Abstract
A new, fast and efficient method was developed for the separation and simultaneous quantification of acesulfame-K, aspartame, cyclamate, neotame and saccharin in food by ultra-performance liquid chromatography (UPLC) and diode array detector (DAD). Univariate strategies were applied for the optimisation of mobile phase pH, proportion of solvents in the mobile phase, flow rate and column temperature. Multivariate techniques were used for the simultaneous optimisation of 13 responses applying the Derringer and Suich desirability function. Many of the models presented lack of fit. However, it was possible to optimise the method using strategies for the restriction of the region to be investigated by the algorithm used in the desirability function. The optimised method was validated and applied to nine food matrices (soft drink, nectar, juice, ready-to-drink tea, jam, barbecue sauce, tomato sauce, instant juice and instant pudding), presenting good resolution, rapid analysis (11 min) and low use of reagents. This indicates that the UPLC is an excellent alternative for the simultaneous analysis of artificial sweeteners in foods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
By the end of the twentieth century, the consumption of low-sugar and low-calorie foods had tripled (Nabors 2001), due to the increasing indices of obesity and diabetes, and the concern about physical appearance. Consequently, the food industry has been producing a great variety of products containing artificial sweeteners as a replacement for sugar. Artificial sweeteners are usually used as blends, combining different sweeteners in small amounts to replicate the texture and flavours of products containing sucrose and without an undesirable aftertaste (Zygler et al. 2009). The blends contain mixed artificial sweeteners in low concentrations when compared with single artificial sweetener use. However, it is extremely important to investigate whether their concentration follows the established food and drug regulations and is declared correctly on the label. Regulatory agencies such as ‘Agência Nacional de Vigilância Sanitária’, Anvisa (National Agency of Health Surveillance from Brazil), US Food and Drugs Administration, FDA, European Food Safety Authority, EFSA, and Food Standards Australia New Zealand, FSANZ, establish the maximum limits for the use of these substances due to their toxicity when in high concentrations in foods and medications (Abhilash et al. 2011; Prasad and Rai 1987).
Among the methods used for separation and analysis of artificial sweeteners, the most commonly used are high-performance liquid chromatography (Chen et al. 1997; Demiralay et al. 2006; Dossi et al. 2006; Wasik et al. 2007; Yang and Chen 2009; Zhao et al. 2012; Zhu et al. 2005) and capillary electrophoresis (Bergamo et al. 2011; Horie et al. 2007). Most of the methods which allow the determination of artificial sweeteners simultaneously combine different detectors and also present long time of analysis and increasing cost and waste. Only the use of mass spectrometry allows the determination of eight to nine sweeteners (Wasik et al. 2007; Yang and Chen 2009); however, this technique is expensive and requires more specialised analysts.
More advanced techniques of separation and determination of different substances simultaneously have been developed with the aim to make sample analysis faster and more efficient. The ultra-performance liquid chromatography (UPLC), based in the principles of the high-performance liquid chromatography (HPLC), is one of the most recent advances in separation technique. The UPLC allows the use of columns with small particle size, enabling an increase in resolution. The use of particles smaller than 2 μm associated with high linear speed of mobile phase increases separation efficiency and detectability and reduces analysis time (Chesnut and Salisbury 2007; Gumustas et al. 2013). As UPLC is a recent and still evolving technique, only a few methods have been developed and validated for food analysis and, among them, none for the simultaneous determination of artificial sweeteners with detection by diode array, the most commonly used detector in laboratories. This makes the UPLC coupled to diode array detector (DAD) a potential object of research for the analysis of artificial sweeteners.
Many factors can significantly affect the interactions between analyte and column, during the development and optimisation of chromatographic methods, modulating the resolution among analytes. When the aim is the simultaneous separation of many analytes, the number of responses to be observed is high, as it is necessary to obtain a good resolution among all the target analytes and the interferences in the matrix. For this reason, multivariate experimental designs are being increasingly used for the optimisation of chromatographic methods, assisting in the interpretation of the effects of interaction among the variables and allowing optimisation with a small number of experiments. The Derringer and Suich (1980) desirability function enables the analysis of several responses simultaneously, assisting the selection of the most appropriate condition to obtain the desired values for all responses analysed (Breitkreitz et al. 2009; Zecevic et al. 2008).
