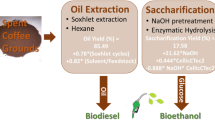
Abstract
This work presents a way to use ethanol and spent coffee grounds (SCG) as feedstocks for biodiesel production to solve ethanol overproduction in Thailand and biodiesel feedstock shortage problems together. Waste coffee oil (SCGO) was ethanolic extracted from SCG; then, ethanol-SCGO mixture was transesterified in supercritical condition without ethanol removal. The ethanolic extraction curves of SCG at a temperature range of 50–70 °C were constructed. Transesterification experiments were studied in a batch reactor and a continuous reactor at various temperatures (275–350 °C) and reaction time (5–40 min) under 15.0 MPa. The molar ratio of ethanol-oil mixture was set at 30:1. The highest ester content of 88.37 ± 3.00 wt% was found in biodiesel obtained at a temperature of 275 °C and a reaction time of 40 min in a batch reactor. Furthermore, excess temperature (< 300 °C) and reaction time (< 20 min) induced thermal degradation and promoted the loss of ethyl linoleate. For continuous reactor, the maximum ester content of 83.38 ± 5.86 wt% was observed at 325 °C and 29.4 min of residence time. Unlike in batch reactors, thermal degradation of ethyl linoleate was not observed in a continuous reactor. The results showed that ethanolic extraction and supercritical transesterification are alternative ways to produce biodiesel from SCG without removing extractant and using catalyst. From a prospective point of view, techno-economic analysis (TEA) and life-cycle assessment (LCA) of invented process should be conducted to ensure profitability and environmental benefits, respectively.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coffee consumption is yearly increasing worldwide that promotes spent coffee grounds (SCG) as a promising feedstock for biodiesel production. It should be informed that the fresh SCG discharged from the coffee brewing machine has a moisture content in the range of 50 wt% to 65 wt%. It was reported that dried SCG (DSCG) contains 5–20 wt% of extractable oil. The oil content in SCG depends on many factors, e.g., source of coffee bean, roasting level, and gridding size [1, 2]. Biodiesel is an environmentally friendly biomass-based fuel because it is produced from various types of renewable feedstock, including waste products from food processing plants [3]. This makes using SCG as a feedstock more appealing. Furthermore, palmitic and linoleic acids are the main fatty acids found in SCG oil, hereafter called “SCGO.” Those fatty acids are equivalent to palm oil, currently commercialized feedstock for biodiesel production in Thailand. For general coffee shops, brewing around 70–75 standard espresso shots produces 1 kg of fresh SCG and moisture content up to 50 wt%. Instant coffee and ready-to-drink coffee production plants generate approximately 500–700 kg of fresh SCG per batch.
Since January 2021, the production of ethanol in Thailand, 2.97 to 5.31 million liters per day, ran ahead of the consumption as liquid fuels, around 3.22 to 4.10 million liters per day. Thus, the anhydrous ethanol stocks in Thailand came over 100 million liters in 2021 (Department of Alternative Energy Development and Efficiency, Thailand). The alternative utilizations of ethanol require to reduce the surplus of ethanol in the stocks. First, it was reported that ethanol is a compatible solvent for extracting oil from soybean [4] and spent coffee grounds [5]. Second, ethanol could be used as a reactant for biodiesel production. Although the commercial process used methanol, it is presently synthesized from non-renewable resources such as natural gas. On the other hand, ethanol is derived from renewable resources, e.g., sugarcane, molasses, and casava via fermentation process. Unfortunately, the presence of water in ethanol induces the saponification between bases and oils. Hence, the alkaline catalytic process is not practical when using ethanol as feedstock for biodiesel production. The novel methods could avoid saponification such as heterogeneous and lipase catalytic processes, including non-catalytic transesterification in supercritical condition [6].
The conventional biodiesel production from SCG consists of extraction, solvent removal, reaction, and purification steps. At the beginning, the SCGO is extracted by organic solvents such as acetone, chloroform, n-hexane, petroleum ether, and toluene [7]. Vacuum distillation is employed to remove and recycle the excess solvents. Among all production steps, the solvent removal consumes the largest amount of energy. The reactions involved in biodiesel production from SCG are esterification and transesterification. The acid-catalyzed pretreatment or esterification is necessary to reduce a high amount of free fatty acid in SCGO before alkali-catalyzed transesterification is performed. After the by-product glycerol is separated by decanting, the catalyst traces in biodiesel are removed by water leaching. Besides the time-consuming biodiesel-water phase separation retraced by the saponified fatty acid, the last step generates a significant amount of wastewater.
The biodiesel production in supercritical ethanol (SCE) is a green alternative method to produce biofuel from SCG. Because ethanol is reactive at supercritical condition, the esterification and transesterification reactions between SCGO and SCE simultaneously take place without any presence of catalyst. Transformations of SCE itself and possible reactions of lipids, carbonyl compounds, and glycerol in SCE at temperature of 380–400 °C and pressure up to 3.0 GPa were recently reported [8]. The acid-catalyzed pretreatment and base-fatty acids saponification are eliminated. The main product is fatty acid ethyl esters (FAEE) or biodiesel, and the by-product is glycerol. The reaction at supercritical condition could reduce the number of used chemicals and amount of generated wastewater. Even though the reaction at supercritical condition takes place at high temperature, the recovery of heat is easier and cleaner than that of chemicals. For example, the heat from hot streams could be recovered to improve the efficiency of SCGO extraction. Using the hot streams to preheat the solvent in the extraction step improves the solubility of SCGO. Besides, the heat duty of the cooling system is dropped due to the reduced temperature.
