition of H2O2 while MnO2 nanoparticles (MnO2 np) react with H2O2 directly (Luo et al. 2004a, b). By using this advantage, various biosensors based on H2O2 monitoring have been constructed (Yao et al. 2006; Hocevar et al. 2004; Lin et al. 2005). It has also been demonstrated that by using MnO2 np, more sensitive results were obtained both for lactate (Xu et al. 2005) and ascorbic acid (AA) (Luo et al. 2004a, b).
AA is a water-soluble vitamin which exists in biological fluids, fruit juice, and vegetables. Pharmaceutical, chemical, cosmetic, and food sectors benefit from it as an antioxidant (Prasad et al. 2011). It is widely used to prevent and treat some diseases such as common cold, mental illness, infertility, and in some manifestations of HIV infection. On the other hand, diseases such as cancer, diabetes mellitus, and hepatic disorders are related to AA concentration in the body fluids (Mbouguen et al. 2011; Mallesha et al. 2011).
Although electrochemical methods are majorly used techniques for AA detection, methods like fluorescence, chemiluminescence, high-performance liquid chromatography, capillary zone electrophoresis, flow-injection spectrophotometry, and colorimetric determination have also been utilized for this purpose (Liu et al. 2015). On the other hand, in electrochemical analyses, traditional electrodes have some disadvantages like low selectivity and sensitivity, poor reproducibility, and electrode fouling.
In present work, MnO2 np-modified glassy carbon paste electrode (GCPE) was firstly used for detection of AA. The experimental parameters like MnO2 np amount and pH were optimized by using modified full factorial design model. Under optimal conditions, the linear range for AA was obtained between 2.64x10−6 and −1.5x10−3 M. Then, analytical characteristics were explored, and developed electrode was applied for AA detection in fruit juice samples.
Experimental
Reagents
Glassy carbon spherical powder (20–50 μM) was purchased from Alfa Aesar (www.merck.de). AA, mineral oil, manganese acetate, and potassium permanganate were purchased from Sigma-Aldrich (www.sigmaaldrich.com). All solutions were prepared by double distilled water.
Apparatus
Differential pulse (DP) measurements were carried out a μ-AUTOLAB type III electrochemical measurement system from ECO CHEMIE Instruments B.V. (Netherlands), driven by GPES software. GCPE and MnO2 np-modified GCPE were used as working electrodes. Ag|AgCl electrode and Pt electrode were used as reference and auxiliary electrode, respectively. The electrodes were inserted into a conventional electrochemical cell. The size of MnO2 np was measured using JEOL-JEM 2100 transmission electron microscopy (TEM). Also during the preparation of MnO2 np, Sigma 3-16 PK-type centrifuge was used.
Preparation of MnO2 np
MnO2 np (20 nm in size) was prepared according to the literature (Luo et al. 2004a, b; Bai et al. 2007). Briefly, 0.10 M potassium permanganate solution (40 mL) was added to 4 mL of 1.5 M manganese acetate solution under ultrasonication at 25 °C. After reaction, the brown solution was centrifuged (about 10,000 rpm). The deposition (MnO2 np) was washed with water repeatedly until the washing solution was neutral. Obtained MnO2 nps were found to be in the size of 20 nm from TEM measurements (Fig. 1). The nanoparticles were kept at 4 °C in distilled water when not used.
Preparation of Electrodes
MnO2 np modified-GCPE was prepared by hand mixing 80:20 (% w/w) glassy carbon spherical powder/mineral oil and 2 μL MnO2. A portion of the resulting paste was then packed firmly into the electrode cavity (3.0 mm in diameter and 5.0 mm in depth) of a PTFE sieve where electrical contact was established via a copper wire.
Sample Application
The contents of AA in orange juice were determined. For this purpose, the sample solutions were stirred for a while and used as stock substrate solutions without any dilution. Then, known amount of sample solution was added to the reaction cell containing working buffer. The signals obtained from the samples were recorded, and AA concentration was calculated by using a calibration graph.