The aim of this study was to optimise and validate a method for the determination of artificial sweeteners by UPLC-DAD. Univariate and multivariate optimisation strategies were used in this study to optimise the separation of five artificial sweeteners by UPLC-DAD. In the multivariate method, matrix interferents were taken into account and alternatives for the methods with lack of fit were suggested. After that, the optimised method was validated and applied for the analysis of 25 food and beverage samples.
Methods
Reagents
Acetonitrile (J. T. Baker, USA), monobasic monohydrate sodium phosphate (Merck, Germany), sodium hydroxide (Carlos Herba, Italy) and orthophosphoric acid (Merck, Germany) were used. All the chemicals were of chromatographic grade with high purity. The water for preparation of all solutions was purified by the Millipore system (Millipore, USA) and presented resistivity of 18.2 MΩ·cm−1. The standards used were aspartame, sodium cyclamate and hydrated sodium saccharin (Sigma, USA), acesulfame-K (Fluka, USA) and neotame (donated by the company Sweetmix). The sodium phosphate buffer (5 mmol L−1) was filtered in hydrophilic membrane polyvinylidene difluoride (PVDF) 0.22-μm pore (Millipore, USA). The acetonitrile was filtered in universal membrane GHP 0.22-μm pore (Pall Corporation, USA).
The stock standard solutions were prepared in water and stored at −18 °C. All solutions used in the optimisation method were filtered in membrane PVDF 0.22-μm pore (Millipore, USA).
Apparatus
The artificial sweeteners were separated using an UPLC system Acquity Waters, model UPA, series J08UPA 905 M, equipped with binary pump model UPB, series K08UPB 444 M, using a reverse phase column C18 Hypersil Gold (Thermo, USA) 50 × 2.1 mm and particle size of 1.9 μm. Flow rate, temperature, mobile phase composition and buffer solution composition were optimised. Detection was performed by diode array detector Acquity (Waters, USA), with wavelength reading capacity from 190 to 500 nm, resolution of 1.2 nm and sampling band of 20 points per second. The monitored wavelengths were 192 nm (for sodium cyclamate, aspartame and neotame), 201 nm (for sodium saccharin) and 227 nm (for acesulfame-K). Constant filter time was normal and exposition time was automatic. Data acquisition was performed by the software Empower Pro (Waters, USA).
Method Optimisation
Univariate Study for the Selection of Variables
From methods described in the literature for determination of artificial sweeteners by HPLC (Dossi et al. 2006; Lawrence and Charboneau 1988; Wasik et al. 2007; Yang and Chen 2009), calculations for the transference of methods to UPLC were done, followed by univariate experiments assessing the effects of flow rate variation (0.3 and 0.7 mL min−1), acetonitrile initial concentration (0 and 4 %), mobile phase pH (3.0 and 7.0) and column temperature (25 and 45 °C). On this experiment, the concentration of the buffer solution sodium phosphate was 5 mmol L−1. Before each new chromatographic condition, the column was conditioned for 15 min. Between runs in the same condition, the re-equilibration was obtained by conditioning for 1 min. Each test was performed in triplicate. The separation was performed in gradient as follows: acetonitrile test concentration (0 and 4 %) for 3 min, 14 % acetonitrile for 2 min followed by 30 % acetonitrile for 2 min.
Each condition was evaluated injecting a standard mix containing 20.5 μg mL−1 acesulfame-K, 1.55 μg mL−1 sodium saccharin, 326.27 μg mL−1 sodium cyclamate, 3.24 μg mL−1 aspartame and 10.41 μg mL−1 neotame.