This work demonstrates the biodiesel production from SCG by ethanol, extracting and reacting at atmospheric pressure and supercritical conditions, respectively. The ethanolic extract could be instantly converted to biodiesel in SCE without ethanol removal. The entire process employs only DSCG and ethanol as feedstock. This research introduces an environmentally friendly method for biodiesel production from SCG by using ethanol as an extracting solvent and reactant that minimized the process complexity and chemical usage. For conducting the reaction between SCGO and SCE, the liquid form of SCG is easier to handle than the solid form, especially when operating at high pressure in a continuous reactor. The solid phase interferes with the fluid flow and causes uncontrollable pressure. In the worst case, the particle could clog in the reactor tube or damage the back-pressure regulator. This process also expresses the alternative option to treat other high lipid wastes such as brown grease collected in a grease trap, fruit seeds spent from food processing plants, and waste lipids left in fish market. This approach was successfully demonstrated for production of fatty acid esters from forage radish (Raphanus sativus L.) and crambe (Crambe abyssinica H.) seeds [9, 10]. The objective of this work is to approve the concept of using ethanol as extracting solvent and reactant for producing biodiesel from SCG.
Materials and Methods
Materials
The wet SCG (WSCG) sample, 2–3 kg per day, was donated by Starbucks coffee house, Square One, and Samyan Mitrtown branches, Bangkok, Thailand, from October to December 2021. The received sample was treated as described in a previous work [11]. Briefly, WSCG was air dried at room temperature (30–35 °C) for 48 h and then dried in a hot-air oven at 105 °C for 24 h. The dried SCG (DSCG) was kept in the desiccator before the ethanolic extraction. The anhydrous ethanol (99.5% v/v) and hydrated ethanol (95.6% v/v) were supplied by The Liquor Distillery Organization, Ministry of Industry, Thailand. The analytical grade n-hexane (99.95%) and n-heptane (99.50%) were bought from RCI Labscan Co., Ltd. External standard compounds, ethyl palmitate (≥ 99%), and ethyl oleate (98%) were provided from Sigma Aldrich. Common chemicals used in characterization of SCGO such as NaOH, HCl, and KHP were purchased from SAC SCI-ENG Co., Ltd.
SCG, SCGO, and Biodiesel Characterizations
The overall experimental protocol is shown in Fig. 1. The moisture contents in fresh and DSCG were determined by the gravimetric method. Total extractable oil in SCG was measured by the Soxhlet apparatus using anhydrous ethanol, hydrated ethanol, and n-hexane. The particle size distribution and the bulk density of DSCG were examined by sieving and gravimetry, respectively.
The apparent density of SCGO was measured by the gravimetric method. The SCGO was characterized by AOCS official methods, including the free fatty acid content (Ca 5a–40), the saponification number (Cd 3c–25), unsaponifiable matter (Ca 6a-40), and the fatty acid profile (Ce 2–66 and Ce 1–62). The average molecular weight of SCGO estimated by the saponification number was in the range of 830–835. Water content in extracted oil was determined by the Karl–Fischer titration (Metrohm Eco KF Titrator). The viscosity of SCGO and biodiesel was measured by a digital viscometer (Brookfield, model LVDV-E).
The FAEE content in biodiesel produced from SCGO was analyzed by a modified method as described in a previous work [11]. Briefly, a gas chromatograph (GC; Shimadzu, model GC2030, Japan) was standardized by the known concentrations (0.625–10.000 wt%) of ethyl palmitate and ethyl oleate solutions in n-heptane. A capillary column (DB-FATWAX, 30 m × 0.25 mm OD., 0.25 µm), an autosampler (Shimadzu, model AOC 20i Plus, Japan), and a flame ionization detector were employed for all analyses. Unknown concentrations of FAEE in the biodiesel solution were calculated from slope and x-intercept of the calibration curves. The developed method was confirmed via spike-and-recovery analysis every 6 months.
Ethanolic Extraction of SCGO
The ethanolic extraction of SCGO was modified from the conditions reported in a literature [5]. The extraction curves of anhydrous- and hydrated-ethanolic extraction of DSCG were constructed by using a 2.5-l glass isothermal extractor heated by a circulating hot-water jacket and stirred by a mechanical stirrer. The experiments were performed at ethanol-to-DSCG mass ratio of 5:1, 1000 mL (783 g) of ethanol with 156.6 g of DSCG as recommended by a literature [12]. Ethanol was heated to the desired temperature (50 °C and 60 °C) before adding DSCG into the extractor. The mixture was constantly agitated at 180 rpm. A 1.0 mL of micellar sample was withdrawn every 5 min within the first 20 min of experiment and every 10 min within the next 20 min. The sample was filtered by a 0.45 mm Nylon syringe filter before measuring the initial weight of SCGO-ethanol mixture. The excess ethanol was removed by nitrogen striping until the constant weight of extracted oil was observed. The extraction yield was measured by a gravimetric method. All extractions were performed in quadruplicates.
