Abstract
A high-performance liquid chromatography–fluorescence detection (HPLC-FLD) method was developed and validated for choline quantification in foods. Samples were extracted by homogenizing in chloroform/methanol/water and hydrolyzing in HCl-acetonitrile. Choline was derivatized using 1-naphthyl isocyanate and quantified by HPLC-fluorescence detection. Average recovery using choline iodide as the standard (n = 6) ranged from 84 ± 6 % for whole-wheat flour to 106 ± 5 % for milk. Recovery after addition of dietary lecithin to two different food matrices faba beans and for whole-wheat flour (n = 6) was 83 ± 5 %. The precision of the analysis (coefficient of variation) ranged from 2 to 13 %. Accuracy was evaluated by analyzing dietary lecithin using HPLC-FLD, liquid chromatography–mass spectrometry, and nuclear magnetic resonance, which across the different methods agreed within 15 %. This method was applied to quantify the choline content in different food matrices, and provides a simple, inexpensive method that could be widely used.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Choline, which occurs widely in nature, is a dietary component that is essential for mammals (Zeisel and Da Costa 2009). Choline is important for the synthesis of phospholipids that are a major constituent of cell membranes, as well as a source of methyl groups in one-carbon metabolism, acetylcholine synthesis, and cholinergic neurotransmission in humans (Zeisel and Blusztajn 1994). Choline can be metabolized to betaine through a two-step oxidation process in the mitochondria. Betaine has two important biological roles: it is a major osmolyte accumulated to high levels to maintain cell volume and it is a methyl group donor for the remethylation of homocysteine to methionine (Craig 2004; Lever and Slow 2010; Ueland 2011). Insufficient dietary choline intake and low choline status have been suggested as possible mechanisms for liver dysfunction, kidney failure, abnormalities in bone formation, and impaired growth (Zeisel and Da Costa 2009). In 1998, the institute of medicine established a recommended dietary intake for choline of 550 mg/day for men and 425 mg/day for women, increasing during pregnancy and lactation to 450 and 550 mg/day, respectively (Institute of Medicine 1998). Although choline can be endogenously synthesised in the body, dietary sources, either through the consumption of choline-rich foods or the use of choline supplements (for example, lecithin), are required to meet the recommended daily intake (Blusztajn 1998).
Despite the health benefits of choline, reference food composition databases for this nutrient are not widely available, except for the USDA database. Further measurements of the choline content of foods, especially those not commonly consumed or covered by the USDA data, are therefore required for both the estimation and the subsequent improvement of dietary intake. In addition, there is a need to measure the choline content of the diets of populations outside the most highly developed countries.
The majority of choline in the diet is found as phospholipids, and only small quantities are present as free choline (Patterson et al. 2008), which makes quantification difficult. The choline molecule itself is lacking native UV absorbance or fluorescence (McEntyre et al. 2009). A number of methods have been developed for choline determination in foods using nuclear magnetic resonance (NMR) spectroscopy (Graham et al. 2009), liquid chromatography–tandem mass spectrometry (LC-MS/MS) (Bruce et al. 2010; Xiong et al. 2012; Zhao et al. 2011), or isotope dilution liquid chromatography-mass spectrometry (LC-MS) (Koc et al. 2002). These methods require expensive equipment, which is not available in all laboratories, especially in the countries in most need of new dietary choline data. Our aim was to develop a simple high-performance liquid chromatography–fluorescence detection (HPLC-FLD) method for choline quantification in foods after acid hydrolysis and derivatization using the commercially available reagent 1-naphthyl isocyanate.
Materials and Methods
Chemicals and Reagents
1-Naphthyl isocyanate (98 %), choline iodide ((2-hydroxyethyl) trimethylammonium iodide), phosphatidylcholine (~99 %, from egg yolk) and phospholipase D ≥50,000 units/mL (buffered aqueous glycerol solution), and chloroform-D were purchased from Sigma-Aldrich (St. Louis, MO, USA). Benzoylcholine chloride was obtained from Alfa Aesar (England). Tetramethylammonium hydroxide and glycolic acid were obtained from Fluka (Buchs, Switzerland). Magnesium oxide was purchased from BDH (Poole, England). Acetonitrile was purchased from Merck (Darmstadt, Germany).