When the best condition was found, matrices of interest were tested to evaluate the presence of substances which could interfere in the analysis. From these studies, the conditions for a multivariate study as well as the test matrix were determined.
A sample of tea ready to drink was then chosen to evaluate the effects of the matrix, because the tea presented the most common interferents found among the matrices studied during the univariate studies. The matrix chosen contained saccharin and cyclamate and was added with acesulfame-K, aspartame and neotame standard solutions. The final concentrations obtained were 20.5 μg mL−1 acesulfame-K, 6.0 μg mL−1 sodium saccharin, 50.0 μg mL−1 sodium cyclamate, 3.24 μg mL−1 aspartame and 10.41 μg mL−1 neotame.
Multivariate Study
The multivariate study was performed using a central composite design 22, with axial points and three true replicates on the central point, resulting in 11 experiments, performed in random order. Based on the results obtained from the univariate experiments, the effects of mobile phase pH (3.0–8.0) and column temperature (30–60 °C)—values corresponding to codified levels −1.41 to 1.41 from the central composite—were investigated.
Separation was performed, maintaining unchanged the variables: mobile phase, consisting in monobasic sodium phosphate buffer 5 mmol L−1 (pH adjusted with orthophosphoric acid or sodium hydroxide solutions) (A) and acetonitrile (B), under flow rate of 0.4 mL min−1. The gradient was carried out as follows: 100 % A for 5 min, 14 % B for 2 min, followed by 25 % B for 3 min. The initial condition was then restored and the column was reconditioned for 1 min between runs. Before each new experimental condition, the column was conditioned for 15 min. Each condition was performed in triplicate.
Processing of Data for Optimisation
Thirteen responses were analysed from the experiments performed on the central composite design, including resolutions between pairs of sweeteners which presented co-elution and sweeteners which co-eluted with interferents from the matrix. The responses analysed were as follows: resolution between saccharin and cyclamate measured at 192- and 201-nm wavelength (Sac-Cyc 192 nm and Sac-Cyc 201 nm, respectively), resolution between interferent 1 and saccharin (I1-Sac), between interferent 2 and saccharin (I2-Sac), interferent 3 and saccharin (I3-Sac), interferent 4 and saccharin (I4-Sac), between saccharin and interferent 4 (Sac-I4), between interferent 5 and saccharin (I5-Sac), between saccharin and interferent 5 (Sac-I5), between acesulfame-K and interferent 4 (Ace-I4), between acesulfame-K and interferent 5 (Ace-I5), between acesulfame-K and interferent-1(Ace-I1) and between interferent 1 and acesulfame-K (I1-Ace).
The resolution was calculated as follows:
where R s represents the resolution, t 1 and t 2 represent the retention time of each substance in the pair analysed and w 1 and w 2 represent the base width for each substance (Siouffi 2000).
The models were evaluated using analysis of variance (ANOVA) with 95 % confidence level, and the best separation conditions for the analytes were optimised using Derringer and Suich desirability function (Ballus et al. 2011; Breitkreitz et al. 2009). The desirability function was defined to maximise the resolutions between pairs of peaks. Hence, the following formula was used:
where
- d i :
-
Desirability i between 0 and 1
- R i :
-
Predicted value by the statistic model for the i resolutions
- R des :
-
Desired values for the resolution
- R i min :
-
Minimum value for the resolution
Desirability criteria were established for the optimisation and the data treatment was performed using the software Design Expert 6.0.10 (Minneapolis, EUA). The condition obtained mathematically was evaluated experimentally to confirm the separation of the substances of interest.
Validation of the Optimised Method
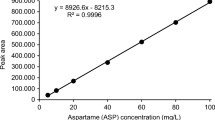
Validation was performed according to the Harmonized Guidelines for Single Laboratory Validation of Methods of Analysis (Thompson et al. 2002). Linearity, limits of detection, limits of quantification and precision were performed on the matrix jam, acquired free from artificial sweeteners and added with standards. This matrix was chosen due to its complexity, both in relation to the number of interferents and to the difficulty in its preparation due to its high viscosity. Concentrations equidistant from each other were used to assess linearity and the analysis was done in true triplicate.