For biodiesel production experiments, the extraction was conducted in a 20-l HDPE (high-density polyethylene) container. Ethanol-to-DSCG mass ratio was set at 5:1. The extraction was attempted at room temperature (30–35 °C) and atmospheric pressure (0.1 MPa) by using hydrated ethanol 10 l, shaking vigorously in every 2–4 h for a total duration of 48 h. The micellar phase was settled overnight and filtrated by filter paper (Whatman No. 42). The final concentration of SCGO in ethanol was observed at approximately 10.0 wt%. When using excess solid loading, SCG-ethanol mixture became a viscous slurry, difficult to mix by magnetic bar. The pure SCGO obtained from the Soxhlet extractor using ethanol as the solvent was added to adjust the desired concentration of 20.0 wt%, which equivalented to ethanol-to-SCGO molar ratio of 30:1. The volumes of make-up SCGO were in the range of 100–200 mL depending on initial SCGO content in each extraction batch. The ethanol-SCGO mixture was accordingly used as feedstock of biodiesel production with SCE in all experiments.
Biodiesel Production with SCE
Batch Reactor
A stainless steel (SUS316) tubing reactor was used to investigate the effects of temperature and reaction time. The general descriptions of equipment were given in the earlier works [11, 13]. The feedstock mass (hydrated ethanol-SCGO mixture) added into the batch reactor was calculated from given pressure of 15.0 MPa, reactor volume of 4.38 cm3, mixture density of 0.85 g/cm3, and ethanol-to-SCGO molar ratio of 30:1, at desired temperatures. The critical properties of SCGO were estimated by the Group-Contribution method as described in the literatures [14, 15]. The estimated properties of SGCO agreed well with the values of saturated triglycerides [16]. The total mass of the mixture was in the range of 2.800–3.100 g to access the pressure greater than 15.0 MPa. The heating interval for the desired temperature was in the range of 5–10 s. All experiments at each reacting condition were evaluated in triplicates.
Continuous Reactor
After the reacting conditions provided a high FAEE content was obtained from “Batch Reactor,” a 140-mL coiled reactor made of stainless steel (SUS316) was employed to reproduce biodiesel at the same condition. The schematic diagram of experimental setup is shown in Fig. 2.
At the beginning, the fluidized sand bath (OMEGA, model FSB-3) and the water-cooling bath circulator (HETO, model CBN 8–30) were set at the desired reaction temperature and 10 °C, respectively. Then, ethanol-SCGO mixture (30:1 molar ratio) was fed through preheater and reactor using an HPLC pump (Jasco, model PU-1580). A magnetic stirrer was employed to create emulsion at the suction of the high-pressure pump. It should be noticed that pre-heating ethanol-SCGO mixture to 127 °C (400 K) under the pressurized condition notably reduces viscosity of vegetable oil-ethanol mixture [17]. Because ethanol-SCGO mixture is viscous, the workable flow rate of HPLC pump was in the range of 2.0–3.0 g mixture/min. After outlet flow rate was steady, a back-pressure regulator (Swagelok, KHB Series) was closed to gently increase pressure to 15.0–16.0 MPa. Further description and operation were given in previous works [11, 13]. The operating temperature and residence time were regulated to increase the FAEE content as well. The excess ethanol in the product was removed by a vacuum evaporator before %FAEE measurements.
Statistical Analysis
Results from this study are presented in terms of means ± standard deviations. All data including acid value, saponification value, water content, unsaponifiable matters in Table 1, FAEE content in Table 3, and ethyl palmitate and ethyl linoleate contents in Fig. 5 were analyzed using Tukey pairwise comparisons with significant differences accepted at the p value < 0.05 level on the Minitab® v20.3 for Windows.
Results and Discussion
Characterizations of DSCG and SCGO
The wet SCG (WSCG) has a moisture content of 58.69 ± 2.40 wt%. After drying in a hot-air oven at 105 °C and 24 h, the moisture content of DSCG was reduced to 1.40 ± 0.42 wt%. Compared to a previous work, WSCG and DSCG had moisture contents of 55.86 ± 0.22 wt% and 14.98 ± 0.25 wt%, respectively, because only a 48-h convective drying at room temperature 30–35 °C was employed [11]. The DSCG sample had particle size distribution in ranges of less than 250 µm (~ 15 wt%), 250–500 µm (~ 80 wt%), and more than 500 µm (~ 5 wt%). The bulk density of DSCG was 0.387 g/cm3. The moisture content of WSCG and the particle distribution and bulk density of DSCG were conformed with the values reported in the literatures as well [18, 19].
Oil yield and characteristics of SCGO samples and their fatty acid profile obtained from anhydrous ethanol, hydrated ethanol, and n-hexane extractions by Soxhlet extractor are shown in Tables 1 and 2, respectively.
According to Table 1, densities of SCGO extracted by all solvents are not significantly different. Acid values (AV) of SCGO extracted by anhydrous ethanol and hydrated ethanol are higher, fivefold than that of SCGO extracted by n-hexane. For organic solvent extraction, it was reported that the extraction yield was increasing with polarity of solvent [7]. Thus, employing ethanol as an extraction solvent provided a higher oil yield in comparison to n-hexane. Nonetheless, ethanol extraction exhibits lower selectivity, potentially leading to increased polar impurities in the extracted oil [20]. This result also agrees well with ethanolic extraction of dried Halamphora coffeaeformis microalga [15]. Furthermore, it was mentioned that the use of ethanol in the extraction of Echium plantagineum L. and Amygdalus scoparia seed oils can effectively extract non-lipid compounds, thereby enhancing the extracted yield. However, this approach may induce polar impurities into the final product [21]. Ethanol is a suitable solvent to extract an acidic oil–lipid containing high AV from biomass. It was reported that free fatty acid could be esterified in SCE and converted to FAEE at supercritical condition [22]. When comparing with transesterification of triglyceride, esterification of free fatty acid required lower ethanol-to-fatty acid ratio (6:1), pressure (10 MPa), temperature (280 °C), and reaction time (10 min). Furthermore, the presence of free fatty acid enhanced the FAEE content in the biodiesel produced from soybean, rice bran, and high oleic sunflower oils [23] and blended waste oil-crambe oil with supercritical ethanol [24].