Food Samples and Extraction
The food items utilized included dried faba beans (Vicia faba), dried, red lentils (Lens culinaris), whole milk (fat 3.25 %), and whole-wheat flour (Triticum aestivum). These foods were selected for analysis because they were expected to be good sources of choline, are commonly consumed globally, and represented different food matrices. Foods were purchased from a local supermarket in Christchurch, New Zealand. Two food samples (whole-wheat flour and dried faba beans) were purchased from a local supermarket in Mansoura, Egypt, and were used for the recovery study using dietary lecithin. Before extraction, foods were minced using a household food processor (Braun, Germany). Dietary lecithin, a nutritional supplement—Thompson’s Super Lecithin (Integria Healthcare, Auckland, New Zealand), was purchased from a Christchurch, New Zealand, pharmacy.
Choline Extraction
Sample extraction was based on the method of Bligh and Dyer (1959). Briefly, 0.25 g of the food samples was homogenized in 2 mL extraction solvent (chloroform/methanol/water, 1:2:0.8) for 5 min then centrifuged at 3000 rpm for 5 min. The supernatant was collected and the extraction procedure repeated a further two times. The supernatant from the three extracts were combined and the total volume was recorded prior to acid hydrolysis or direct derivatization for free choline quantification.
Acid Hydrolysis of Phospholipids
A 1 M solution of HCl was freshly prepared in acetonitrile-water (9 mL acetonitrile/1 mL water) or water, and the solution was used for hydrolysis at 115 °C. Different digestion times were tested (30, 45, and 60 min). To ensure complete hydrolysis when larger amounts of phospholipid were used, different concentrations of dietary lecithin (containing 10.4 to 104 μg/mL choline) and phosphatidylcholine were hydrolyzed at 115 °C for 30 min in solutions of either HCl-acetonitrile or HCl-water.
The final protocol of acid hydrolysis was to add 3 mL of the HCl-acetonitrile solution to 0.5 or 1 mL of the extract. The mixture was heated at 115 °C for 30 min in a block heater. The caps of the tubes were occasionally loosened during the first 2 min of heating to avoid excessive pressure build-up. Thereafter, samples were cooled to room temperature, neutralized using NaOH (10 M), and made up to a final volume of 5 mL using distilled water.
Phospholipase D Treatment
A phospholipase D solution (400 U/mL) was prepared using 50 mM Tris-HCL buffer (pH = 8). To check the efficiency of the acid hydrolysis, an aliquot from the hydrolyzate (400 μL) was incubated at 37 °C for 15 min with phospholipase D (125 μL, 50 U) according to the method of Fu et al. (2012).
Derivatization
Derivatization was carried out according to McEntyre et al. (2009) with a minor modification. Sodium hydroxide improved the derivatization reaction and therefore different volumes of 1 M NaOH (5–100 μL) were tested. A “blank” was also included, which containing water only was also included, with the aim to show that the improvement in the derivatization reaction was mainly caused by the sodium hydroxide and not from the water. Sixty microliters of 1 M NaOH was found to be the optimum volume and thereafter was used for subsequent derivatization reactions. The final protocol was to add 1 mL of acetonitrile to 20 μL of sample (or standard or water for the negative control) in a 1.5-mL microcentrifuge tube, then the tubes were half filled with dry magnesium oxide (approximately 80 mg was added) and vortex mixed. After adding 60 μL of NaOH (1 M), samples were vortexed and 1-naphthyl isocyanate (20 μL) was added. The samples were shaken at room temperature for 15 min. Sixty microliters of water was added and the mixture was vortexed and centrifuged at 13,000×g for 5 min. Two hundred microliters of the supernatant was transferred to HPLC vials and capped for analysis. Each batch of samples derivatized included a negative control containing water in place of the sample.
Choline Quantification by HPLC
The quantification of choline was carried out by HPLC with fluorescence detection (Shimadzu LC20AD, Kyoto, Japan) (McEntyre et al. 2009). The choline derivative was separated on a Phenosphere strong cation exchange column (SCX) (125 mm × 4 mm × 5 μm) (Phenomenex, CA, USA) with a guard column containing the same packing. The injection volume was 50 μL, the column temperature was maintained at 40 °C, and the run time was 10 min. Separation was performed isocratically using a mobile phase containing 10 mmol/L tetramethylammonium hydroxide and 20 mmol/L glycolic acid and 15 % water in acetonitrile. Ten millilitres of 1 mol/L aqueous tetramethylammonium hydroxide and 20 mL of 1 mol/L aqueous glycolic acid were mixed with 120 mL of distilled water, transferred into 1 L volumetric flask, and made up to 1 L with acetonitrile. The flow rate was 1 mL/min. Quantification was based on fluorescence detection (ex/em 220/350 nm) using a multilevel (n = 10) external calibration curve. Calibration curves were constructed using aqueous calibration standards containing 2.5, 5, 10, 50, 100, 200, 400, 600, 800, or 1000 μmol/L choline.