The limits of detection were determined with the serial dilution of the matrix-standard solution and defined as the concentration necessary to produce a signal three times greater than the amplitude of the noise, while the limits of quantification were defined as two times the limit of detection. Precision intra-day was evaluated by successive measurements (n = 10) on the same day using samples added with standard concentrations of 1.79 μg mL−1 acesulfame-K, 2.02 μg mL−1 saccharin, 10.07 μg mL−1 cyclamate, 4.30 μg mL−1 aspartame and 1.20 μg mL−1 neotame. Precision inter-day was evaluated on 3 different days using the same conditions and standard concentrations.
For accuracy (recovery), different matrices were studied (soft drink, nectar, juice, tea ready to drink, jam, barbecue sauce, tomato sauce, instant pudding and instant juice). Samples were acquired free from artificial sweeteners and added with standards.
Sample Analysis
The method was applied to nine different food matrices. Samples of products labelled as diet, light and zero were acquired at supermarkets in Campinas and Sao Paulo (SP, Brazil). Three lots from 25 samples were analysed in triplicate: nectar (6), juice (1), soft drink (6), tea ready to drink (2), instant juice (4), instant pudding (2), jam (2), barbecue sauce (1) and tomato sauce (1).
All samples were diluted in Mili-Q water to concentrations within the linear range. Soft drinks were degassed using a bath with ultrasound for 5 min before dilution. Instant products were solubilised in water using a bath with ultrasound for 15 min. After dissolution in water, some samples (nectars, juice, instant pudding, barbecue sauce, tomato sauce and jam) were centrifuged at 2415 g s−1 for 5 min to precipitate solids and facilitate the filtration. All samples were filtered using PVDF membrane 0.22-μm pore (Millipore, USA) and VS cellulose ester membrane (VSWP) 0.025-μm pore (Millipore, USA).
Results and Discussion
Method Optimisation
Univariate Study
The variables pH of the buffer solution and column temperature were shown to affect the separation of analytes and interferents during preliminary tests the most. The pH variation caused the most significant change in the retention time for the interferents. The temperature increase caused an increase in the resolution of the compounds, due to an alteration in the mobile phase density and the interaction of the compounds with the mobile phase and the stationary phase. However, it was not possible to obtain a satisfactory resolution for all compounds with the levels studied univariately. An increase in acetonitrile concentration and flow rate caused a reduction in the resolution between pairs of peaks, due to a lesser time of interaction between compounds and stationary phase; thus, both parameters were maintained at minimum levels during the central composite design. When the flow rate was lower than 0.4 mL min−1, all peaks were enlarged, which caused co-elution between peaks. Therefore, acetonitrile initial concentration was set to 0 % and flow rate was set to 0.4 mL min−1. Among the samples analysed, ready-to-drink tea presented the most common interferents found among the matrices studied in the univariate studies and was thus chosen as test sample for the central composite design.
Multivariate Study
Table 1 contains the codified experimental conditions for the central composite design as well as the 13 responses investigated. The responses correspond to pairs of peaks which co-eluted in one or more experiment.
Table 2 contains the coefficients to the mathematic models established after the variance analysis, as well as their standard errors, study of the evidence of lack of fit of the models and the regression significance. There was lack of fit of the model of several responses, indicating the great complexity of the effects of the variables for the studied compounds. There was fit of the model for only three responses (Sac-I5, I5-Sac and Ace-I5). Only three models among the 13 studied explained the variance of the data.