Results and Discussion
Effect of MnO2 np
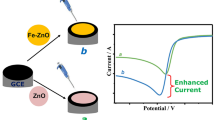
It has been known that nanoparticles provide fast mass transport, sensitivity, and electrocatalytic activity when used in electrodes (Cevik and Anik 2010). To compare the performances and observe the difference, electrochemical behavior of plain GCPE and MnO2 np-modified GCPE were compared for the oxidation of AA. As can be seen clearly from Fig. 2, the best current value was obtained with MnO2 np-modified GCPE. This result proves that the usage of MnO2 np provides better sensitivity compared to bare GCPE. Considering the oxidation reaction of AA to dehydroascorbic acid, it can be stated that MnO2 np could be fastened by this reaction (Luo et al. 2004a, b).
Optimization of pH and Electrode Structure
The experimental parameters like MnO2 np amount and pH of acetate buffer were optimized by using a full factorial experimental design modified added overall central point. Experimental running ranges and measured current values as response were given in Table 1. Analysis of the experimental design was conducted by Design-Expert software. Quadratic response surface function chosen for optimization was given as follows:
Response surface function for optimized processes parameters was plotted on Fig. 3, and the optimization zone on the figure could be seen in detail.
Analytical Characteristics
The linear range for MnO2 np-modified GCPE was found between 2.64 × 10−6 and −1.5 × 10−3 M AA with the equation of y = 0.0062x + 0.3676 and R 2 of 0.991 (Fig. 4). This range is better than hydroquinone-modified chitosan/carbon film electrode (Jirimali et al. 2013), polyaniline-modified screen-printed carbon electrode (Kit-Anan et al. 2012), poly(bromocresol purple)-modified glassy carbon electrode (Zhang et al. 2013), and gold nanoparticles-TiO2 nanotubes-Ti electrode (Hosseini et al. 2011). At higher concentrations, a standard curve showed a deviation from linearity. LOD was calculated 8 × 10−7 M. The repeatability of the biosensor was tested for 25 μM of AA (n = 4), and the relative standard deviation (RSD) was calculated as 4.6 %.
The obtained linear range for AA was compared with other reported literature methods for AA determination (Table 2). As can be seen from Table 2, MnO2 np-modified GCPE exhibits lower linear range.
Interference Study
Determination of AA in the presence of uric acid (UA) was also studied using MnO2 np-modified glassy carbon paste electrode. For this purpose, interference studies were performed in acetate buffer containing 25 μM AA-25 μM UA, 25 μM AA-50 μM UA, and 25 μM AA-100 μM UA. Obtained current values were compared to 25 μM AA results. Current values showed a difference lower than 1 %. As a result, it can be concluded that UA did not show interference for detection of AA.
AA Determination in Real Samples
The sample solutions were prepared as described in section 2.5. Two different AA concentrations, 25 and 50 μM, were tried, and experiments were repeated for three times. All the results are given in Table 3. Obtained results from the biosensor were compared to the AA content of fruit juice that was calculated from product label written by the manufacturer. As can be seen from Table 3, very promising recovery values were obtained demonstrating that designed system is suitable for the detection of AA in real samples.
Conclusion
MnO2 np-modified GCPE was prepared, and its performances were examined for AA detection. The experimental parameters like MnO2 np amount and pH were optimized by using a modified full factorial design model. Under optimum conditions, the current values display a linear relationship with respect to the concentrations of AA in the range from 2.64 × 10−6 to −1.5 × 10−3. Meanwhile, the detection limit was 8 × 10−7 M. Developed sensor was applied to AA detection in fruit juice samples, and good recovery values were obtained.