Choline Quantification by LC-MS
The choline content of the food extracts was also measured by LC-MS and the results were used for method validation. In a 1.5-mL microcentrifuge tube, 50 μL from the sample or the standard was added to 1 mL of extraction solvent (90 mL acetonitrile and 10 mL methanol) containing D9-choline. Two hundred microliters of the mixture was transferred to HPLC vials and capped for analysis. Separation of choline was performed on a LC-MS (Agilent 1100, Agilent Technologies, USA) using A Cogent Diamond Hydride silica column (100 × 2.1 mm, 4 μm, MicroSolv Technology, Eatontown, NJ, USA) (Lenky et al. 2012). The injection volume was 5 μL and the column was maintained at 40 °C. The total run time was 11 min, which consisted of a pre-equilibration time of 3 min and a sample run time of 8 min. A gradient system was used with solvent A containing 50 % (v/v) distilled water with 10 mM ammonium formate and 10 mM formic acid, and 50 % (v/v) acetonitrile. Solvent B contained 90 % acetonitrile and 10 % distilled water (v/v). The gradient used for the analysis consisted of 0 min of 50 % A and 50 % B, 7 min of 100 % A and 0 % B, and 7.1 min of 50 % A and 50 % B. The mobile phase was delivered linearly at a flow rate of 0.3 mL/min. An Agilent 6120 quadrupole mass spectrometer (Agilent 1100, Agilent Technologies, USA) was used for detection using an electrospray ion source (ESI) and selected ion monitoring (SIM). Choline was measured in positive ion mode using m/z = 104, and D9-choline was used as the internal standard using m/z = 113.
Choline Quantification Using NMR
Dietary lecithin was used as a secondary standard and the total choline content was quantified using NMR based on the method of Lee et al. (2006). Briefly, lecithin (0.01 g) was dissolved in 1 mL of CDCl3 and 20 μL of acetonitrile was added as an internal standard. Calibration standards containing 1, 5, and 10 mmol/L of benzoylcholine were dissolved in CDCl3. NMR was performed using a Varian INOVA 500 MHz instrument in 5 mm NMR tubes. The lecithin methyl group singlet was at 3.28 ppm, whereas the benzoylcholine methyl group singlet was observed at 3.58 ppm.
Quality Control
Results obtained by HPLC-FLD were compared with LC-MS for total choline content. The following criteria have been also evaluated:
Precision and Accuracy
NMR was used to measure the choline content in dietary lecithin, validated, and used as a secondary standard. The choline content in dietary lecithin was quantified using HPLC-FLD, LC-MS, and compared with results from NMR. For method validation, phosphatidylcholine (as reference material) was acid hydrolyzed and the total choline content was quantified using HPLC-FLD.
To check day-to-day variation in the assay, an extract of whole-wheat flour was used as an in-house control sample and was stored at −20 °C and included for the analysis in each batch of samples run (n = 10). The within-batch- and between-batch coefficients of variation (CVs) were calculated from replicate samples.
Recovery
Recovery using lecithin was also estimated to check for hydrolysis efficiency and losses during sample extraction. Lecithin was dissolved in extraction solvent (chloroform/methanol/water, 1:2:0.8 v/v) and added to different food samples (whole-wheat flour and faba bean) at concentrations of 50 and 100 % of the initial choline content.
In addition, to check for losses during sample preparation (including extraction and acid hydrolysis), choline iodide was added to different food matrices (faba bean, whole-wheat flour, and milk) at concentrations of 50 and 100 % of the initial choline content before extraction. The recovery (R) was calculated according to the following equation: R = (C found − C sample) / C added, where C found is the measured content in the spiked sample, C sample is the measured content in the sample before spiking, and C added is the added concentration.
Linearity
Different concentrations (0, 2.5, 5, 10, 50, 100, 200, 400, 600, 800, or 1000 μmol/L) of choline standard were derivatized and quantified using HPLC in triplicate. The linearity of the method was determined by linear regression analysis.