Saponification value (SV) of SCGO extracted by all solvents is not significantly different. Thus, the average molecular weight (MW) of SCGO is in a narrow range of 830–835 g/mol. Water content (WC) in SCGO samples is ranked from low-polarity to high-polarity of extracted solvents, n-hexane, anhydrous ethanol, and hydrated ethanol, respectively. However, WC in all SCGO samples, below 5.0 g/100 g oil, has no significant effects on transesterification in SCE [15].
Major fatty acids in SCGO from this work are linoleic (C18:2) and palmitic (C16:0) acids which agree with the literatures, Colombian coffee beans [25, 26]. The SCGO from Portuguese SCG has high palmitic acid (C16:0) content [27], while SCGO from Vietnamese SCG has high oleic acid (C18:1) content than other SCGO samples [26].
Unsaponifiable matters (UM) in coffee oil are neither glycerides nor fatty acids that could be divided into 3 groups: diterpene alcohols, sterols, and tocopherols [28]. Hence, UM could not convert to FAEE. It was reported that UM in SCGO extracted by hexane is 1.9–4.9% and 21.4% for Vietnamese and Colombia SCGs, respectively [26]. Furthermore, ethanolic extracted of SCG from 6 sources had an average UM of only 1.40 wt%, which was lower than UM in Arabica roasted coffee beans [29]. The UM found in this work was in the range of 1.47–1.89 g/ 100 g oil which agrees well with literatures. Because of the high biological activity of UM, the extraction of UM to use as value-added product in cosmetics industry is remarkably interesting.
Ethanolic Extraction of SCGO
Figure 3 depicts the oil yield of SCGO extracted by anhydrous- and hydrated-ethanol as a function of extraction time.
Anhydrous-ethanolic extraction of DSCG supplies higher extracted oil yield than hydrated-ethanolic extraction. Ethanol could not completely extract SCGO from DSCG because all extraction curves in Fig. 3 reach a plateau after an hour of extraction. As aforementioned in Table 1, SCGO content in DSGC was 0.22 g/g DSCG. The maximum extraction yield in Fig. 3 is only 10% of SCGO content. In other words, ethanolic extraction at atmospheric room condition could recover a small amount of SCGO. Otherwise, pressurized ethanolic extraction which is demonstrated in a 250-mL extractor could shorten the extraction time and enhance the extracted oil yield up to 96.8% [5]. Despite improving extraction yield, a high investment cost could decline the economic viability of the process. However, the pressurized extraction enhances yield and reduces the duration of the overall process. This conflict could be debated in two scenarios of economic analysis, using atmospheric extraction versus pressurized extraction in the future study. Ultrasonic-assisted extraction is a promising method to enhance the efficiency of SCG ethanolic extraction [30]. Recently, the design of the continuous countercurrent spent coffee ground oil extraction by hydrated ethanol was reported as well [12]. Hence, the make-up SCGO needs to fulfill the suitable ethanol-to-SCGO molar ratio of 30:1 and to maximize the conversion in supercritical transesterification.
The ultimate goal of this project is to create a zero-waste process for valorization of SCGs and other wastes from coffee industries. From a prospective point of view, the solid residue discharged from SCGO extractor could be utilized as feedstock for pyrolysis [31], gasification [32], and anaerobic digestion [33]. The profitability and environmental impacts of the designed process could be estimated by a TEA and LCA, respectively.
Biodiesel Production in Supercritical Ethanol (SCE)
Biodiesel production in SCE was first conducted in batch reactor to preliminary investigate the effects of temperature and reaction time because it had uniform temperature distribution and exact reaction time. After the effects of temperature and reaction time were concluded, the reactions were investigated in a continuous reactor to compare the conditions that resulted in the highest FAEE.
Batch Reactor
Figure 4 shows the ester content as a function of reaction time at various temperatures. Depending on temperature, reaction time dominates the ester content in biodiesel obtained from transesterification in supercritical conditions. Effects of reaction time on FAEE content were categorized into 3 groups. First, at 275 °C, FAEE content continuously enhanced with increasing reaction time; then, the maximum FAEE content was reached at 40 min of reaction time. Second, FAEE contents drop to 55–60 wt% when reaction time over 20 min at 300 °C and 325 °C is equivalent to total saturated fatty acids (C16:0, C18:0, and C20:0) in SCGO (45–55 wt%) as shown in Table 2. For high unsaturated microalga oil containing palmitoleic (C16:1) of 27.8–32.2 wt% and eicosapentaenoic acid (C20:5) of 17.6–21.3 wt%, the maximum FAEE contents of 56.1–71.7 wt% were found at 305 °C and 40 min [15]. Third, FAEE content at 325 °C reached the maximum value at 10 min of reaction time and slightly dropped.