To fulfil the aim of predicting the optimised condition, it was necessary to use two fundamental strategies. The first was accepting the models with slight lack of fit to perform the prediction. The models with F calculated values up to 10 times the F critical value were accepted. Some authors cited this procedure as a useful tool to avoid loss of experimental data, although it may introduce some prediction errors (Ballus et al. 2014). Thus, nine models (Sac-Cyc 192 nm, Sac-Cyc 201 nm, I1-Sac, I2-Sac, I5-Sac, Sac-I5, Ace-I5, Ace-I1 and I1-Ace) were included in the simultaneous optimisation process. Three other models could not be used due to the high lack of fit, and the fit for one of the models could not be calculated due to the absence of error in the central point (the pair of compounds co-eluted completely in the central point). The second strategy included the delimitation of the experimental region for the optimisation. Initially, the experimental responses were assessed for the resolutions in which the lack of fit was high or could not be evaluated (I3-Sac, I4-Sac, Sac-I4 and Ace-I4). This assessment identified the variable levels in which those four pairs of peaks presented a resolution greater than 1. The variable levels were then limited in a way that the algorithm would select responses in the region where the compounds presented acceptable resolutions, during the simultaneous optimisation of responses. In this way, due to the low resolution between I4 and Sac and between Sac and I4 in the central point region (0,0) and in the axial points for temperature (0,1.41 and −1.41,0), the algorithm was limited to search for optimal responses only in the other experimental conditions. Likewise, due to a low resolution between I3 and Sac on the experiments with maximum pH and temperature (1,1), this condition was also limited. Also, high pH levels were restricted, due to co-elution between Ace and I4.
Considering the restrictions of the fit of the models and the experimental results, the variables temperature and pH were allowed to range between the levels in which there would be no co-elution between pairs of peaks (resolution greater than 1.0) with excessive lack of fit. Therefore, the experimental region for algorithm prediction was limited to pH between central point and negative axial (0 and −1.41) and temperature from the lowest levels to the highest except for the maximum axil (−1.41 and 1). Considering that the levels −1.41 and −1 for temperature increased the analysis time, the levels between 0 and 1 were chosen.
Despite the limitations, the algorithms predicted two analytical conditions which fulfilled the desirability of obtaining resolutions greater than 2.0 for all pairs of peaks. Table 3 contains the criteria established to achieve the optimised conditions and the optimal condition predicted by the models, as well as the predicted responses. The two conditions were evaluated experimentally and their responses are also presented in Table 3. The majority of the values obtained were similar to those predicted by the statistical model, although some models presented lack of fit.
Based on those values and observing the chromatograms, the second algorithm prevision was chosen. The chromatograms for the method chosen can be seen in Fig. 1. In this method, the cyclamate presented a better resolution, while the other artificial sweeteners were distributed in a similar way in both methods. Cyclamate was a recurring problem for the artificial sweetener separation in this study. It presents a low signal and, due to its own characteristics, tends to have a posterior tail, which was observed in all the tests performed. Indeed, other authors used different methods of detection and confirmation of the compound identity, such as the mass spectrometer (Wasik et al. 2007; Yang and Chen 2009) or co-chromatography (Lawrence and Charboneau 1988), among other methods, or simply did not identify the cyclamate (Dossi et al. 2006; Ji et al. 2009).
Chromatograms of the best condition for the separation of five artificial sweeteners in black tea, ready to drink, at wavelengths 192 nm (a), 201 nm (b) and 227 nm (c). ACE acesulfame-K, SAC saccharin, CYC cyclamate, ASP aspartame, NEO neotame, I1 interferent compound 1, I3 interferent compound 3, I5 interferent compound 5
The analytical condition optimised consisted of gradient elution, with acetonitrile and buffer solution monobasic sodium phosphate/orthophosphoric acid (pH = 3.0) as mobile phase, flow rate of 0.4 mL min−1 and temperature of 56 °C, using a reversed phase C18 column (50 mm × 2.1 mm × 1.9 μm). Using the Derringer and Suich desirability function allowed us to correlate several responses to obtain the most appropriate methodology, performing only few experiments.