Results
Optimization of Acid Hydrolysis
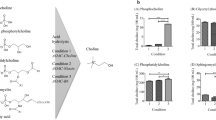
HCl-acetonitrile produced a rapid release of choline from phospholipids compared to HCl-water. A comparison of the efficacy of hydrolysis using available dietary lecithin (containing 19 mg/g choline) and phosphatidylcholine was conducted using both acid solutions. This showed that the measured choline content after hydrolysis with HCl-acetonitrile was higher than HCl-water (Fig. 1a, b). A complete hydrolysis of the choline-phospholipid components using HCl-acetonitrile was achieved after 30 min at 115 °C (Table 1). Increasing the heating time from 30 to 45 or 60 min did not result in a significant increase of measurable choline content (Table 1) in whole-wheat flour or lecithin. However, the relative number of moles of acid to moles of esterified choline was the most important factor for full recovery, where 3 mL of 1 M HCl-acetonitrile at 115 °C for 30 min could hydrolyse up to 767 μg/mL phosphatidylcholine (Fig. 1a, b).
Enzyme-Assisted Hydrolysis
Treatment with phospholipase D after acid hydrolysis (HCl-acetonitrile) did not increase the measurable choline content, confirming that the choline-phospholipids were fully hydrolyzed in the HCl-acetonitrile solution. The enzymatic hydrolysis step was therefore excluded from the final method for sample hydrolysis, thereby simplifying and reducing the cost of extraction. The overall time required for complete hydrolysis using the HCl-acetonitrile solution was 30 min, which is significantly shorter than the 3 h needed for the previously reported method of Fu et al. (2012), when a solution of HCl in water was used.
Optimization of Derivatization
The addition of sodium hydroxide was found to be required for successful derivatization to neutralize the acid from the sample hydrolysis. Nothing was detected if the reaction medium was acidic (data not shown). Moreover, the use of sodium hydroxide during the derivatization reaction of the samples with 1-naphthyl isocyante improved the reaction and increased the peak area fivefold when compared to derivatization without sodium hydroxide (Fig. 2). Results from the “blank” where water was used instead of sodium hydroxide showed no increase in peak area. This confirmed that the improvement in the derivatization reaction was caused by the alkali (NaOH) and not the water. Different volumes of 1 M NaOH were tested (Fig. 3) and the addition of 60 μL was found to give the highest peak area and the most reproducible results (CV = 0.76, n = 3) (Fig. 3). Adding less than 60 μL of 1 M NaOH decreased the peak area of the choline derivative, while using more than 60 μL resulted in a peak area, which was almost the same.
Precision, Accuracy, Recovery, and Linearity of the Assays
The intra and inter-assay CV for the control sample (whole-wheat flour) was 8 % (n = 12) and 7 % (n = 6), respectively. The method was validated by analyzing two reference samples at various concentrations; dietary supplement lecithin (containing ~19 mg/g choline, according to the labelling information) and phosphatidylcholine (Sigma-Aldrich). The accuracy of measurements ranged between 90 and 110 % for lecithin and 88 and 106 % for phosphatidylcholine (Fig. 1a, b). The choline content in the dietary supplement lecithin was quantified using three different methods (NMR, HPC-FLD, and LC-MS). The results, expressed as milligram/gram, ranged from 28 ± 0.6 (n = 3), 21 ± 1 (n = 6), and 22 ± 1 (n = 6) for NMR, HPC-FLD, and LC-MS, respectively. The results for all methods were comparable with less than 15 % variation, thus confirming the efficacy of the acid hydrolysis and fluorescence detection for measuring the choline content.
The relative recovery of choline at two different concentrations (50 and 100 % of the initial choline content before extraction) (n = 6) using choline iodide was 93 ± 9 % for faba beans, 84 ± 6 % for whole-wheat flour, and 106 ± 5 % for milk (Table 2). Average recovery after addition of dietary lecithin to two different food matrices faba beans and whole-wheat flour (n = 6) was 84 ± 7 and 82 ± 3 %, respectively (Table 2). The use of sodium hydroxide (60 μL, 1 M) during derivatization improved the limit of detection from 1 to 0.25 μmol/L confirming that the method had sufficient sensitivity for choline quantification in foods. The calibration curve for the choline standards prepared with 60 μL NaOH was linear in the range from 2.5 to 1000 μmol/L (R 2 = 1) (data not shown).