It was reported that the homogeneous catalytic transesterification of SCGO with methanol resulted in a maximum conversion of 85% without pretreatment [7]. Because the base-catalyzed transesterification was inhibited by a high FFA in SCGO, the final biodiesel yield of 96–100% was reported when employing acid-catalyzed pretreatment before KOH-catalyzed transesterification. Furthermore, lipase-catalyzed transesterification was introduced for producing biodiesel from SCGs and ethanol. It was reported that lipase catalyst provided FAEE yield in the range of 72–88% within 24–48 h [1].
The FAEE content in other samples obtained from 350 °C and reaction time higher than 15 min could not be analyzed by GC because it formed a solid phase at room temperature (30–35 °C). The solid fraction could be derived from condensation reaction of minor compounds such as phenolic compounds and furans at high temperature [34] and/or monoglycerides and diglycerides in SCE [35]. On the other hand, thermal degradation of unsaturated fatty acids caused decreasing of FAEE as mentioned in various literatures [36,37,38,39]. The amounts of ethyl palmitate and ethyl linoleate at various temperatures and reaction times are shown in Fig. 5.
(Blue box) ethyl palmitate and (orange box) ethyl linoleate contents in SCG biodiesel obtained from various reaction temperatures and reaction times at pressure of 15 MPa and oil-to-ethanol molar ratio of 1:30 in a 4.5-mL batch reactor. Significant differences between the yields at the same temperature are indicated by different alphabets at a 5% confidence level
According to Fig. 5, ethyl palmitate contents in SCG biodiesel obtained from 275 °C increase with the reaction time, while they reach the plateau after a reaction time of 20 min at 300 °C and 325 °C. In contrast, ethyl linoleate contents rise with increasing reaction time at 275 °C, but they drop at 300 °C and 325 °C after 20 min of reaction time. Thus, it could be concluded that a suitable temperature for transesterified SCGO by SCE to obtain a high FAEE content in a batch reactor is between 300 and 325 °C at 20 min of reaction time.
Continuous Reactor
Figure 6 depicts the FAEE content in SCG biodiesel obtained from a continuous reactor at various conditions.
It is clear that increasing residence time enhances the FAEE contents in SCG biodiesel obtained from all reaction temperatures. According to critical points of ethanol (Tc = 241 °C and Pc = 6.3 MPa) and SCGO (Tc = 684 °C and Pc = 0.5 MPa, see “Batch Reactor”), the critical temperature and pressure of ethanol-SCGO at molar ratio of 30:1 estimated by the second-order modified Huron-Vidal (MHV2) mixing rules [40] were 255 °C and 6.1 MPa, respectively. Vapor–liquid equilibrium found from the experimental measurements for a non-edible vegetable oil-ethanol was in the temperature range of 160–280 °C and pressure range of 1.1–7.2 MPa [41]. Hence, it could be deduced that the SCGO-ethanol mixture is not a single-phase mixture at 255 °C and 6.1 MPa. The reduced temperatures (Tr) could be calculated from the reaction temperatures divided by the critical temperature of ethanol-SCGO mixture.
In batch reactor, reaction at 275 °C (Tr = 1.08) and prolonged reaction time up to 40 min provided high FAEE content (see Fig. 4). Unlike the reaction in batch reactor, when the reaction took place at 300 °C (Tr = 1.17) and 325 °C (Tr = 1.27) in the continuous reactor, FAEE content still increased with increasing residence time even though the residence time over 20 min. This phenomenon indicated the different heat transfer in batch and continuous reactors. In batch reactor, ethanol-SCGO mixture contacted the hot-surface of reactor and moved diagonally by the mechanical shaker. Hence, thermal degradation was favorable to occur at reactor wall where the temperature and the contact time were higher than at the reactor center. For continuous reactors, the fresh reactants continuously flow along the tubular reactor and mixed by fluid flowing force. The axial dispersion of components and thermodynamic effect on reaction in supercritical ethanol was described in a literature [42]. Ethyl palmitate and ethyl linoleate contents in biodiesel synthesized from a continuous reactor are shown in Table 3.
According to Table 3, thermal degradation of ethyl linoleate due to excess residence time was not observed at all reaction temperatures. In batch reactor, thermal degradation of ethyl linoleate was detected at temperature above 300 °C and reaction time over 20 min (see Fig. 5). At temperature below 325 °C, ethyl palmitate was thermal stable in both batch and continuous reactors. According to maximum extractable oil yields, approximately 22 g/100 g DSCG and the maximum FAEE content of 83.35 wt% as observed in the continuous reactor, it could be estimated that 1 kg of DSCG can be converted to ~ 200 mL of biodiesel with the standard specific gravity of 0.88. However, only 3 g SCGO/100 g of DSCG was obtained in the ethanolic extraction experiments. The improvement of ethanolic extraction is crucial for developing this process.