References
Amiri M, Imanzadeh H, Banaei A (2015) Carbon nanoparticles with tosyl functional group for distinguishing voltammetric peaks of ascorbic acid and uric acid. Mater Sci Eng C 47:189–195
Bai YH, Du Y, Xu JJ, Chen HY (2007) Choline biosensors based on a bi-electrocatalytic property of MnO2 nanoparticles modified electrodes to H2O2. Electrochem Commun 9:2611–2616
Cevik S, Anik U (2010) Banana tissue-nanoparticle/nanotube based glassy carbon paste electrode biosensors for catechol detection. Sens Lett 8:667–671
Habibi B, Jahanbakhshi M, Pournaghi-Azar HM (2011) Differential pulse voltammetric simultaneous determination of acetaminophen and ascorbic acid using single-walled carbon nanotube-modified carbon–ceramic electrode. Anal Biochem 411:167–175
Hocevar SB, Ogorevc B, Schachl K, Kalcher K (2004) Glucose microbiosensor based on MnO2 and glucose oxidase modified carbon fiber microelectrode. Electroanalysis 16:1711–1716
Hosseini MG, Faraji M, Momeni MM (2011) Application of titanium oxide nanotube films containing gold nanoparticles for the electroanalytical determination of ascorbic acid. Thin Solid Films 519:3457–3461
Hu G, Ma Y, Guo Y, Shao (2008) Electrocatalytic oxidation and simultaneous determination of uric acid and ascorbic acid on the gold nanoparticles-modified glassy carbon electrode. Electrochim Acta 53:6610–6615
Jirimali HD, Nagarale RK, Saravanakumar D, Lee JM, Shin W (2013) Hydroquinone modified chitosan/carbon film electrode for the selective detection of ascorbic acid. Carbohydr Polym 92:641–644
Kit-Anan W, Olarnwanich A, Sriprachuabwong C, Karuwan C, Tuantranont A, Wisitsoraat A, Srituravanich W, Pimpin A (2012) Disposable paper-based electrochemical sensor utilizing inkjet-printed polyaniline modified screen-printed carbon electrode for ascorbic acid detection. J Electroanal Chem 685:72–78
Lin Y, Cui X, Liyu L (2005) Low-potential amperometric determination of hydrogen peroxide with a carbon paste electrode modified with nanostructured cryptomelane-type manganese oxides. Electrochem Commun 7:166–172
Liu J-J, Chenc Z-T, Tanga D-S, Wanga Y-B, Kanga L-T, Yaoe J-N (2015) Graphene quantum dots-based fluorescent probe for turn-on sensing of ascorbic acid. Sensors Actuators B 212:214–219
Luo XL, Xu JJ, Zhao W, Chen HY (2004a) A novel glucose ENFET based on the special reactivity of MnO2 nanoparticles. Biosens Bioelectron 19:1295–1300
Luo XL, Xu JJ, Zhao W, Chen HY (2004b) Ascorbic acid sensor based on ion-sensitive field-effect transistor modified with MnO2 nanoparticles. Anal Chim Acta 512:57–61
Mallesha M, Manjunatha R, Nethravathi C, Suresh GS, Rajamathi M, Melo JS, Venkatesha TV (2011) Functionalized-graphene modified graphite electrode for the selective determination of dopamine in presence of uric acid and ascorbic acid. Bioelectrochemistry 81:104–108
Mbouguen JCK, Kenfacka IT, Walcarius A, Ngameni E (2011) Electrochemical response of ascorbic and uric acids at organoclay film modified glassy carbon electrodes and sensing applications. Talanta 85:754–762
Prasad BB, Kumar D, Madhuri R, Tiwari MP (2011) Ascorbic acid imprinted polymer-modified graphite electrode: a diagnostic sensor for hypovitaminosis C at ultra trace ascorbic acid level. Sens Actuators B 160:418–427
Wang J (2005) Nanomaterial-based electrochemical biosensors. Analyst 130:421–425
Xu JJ, Zhao W, Luo XL, Chen HY (2005) A sensitive biosensor for lactate based on layer-by-layer assembling MnO2 nanoparticles and lactate oxidase on ion-sensitive field-effect transistors. Chem Commun 3:792–794
Yao S, Xu J, Wang Y, Chen X, Xu Y, Hu S (2006) A highly sensitive hydrogen peroxide amperometric sensor based on MnO2 nanoparticles and dihexadecyl hydrogen phosphate composite film. Anal Chim Acta 557:78–84
Yu Z, Li H, Lu J, Zhang X, Liu N, Zhang Xu Z (2015) Hydrothermal synthesis of Fe2O3/graphene nanocomposite for selective determination of ascorbic acid in the presence of uric acid. Electrochim Acta 158:264–270
Zhang R, Liu S, Wang L, Yang G (2013) Electroanalysis of ascorbic acid using poly(bromocresolpurple) film modified glassy carbon electrode. Measurement 46:1089–1093
Compliance with Ethical Standards
Conflict of Interest
Serdar Çevik declares that he has no conflict of interest. Ülkü Anık declares that she has no conflict of interest. Oğuz Akpolat declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Informed Consent
Informed consent was not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Çevik, S., Akpolat, O. & Anik, Ü. Ascorbic Acid Detection with MnO2-Modified GCPE. Food Anal. Methods 9, 500–504 (2016). https://doi.org/10.1007/s12161-015-0221-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0221-8