Choline Content in Selected Foods
The quantitative results for the choline content in the analyzed foods using HPLC-FLD were expressed as milligram/100 g fresh weight and compared with LC-MS. Phosphatidylcholine and lecithin were included for analysis with each batch of samples to ensure complete hydrolysis and used as reference samples. Quantitative results of both foods and reference materials (phosphatidylcholine and lecithin) analyzed for choline content using LC-MS were comparable to HPLC-FLD, with differences less than 10 % (Table 3). The results of the analysis for both methods were therefore comparable and provide near-identical determinations. The highest total choline content (198 mg/100 g) was found in faba beans, followed by lentils (186 mg/100 g). The lowest content (42 and 11 mg/100 g) was found in whole-wheat flour and milk (containing 3.26 % fat), respectively. The free choline content varied, depending on the food type, from 8 % in whole-wheat flour up to 55 % in faba beans (Table 3).
Discussion
Although free choline is highly water soluble, the majority of choline in food is esterified; therefore, choline is usually quantified after the hydrolysis of the phospholipids. Previous methods for acid hydrolysis involved heating the food samples in 1 M HCl for 3 h at 70 °C (Andrieux et al. 2008; Fu et al. 2012; Laikhtman and Rohrer 1999), or 45 min at 110 °C using a microwave-assisted hydrolysis procedure (Phillips and Sander 2012) followed by pH adjustment and enzymatic hydrolysis to fully release the choline that may be still bound to the lipids. In this study, we have simplified the sample preparation procedure by using HCl-acetonitrile instead of HCl-water. The efficiency of using HCl-acetonitrile for lipid hydrolysis and fatty acids quantification was previously explored (Aveldaño and Horrocks 1983). Here, we have optimized the hydrolysis approach using HCl-acetonitrile to fully release the bound choline from phospholipids. We have shown that hydrolysis using HCl-acetonitrile is reliable and gives the same results with or without the additional enzymatic hydrolysis with phospholipase D (data not shown).
Choline lacks UV absorbance or fluorescence (McEntyre et al. 2009), and therefore most choline detection methods use NMR spectroscopy (Graham et al. 2009), mass spectrometry (LC-MS/MS) (Bruce et al. 2010; Xiong et al. 2012; Zhao et al. 2011), or isotope dilution LC-MS (Koc et al. 2002). However, these detection methods are not available in all laboratories. To be able to assess the choline intake both within and between different populations, it is important to be able to measure the choline content of a wide variety of foods in diverse regions and cultures. It is therefore essential there is a method that is not only accurate and precise, but also uses more generally available instrumentation. Here, we have shown that the choline content of foods could be easily measured through fluorescence detection after a simple derivatization procedure using 1-naphthyl isocyanate. The ability of 1-naphthyl isocyanate to react with choline has previously been reported (McEntyre et al. 2009) and used for free plasma choline quantification. This reagent gives a cationic derivative that can be separated on an ion-exchange column and quantified by fluorescence detection. We obtained nearly identical results for the choline content of lecithin using this method (HPLC-FLD), NMR and LC-MS, with less than 15 % variation between the different methods. The proposed HPLC-FLD method is less technically demanding and the costs are lower, making it possible to determine the choline content of dietary components in countries where NMR and LC-MS systems are not widely available.The HPLC-FLD method was successfully applied to the determination of free and total choline in different foods. The measured choline content of whole-wheat flour and milk was found to be in good agreement with values obtained from the USDA choline database (Table 3). There is no data available in the USDA choline database for faba beans and lentil.
Conclusion
A simple acid hydrolysis procedure was developed and used for quantifying the choline content of foods. Our method has the advantage of being less time consuming than that of previously reported methods because the acid hydrolysis step for extracting choline from food has been reduced from 3 h to 30 min. HCl-acetonitrile was found to fully release the bound choline content after 30 min but not HCl-water. Our method also shows high linearity, precision, and accuracy. Through the derivatization of the extracted choline samples with 1-naphthyl isocyanate and simple chromatographic separation coupled with fluorescence detection, we have developed a method that makes the routine analysis of choline in foods more widely available, without a requirement for expensive equipment.