Validation
The limits of detection and quantification, as well as the linearity range, precision and accuracy are presented in Table 4. The limits of detection varied between 0.008 μg mL−1 (sodium saccharin) and 0.609 μg mL−1 (sodium cyclamate). The highest limit of detection was observed for sodium cyclamate due to its lower absorptivity at 192 nm. The results for repeatability and intermediate precision were lower than 5 and 10 % respectively and therefore satisfactory. Recovery was analysed in two levels and ranged between 83.5 % (cyclamate in nectar) and 113.5 % (neotame in barbecue sauce). Cyclamate was an exception presenting recovery of 73.9 % in barbecue sauce.
Application in Samples
The method developed and optimised was applied for ready-to-drink tea, soft drink, nectar, juice, instant juice, instant pudding, jam, tomato sauce and barbecue sauce. Table 5 contains the means and standard deviations for all samples analysed and Fig. 2 presents chromatograms for some of the samples. Among the analysed samples, tea, soft drinks, nectars, juice and instant juice presented reference to the quantities of artificial sweeteners on their label. Up to four artificial sweeteners combined were found in some samples, and acesulfame-K was the most commonly used artificial sweetener, especially in instant juice, instant pudding, jam, sauces and nectars. Aspartame was the most commonly used artificial sweetener in the soft drinks analysed.
The concentration levels of artificial sweeteners were analysed according to the ‘Regulamento Técnico Referente a Alimentos para Fins Especiais’ (Technical Regulation on Food for Special Purposes) (Brasil 1998) and the ‘Regulamento Técnico que autoriza o uso de aditivos edulcorantes em alimentos, com seus respectivos limites máximos’ (Technical regulation authorising use of artificial sweeteners in foods, and their maximum levels) (Brasil 2008). Comparison with studies in the literature is difficult due to great variation among different studies, few studies on Brazilian products and lack of comparison between experimental data, legislation and value declared by manufacturers.
The homogeneity among lots from the same product was evaluated using the coefficient of variation among the three lots analysed for each product. The variation among lots for acesulfame ranged from 1.5 % (low-calorie soft drink lemon flavour) to 79.3 % (zero instant pudding vanilla flavour), while for saccharin, it ranged from 0.4 % (zero ready-to-drink tea peach flavour) to 10.4 % (zero instant pudding vanilla flavour). The amount of cyclamate ranged from 0.2 % (zero ready-to-drink tea peach flavour) to 35.1 % (diet instant pudding vanilla flavour), and the aspartame ranged from 2.1 % (instant juice orange flavour) to 22.9 % (diet strawberry jam). The greater variation was observed for artificial sweeteners in instant products, suggesting a lack of standardisation. The lack of standardisation on powder-like products was discussed by Cuq et al. (2011). The variation in quantity of artificial sweeteners among different lots for the same manufacturer and brand highlights the difficulty in maintaining the same concentrations in the final product.
Among the 25 samples analysed, 5 presented one or more artificial sweeteners above the legal limit and 11 presented one or more artificial sweeteners above the values declared by the manufacturer.
The results obtained clearly indicate the need for a greater supervision regarding the amount of artificial sweeteners used, as well as the amount declared by the manufacturers and standardisation processes. This also highlights the importance for the use of a faster, less expensive and more efficient method for simultaneous determination of sweeteners to allow the analysis of a greater number of samples in a shorter period of time. The analytical method developed presented lower analytical time (11 min), use of reagents and waste generation than the existing methods by HPLC-DAD. The method developed also is a less expensive technique and requires less specialised analysts, when compared with mass spectrometry methods. Therefore, the method developed would permit an efficient control of artificial sweeteners in foods produced in Brazil and imported from other countries, as limits can be different for different countries.
Neotame was not found in any of the samples analysed possibly due to its relatively recent discovery and appearance in the market. However, it may have its use increased in the future, due to its very high sweetness compared to other sweeteners which allows its use in very small amounts (Witt 1999).