The HPLC pump used in this work is a double-syringe pump which is designed for low viscosity fluid such as water, methanol, and acetonitrile; thus, it is inappropriate for a viscous fluid like ethanol-SCGO mixture. Scale-up study on continuous production biodiesel in supercritical condition is presently lacking. In a previous work on scale-up tubular reactor, adding 20%vol of hexane in palm kernel oil stabilized the flow rate of double-syringe pump up to 50 g/min [43]. It should be noticed that viscosities of palm kernel oil and anhydrous- and hydrated-ethanolic extracted SCGO are ~ 35, ~ 112, and ~ 168 mm2/s, respectively. The free fatty acid content determines the SCGO viscosity, and a high free fatty acid is leading to a high viscosity [7]. Hence, SCGO needs a high volume of hexane to sufficiently reduce its viscosity. Although addition of hexane did not impact the conversion of triglyceride, the production yield, produced biodiesel per fed vegetable oil was decreased by 20% as well. Engineering design of a high-pressure pump for transporting a viscous fluid into a supercritical reactor is the best solution. The viscosity of biodiesel obtained from a continuous reactor (FAEE content of 83.35%) was 7–10 mm2/s, which is higher than the value of biodiesel specification. This value indicates that enhancing FAEE content and reducing AV of biodiesel are necessary for implementation of this process. On the other hand, purification of biodiesel obtained from the supercritical transesterification could improve the fuel properties of biodiesel. It should be considered that glycerol by-products could affect the viscosity of biodiesel product as well. Reactions between alcohol and glycerol at supercritical condition were described elsewhere [13].
Ethanol-to-SCGO molar ratio of 30:1 is higher than that used in the industrial biodiesel production, for example, the homogeneous catalytic transesterification employs alcohol-to-oil ratios in range of 6:1 to 9:1. According to a previous work, the lowest ethanol-to-oil molar ratio of 12:1 could be used for transesterification in supercritical ethanol [44]. Although ~ 70 wt% FAEE content in biodiesel was observed at the molar ratio of 12:1, the distillation characteristics of biodiesel obtained from molar ratios between 9:1 and 18:1 were similar. Recycling excess alcohol at high molar ratio consumed large amount of energy, generated high environmental load in a LCA study [45], and reduced the profitability of the production process [46].
However, the economic viability of this proposed process must be investigated by TEA in the future work. The process design and computer simulation were applied for biodiesel production from SCGs to estimate the feasibility of the process [47]. The results showed that using SCGs as feedstock for biodiesel production by KOH catalytic process becomes more economically feasible when the co-products such as glycerol and discharged SCGs are converted into value-added products.
Conclusions
The ethanolic extraction of SCGO is favorable at a DSCG-to-ethanol mass ratio of 1:5 and escalated temperature. Anhydrous ethanol (99.5%vol) provided a higher oil yield (g/g DSCG) twofold than hydrous ethanol (95.6%vol) did. The saturated SCGO concentration in ethanol reached a plateau in 30 min. Maximum FAEE contents in batch and continuous reactors are found at different conditions because of mixing intensity. Thermal degradation reduced ethyl linoleate at < 300 °C and < 20 min in batch reactor. In contrast, FAEE content in continuous reactor steadily rose to a maximum value of 83.35 wt% with increasing residence time, even though the reaction temperature reached 325 °C. Further study on decomposition products and ethanol recyclability will be conducted in the future. An outcome of this work was to demonstrate the utilization of ethanol as extracting solvent and reactant for biodiesel production using SCGs as a model for high lipid feedstock. Other high lipid wastes such as brown grease, fruit seed, and fishery waste could be used as feedstock for this process.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Karmee SK (2018) A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manage 72:240–254. https://doi.org/10.1016/j.wasman.2017.10.042
Battista F, Barampouti EM, Mai S, Bolzonella D, Malamis D, Moustakas K, Loizidou M (2020) Added-value molecules recovery and biofuels production from spent coffee grounds. Renew Sustain Energy Rev 131:110007. https://doi.org/10.1016/j.rser.2020.110007
Nurul Aina Nasriqah Binti Ma, Noor H, Siti Norhafiza Mohd K, Prakash B, Mohd Hasbi Ab R, Gaanty Pragas M (2021) Biodiesel (methyl esters). MIJEEC 3(1):30–43. https://doi.org/10.54279/mijeec.v3i1.245153
Toda TA, Sawada MM, Rodrigues CEC (2016) Kinetics of soybean oil extraction using ethanol as solvent: experimental data and modeling. Food Bioprod Process 98:1–10. https://doi.org/10.1016/j.fbp.2015.12.003