References
Andrieux P, Kilinc T, Perrin C, Campos-Gimenez E (2008) Simultaneous determination of free carnitine and total choline by liquid chromatography/mass spectrometry in infant formula and health-care products: single-laboratory validation. J AOAC Int 91:777–785
Aveldaño MI, Horrocks LA (1983) Quantitative release of fatty acids from lipids by a simple hydrolysis procedure. J Lipid Res 24:1101–1105
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Blusztajn JK (1998) Choline, a vital amine. Science 281:794–795. doi:10.1126/science.281.5378.794
Bruce SJ, Guy PA, Rezzi S, Ross AB (2010) Quantitative measurement of betaine and free choline in plasma, cereals and cereal products by isotope dilution LC-MS/MS. J Agric Food Chem 58:2055–2061. doi:10.1021/jf903930k
Craig SAS (2004) Betaine in human nutrition. Am J Clin Nutr 80:539–549
Fu S, Tao B, Lai S, Zhang J, Yiping R (2012) Determination of total choline by liquid chromatography-electrospray ionization-tandem mass spectrometry in infant formulas. J AOAC Int 95:157–162
Graham SF, Hollis JH, Migaud M, Browne RA (2009) Analysis of betaine and choline contents of aleurone, bran, and flour fractions of wheat (Triticum aestivum L.) using 1H nuclear magnetic resonance (NMR) spectroscopy. J Agric Food Chem 57:1948–1951. doi:10.1021/jf802885m
Institute of Medicine. Standing Committee on the Scientific Evaluation Of Dietary Reference Intakes and Its Panel on Folate, other B vitamins, and Choline. (1998) Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press (US); Available from: http://www.ncbi.nlm.nih.gov/books/NBK114310/
Koc H, Mar M-H, Ranasinghe A, Swenberg JA, Zeisel SH (2002) Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem 74:4734–4740. doi:10.1021/ac025624x
Laikhtman M, Rohrer JS (1999) Determination of choline in infant formula by ion chromatography. J AOAC Int 82:115611–115662
Lee MB, Storer MK, Blunt JW, Lever M (2006) Validation of 1H NMR spectroscopy as an analytical tool for methylamine metabolites in urine. Clin Chim Acta 365:264–269
Lenky CC, McEntyre CJ, Lever M (2012) Measurement of marine osmolytes in mammalian serum by liquid chromatography-tandem mass spectrometry. Anal Biochem 420:7–12. doi:10.1016/j.ab.2011.09.013
Lever M, Slow S (2010) The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem 43:732–744. doi:10.1016/j.clinbiochem.2010.03.009
McEntyre CJ, Slow S, Lever M (2009) Measurement of plasma free choline by high performance liquid chromatography with fluorescence detection following derivatization with 1-naphthyl isocyanate. Anal Chim Acta 644:90–94
Patterson KY, Bhagwat SA, Williams JR, Howe JC, Holden JM (2008) USDA database for the choline content of common foods. Release Two. Agricultural Research Service, Beltsville Maryland
Phillips MM, Sander LC (2012) Microwave-assisted extraction and quantitative LC/ID-MS measurement of total choline and free carnitine in food standard reference materials. J AOAC Int 95:1479–1486
Ueland P (2011) Choline and betaine in health and disease. J Inherit Metab Dis 34:3–15. doi:10.1007/s10545-010-9088-4
Xiong Y, Zhao Y-Y, Goruk S, Oilund K, Field CJ, Jacobs RL, Curtis JM (2012) Validation of an LC–MS/MS method for the quantification of choline-related compounds and phospholipids in foods and tissues. J Chromatogr B 911:170–179
Zeisel SH, Blusztajn JK (1994) Choline and human nutrition. Annu Rev Nutr 14:269–296. doi:10.1146/annurev.nu.14.070194.001413
Zeisel SH, Da Costa K-A (2009) Choline: an essential nutrient for public health. Nutr Rev 67:615–623
Zhao Y-Y, Xiong Y, Curtis JM (2011) Measurement of phospholipids by hydrophilic interaction liquid chromatography coupled to tandem mass spectrometry: the determination of choline containing compounds in foods. J Chromatogr A 1218:5470–5479. doi:10.1016/j.chroma.2011.06.025
Acknowledgments
Scholarship funding from the Partner Ownership initiative program (ParOwn, grant number 20150), the Ministry of Higher Education, Egypt, is gratefully acknowledged. Assistance from the National Heart Foundation of New Zealand and from the Maurice & Phyllis Paykel Trust is also acknowledged.
Conflict of Interest
Mohammed Hefni declares that he has no conflict of interest. Christopher McEntyre declares he has no conflict of interest. Michael Lever declares that he has no conflict of interest. Sandy Slow declares she has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hefni, M., McEntyre, C., Lever, M. et al. A Simple HPLC Method with Fluorescence Detection for Choline Quantification in Foods. Food Anal. Methods 8, 2401–2408 (2015). https://doi.org/10.1007/s12161-015-0131-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0131-9