Conclusion
The central composite design and the Derringer and Suich desirability function were successfully applied for the optimisation of a method for the determination of five artificial sweeteners by ultra-performance liquid chromatography and diode array detector, resulting in a method with good resolution among sweeteners and matrix interferents. The method was validated and presented consistent results for quantification of artificial sweeteners in 25 samples from nine food matrices (soft drink, nectar, juice, ready to drink tea, jam, barbecue sauce, tomato sauce, instant pudding and instant juice), as well as good resolution, low analysis time (11 min), low use of reagents and low waste. Among the 25 samples analysed, 5 presented one or more artificial sweeteners above the legal limit and 11 presented one or more artificial sweeteners above the amounts declared by the manufactures. These results indicate the need for greater supervision over the amount of artificial sweeteners used and declared by the manufacturers.
References
Abhilash M, Paul MVS, Varghese MV, Nair RH (2011) Effect of long term intake of aspartame on antioxidant defense status in liver. Food Chem Toxicol 49:1203–1207. doi:10.1016/j.fct.2011.02.019
Ballus CA, Meinhart AD, Bruns RE, Godoy HT (2011) Use of multivariate statistical techniques to optimize the simultaneous separation of 13 phenolic compounds from extra-virgin olive oil by capillary electrophoresis. Talanta 83:1181–1187. doi:10.1016/j.talanta.2010.07.013
Ballus CA, Meinhart AD, de Souza Campos FA Jr, Bruns RE, Godoy HT (2014) Doehlert design-desirability function multi-criteria optimal separation of 17 phenolic compounds from extra-virgin olive oil by capillary zone electrophoresis. Food Chem 146:558–568
Bergamo AB, Silva JAF, Jesus DP (2011) Simultaneous determination of aspartame, cyclamate, saccharin and acesulfame-K in soft drinks and tabletop sweetener formulations by capillary electrophoresis with capacitively coupled contactless conductivity detection. Food Chem 124:1714–1717. doi:10.1016/j.foodchem.2010.07.107
Brasil (1998) Regulamento técnico referente a alimentos para fins especiais vol portaria n. 29, de 13 de janeiro de 1998 poder executivo, diário oficial da união
Brasil (2008) Regulamento técnico que autoriza o uso de aditivos edulcorantes em alimentos, com seus respectivos limites máximos vol Resolução RDC n. 18 de 24 de março de 2008. Poder executivo, diário oficial da união
Breitkreitz MC, Jardim IC, Bruns RE (2009) Combined column-mobile phase mixture statistical design optimization of high-performance liquid chromatographic analysis of multicomponent systems. J Chromatogr A 1216:1439–1449. doi:10.1016/j.chroma.2008.12.093
Chen Q-c, Mou S-f, Liu K-n, Yang Z-y, Ni Z-m (1997) Separation and determination of four artificial sweeteners and citric acid by high-performance anion-exchange chromatography. J Chromatogr A 771:135–143
Chesnut SM, Salisbury JJ (2007) The role of UHPLC in pharmaceutical development. J Sep Sci 30:1183–1190. doi:10.1002/jssc.200600505
Cuq B, Rondet E, Abecassis J (2011) Food powders engineering, between knowhow and science: constraints, stakes and opportunities. Powder Technol 208:244–251. doi:10.1016/j.powtec.2010.08.012
Demiralay EÇ, Özkan G, Guzel-Seydim Z (2006) Isocratic separation of some food additives by reversed phase liquid. Chromatogr Chromatographia 63:215–219. doi:10.1365/s10337-005-0683-8
Derringer G, Suich R (1980) Simultaneous optimization of several responses variables. J Qual Technol 12:214–219
Dossi N, Toniolo R, Susmel S, Pizzariello A, Bontempelli G (2006) Simultaneous RP-LC determination of additives in soft drinks. Chromatographia 63:557–562. doi:10.1365/s10337-006-0793-y