Toda TA, Franco Visioli PdC, de Oliveira AL, da Costa Rodrigues CE (2021) Conventional and pressurized ethanolic extraction of oil from spent coffee grounds: kinetics study and evaluation of lipid and defatted solid fractions. J Supercrit Fluids 177:105332. https://doi.org/10.1016/j.supflu.2021.105332
Farobie O, Matsumura Y (2017) State of the art of biodiesel production under supercritical conditions. Prog Energy Combust Sci 63:173–203. https://doi.org/10.1016/j.pecs.2017.08.001
Al-Hamamre Z, Foerster S, Hartmann F, Kröger M, Kaltschmitt M (2012) Oil extracted from spent coffee grounds as a renewable source for fatty acid methyl ester manufacturing. Fuel 96:70–76. https://doi.org/10.1016/j.fuel.2012.01.023
Vol’eva VB, Koverzanova EV, Ovsyannikova MN, Ryzhakova AV, Gumerov FM, Usmanov RA, Hounkpatin DD (2021) Transformations of ethanol supercritical fluid. Russ J Org Chem 57(9):1466–1470. https://doi.org/10.1134/s1070428021090128
Stevanato N, de Mello BTF, Saldaña MDA, Cardozo-Filho L, da Silva C (2023) Production of ethyl esters from forage radish seed: an integrated sequential route using pressurized ethanol and ethyl acetate. Fuel 332:126075. https://doi.org/10.1016/j.fuel.2022.126075
Trentini CP, de Mello BTF, Postaue N, Raspe DT, da Silva C, Cabral VF (2022) Sequential process to obtain fatty acid esters from crambe oil using a mixture of acyl acceptors under pressurized conditions. J Supercrit Fluids 188:105664. https://doi.org/10.1016/j.supflu.2022.105664
Supang W, Ngamprasertsith S, Sakdasri W, Sawangkeaw R (2022) Ethyl acetate as extracting solvent and reactant for producing biodiesel from spent coffee grounds: a catalyst- and glycerol-free process. J Supercrit Fluids 186:105586-91. https://doi.org/10.1016/j.supflu.2022.105586
Toda TA, Barreiro MM, da Cunha GB, da Costa Rodrigues CE (2023) Performance of alcoholic solvents in the continuous countercurrent spent coffee grounds oil extraction. J Ind Eng Chem 118:268–279.https://doi.org/10.1016/j.jiec.2022.11.012
Sakdasri W, Ngamprasertsith S, Saengsuk P, Sawangkeaw R (2021) Supercritical reaction between methanol and glycerol: the effects of reaction products on biodiesel properties. Energy Convers Manag 12:100145. https://doi.org/10.1016/j.ecmx.2021.100145
Hegel PE, Martín LA, Popovich CA, Damiani C, Pancaldi S, Pereda S, Leonardi PI (2017) Biodiesel production from Neochloris oleoabundans by supercritical technology. Chem Eng Process: Process Intensif 121:232–239. https://doi.org/10.1016/j.cep.2017.08.018
Hegel PE, Martín LA, Popovich CA, Damiani C, Leonardi PI (2019) Biodiesel production from Halamphora coffeaeformis microalga oil by supercritical ethanol transesterification. Chem Eng Process: Process Intensif 145:107670. https://doi.org/10.1016/j.cep.2019.107670
Bogatishcheva NS, Faizullin MZ, Popov AP, Nikitin ED (2017) Critical properties, heat capacities, and thermal diffusivities of four saturated triglycerides. J Chem Thermodyn 113:308–314. https://doi.org/10.1016/j.jct.2017.07.006
Velez A, Soto G, Hegel P, Mabe G, Pereda S (2012) Continuous production of fatty acid ethyl esters from sunflower oil using supercritical ethanol. Fuel 97:703–709. https://doi.org/10.1016/j.fuel.2012.02.024
Woo D-G, Kim SH, Kim TH (2021) Solid fuel characteristics of pellets comprising spent coffee grounds and wood powder. Energies 14(2):371. https://doi.org/10.3390/en14020371
Mohamed G, Djamila B (2018) Properties of dune sand concrete containing coffee waste. MATEC Web Conf 149:01039. https://doi.org/10.1051/matecconf/201814901039
Stevanato N, de Mello BTF, Saldaña MDA, Cardozo-Filho L, da Silva C (2023) Production of ethyl esters from forage radish seed: an integrated sequential route using pressurized ethanol and ethyl acetate. Fuel 332. https://doi.org/10.1016/j.fuel.2022.126075
Postaue N, Eduardo Borba C, da Silva C (2022) Ethanol and dimethyl carbonate as solvents under pressurized conditions to obtain oil and active compounds from crambe seeds. Fuel 324:124827. https://doi.org/10.1016/j.fuel.2022.124827
dos Santos PRS, Voll FAP, Ramos LP, Corazza ML (2017) Esterification of fatty acids with supercritical ethanol in a continuous tubular reactor. J Supercrit Fluids 126:25–36. https://doi.org/10.1016/j.supflu.2017.03.002
Vieitez I, Irigaray B, Casullo P, Pardo MJ, Grompone MA, Jachmanián I (2012) Effect of free fatty acids on the efficiency of the supercritical ethanolysis of vegetable oils from different origins. Energ Fuel 26(3):1946–1951. https://doi.org/10.1021/ef201977s
Trentini CP, Postaue N, Cardozo-Filho L, da Silva C (2020) Waste oil/crambe oil blends for ethyl ester production under supercritical conditions. J Supercrit Fluids 163:104889–98. https://doi.org/10.1016/j.supflu.2020.104889
de Melo MMR, Barbosa HMA, Passos CP, Silva CM (2014) Supercritical fluid extraction of spent coffee grounds: measurement of extraction curves, oil characterization and economic analysis. J Supercrit Fluids 86:150–159. https://doi.org/10.1016/j.supflu.2013.12.016
Jenkins RW, Ellis EH, Lewis EJ, Paterson M, Le CD, Ting VP, Chuck CJ (2017) Production of biodiesel from Vietnamese waste coffee beans: biofuel yield, saturation and stability are all elevated compared with conventional coffee biodiesel. Waste Biomass Valori 8(4):1237–1245. https://doi.org/10.1007/s12649-016-9715-x