Gumustas M, Kurbanoglu S, Uslu B, Ozkan SA (2013) UPLC versus HPLC on drug analysis: advantageous, applications and their validation. Parameters 76:1365–1427. doi:10.1007/s10337-013-2477-8
Horie M, Ishikawa F, Oishi M, Shindo T, Yasui A, Ito K (2007) Rapid determination of cyclamate in foods by solid-phase extraction and capillary electrophoresis. J Chromatogr A 1154:423–428. doi:10.1016/j.chroma.2007.03.094
Ji C, Sun Y, Li X, Chu X, Chen Z (2009) Simultaneous determination of artificial sweeteners in beverage by ultra performance liquid. Chromatogr Sepu 27:111–113
Lawrence J, Charboneau C (1988) Determination of seven artificial sweeteners in diet food preparation. J Assoc Off Anal Chem 71:934–937
Nabors LO (2001) Alternative Sweeteners. Marcel Dekker, Switzerland
Prasad OM, Rai G (1987) Haematological abnormalities induced by feeding a common artificial sweetener, saccharin, in icr swiss mice. Toxicol Lett 36:81–88
Siouffi A-M (2000) HPLC. In: Nollet LML (ed) Food Analysis by HPLC, 2nd edn. Marcel Dekker, Inc., New York, p 1049
Thompson M, Ellison SLR, Wood R (2002) Harmonized guidelines for single laboratory validation of methods of analysis. IUPAC 74:835–855
Wasik A, McCourt J, Buchgraber M (2007) Simultaneous determination of nine intense sweeteners in foodstuffs by high performance liquid chromatography and evaporative light scattering detection—development and single-laboratory validation. J Chromatogr A 1157:187–196. doi:10.1016/j.chroma.2007.04.068
Witt J (1999) Discovery and developmente of Neotame. In: CORTI A (ed) Low-calorie sweeteners: Present and Future., vol 85. World Review of Nutrition and Dietetics, vol 85. A. P. Simopoulus, Barcelona, pp 52–57
Yang DJ, Chen B (2009) Simultaneous determination of nonnutritive sweeteners in foods by HPLC/ESI-MS. J Agric Food Chem 57:3022–3027. doi:10.1021/jf803988u
Zecevic M, Jocic B, Zivanovic L, Protic A (2008) Application of multicriteria methodology in the development of improved RP-LC-DAD for determination of rizatriptan and its degradation products. Chromatographia 68:911–918. doi:10.1365/s10337-008-0823-z
Zhao Y-G, Chen X-H, Yao S-S, Pan S-D, Li X-P, Jin M-C (2012) Analysis of nine food additives in red wine by ion-suppression reversed-phase high-performance liquid chromatography using trifluoroacetic acid and ammonium acetate as ion-suppressors. Anal Sci 28:967–971
Zhu Y, Guo Y, Ye M, James FS (2005) Separation and simultaneous determination of four artificial sweeteners in food and beverages by ion chromatography. J Chromatogr A 1085:143–146. doi:10.1016/j.chroma.2004.12.042
Zygler A, Wasik A, Namieśnik J (2009) Analytical methodologies for determination of artificial sweeteners in foodstuffs. TrAC Trends Anal Chem 28:1082–1102. doi:10.1016/j.trac.2009.06.008
Acknowledgments
The authors are grateful to Sweetmix (Brazil) for providing, free of charge, the neotame standard. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, FAPESP, under grant 08/51190-6; Coordenação Nacional de Desenvolvimento Científico e Tecnológico—CNPq grant 131101/2009-9; and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Capes. C. A. Ballus also would like to thank FAPESP for the post-doctoral scholarship (grant no. 13/25242-7).
Conflict of Interest
Cíntia Botelho Dias, Adriana Dillenburg Meinhart, Daniela Queiroz Pane, Cristiano Augusto Ballus and Helena Teixeira Godoy declare they have no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dias, C.B., Meinhart, A.D., Pane, D.Q. et al. Multivariate Optimisation and Validation of a Method for the Separation of Five Artificial Sweeteners by UPLC-DAD in Nine Food Matrices. Food Anal. Methods 8, 1824–1835 (2015). https://doi.org/10.1007/s12161-014-0056-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-0056-8