Couto RM, Fernandes J, da Silva MDRG, Simões PC (2009) Supercritical fluid extraction of lipids from spent coffee grounds. J Supercrit Fluids 51(2):159–166. https://doi.org/10.1016/j.supflu.2009.09.009
Pacetti D, Lucci P, Frega NG (2015) Unsaponifiable matter of coffee. In: Preedy VR (ed) Coffee Health Dis Prev. Academic Press, San Diego, pp 119–127. https://doi.org/10.1016/b978-0-12-409517-5.00013-9
Page JC, Arruda NP, Freitas SP (2017) Crude ethanolic extract from spent coffee grounds: volatile and functional properties. Waste Manag 69:463–469. https://doi.org/10.1016/j.wasman.2017.08.043
Caballero-Galván AS, Restrepo-Serna DL, Ortiz-Sánchez M, Cardona-Alzate CA (2018) Analysis of extraction kinetics of bioactive compounds from spent coffee grounds (Coffea arábica). Waste Biomass Valori 9(12):2381–2389. https://doi.org/10.1007/s12649-018-0332-8
Gu J, Lee A, Choe C, Lim H (2023) Comparative study of biofuel production based on spent coffee grounds transesterification and pyrolysis: process simulation, techno-economic, and life cycle assessment. J Clean Prod 428:139308. https://doi.org/10.1016/j.jclepro.2023.139308
Tinoco-Caicedo DL, Mero-Benavides M, Santos-Torres M, Lozano-Medina A, Blanco-Marigorta AM (2021) Simulation and exergoeconomic analysis of the syngas and biodiesel production process from spent coffee grounds. Case Stud Therm Eng 28:101556. https://doi.org/10.1016/j.csite.2021.101556
Mahmoud E, Atabani AE, Badruddin IA (2022) Valorization of spent coffee grounds for biogas production: a circular bioeconomy approach for a biorefinery. Fuel 328. https://doi.org/10.1016/j.fuel.2022.125296
Hu X, Wang Y, Mourant D, Gunawan R, Lievens C, Chaiwat W, Gholizadeh M, Wu L, Li X, Li C-Z (2013) Polymerization on heating up of bio-oil: a model compound study. AIChE Journal 59(3):888–900. https://doi.org/10.1002/aic.13857
Varfolomeev SD, Vol’eva VB, Komissarova NL, Kurkovskaya LN, Malkova AV, Ovsyannikova MN, Gumerov FM, Usmanov RA (2019) New possibilities in the synthesis of fuel oxygenates from renewable sources. Russ Chem Bull 68(4):717–724. https://doi.org/10.1007/s11172-019-2478-3
Olivares-Carrillo P, Quesada-Medina J (2012) Thermal decomposition of fatty acid chains during the supercritical methanol transesterification of soybean oil to biodiesel. J Supercrit Fluids 72:52–58. https://doi.org/10.1016/j.supflu.2012.08.012
Niza NM, Tan KT, Lee KT, Ahmad Z (2013) Biodiesel production by non-catalytic supercritical methyl acetate: thermal stability study. Appl Energy 101:198–202. https://doi.org/10.1016/j.apenergy.2012.03.033
Lim S, Lee KT (2014) Investigation of impurity tolerance and thermal stability for biodiesel production from Jatropha curcas L. seeds using supercritical reactive extraction. Energy 68:71–79. https://doi.org/10.1016/j.energy.2014.02.056
Salar-García MJ, Ortiz-Martínez VM, Olivares-Carrillo P, Quesada-Medina J, de los Ríos AP, Hernández-Fernández FJ (2016) Analysis of optimal conditions for biodiesel production from Jatropha oil in supercritical methanol: quantification of thermal decomposition degree and analysis of FAMEs. J Supercrit Fluids 112:1–6. https://doi.org/10.1016/j.supflu.2016.02.004
Sakdasri W, Sawangkeaw R, Medina-Gonzalez Y, Camy S, Condoret JS, Ngamprasertsith S (2016) Experimental study and modeling of phase equilibrium of the methanol-tripalmitin system: application to palm oil transesterification with supercritical methanol. Ind Eng Chem Res 55(18):5190–5199. https://doi.org/10.1021/acs.iecr.5b04588
Valle P, Velez A, Hegel P, Mabe G, Brignole EA (2010) Biodiesel production using supercritical alcohols with a non-edible vegetable oil in a batch reactor. J Supercrit Fluids 54(1):61–70. https://doi.org/10.1016/j.supflu.2010.03.009
de Jesus AA, de Santana Souza DF, de Oliveira JA, de Deus MS, da Silva MG, Franceschi E, da Silva Egues SM, Dariva C (2018) Mathematical modeling and experimental esterification at supercritical conditions for biodiesel production in a tubular reactor. Energy Convers Manag 171:1697–1703. https://doi.org/10.1016/j.enconman.2018.06.108
Sawangkeaw R, Bunyakiat K, Ngamprasertsith S (2011) Continuous production of biodiesel with supercritical methanol: optimization of a scale-up plug flow reactor by response surface methodology. Fuel Process Technol 92(12):2285–2292. https://doi.org/10.1016/j.fuproc.2011.07.014
Sakdasri W, Sawangkeaw R, Ngamprasertsith S (2017) An entirely renewable biofuel production from used palm oil with supercritical ethanol at low molar ratio. Braz J Chem Eng 34(4):1023–1034. https://doi.org/10.1590/0104-6632.20170344s20150660
Sawangkeaw R, Teeravitud S, Piumsomboon P, Ngamprasertsith S (2012) Biofuel production from crude palm oil with supercritical alcohols: comparative LCA studies. Bioresour Technol 120:6–12. https://doi.org/10.1016/j.biortech.2012.06.014
Sakdasri W, Sawangkeaw R, Ngamprasertsith S (2018) Techno-economic analysis of biodiesel production from palm oil with supercritical methanol at a low molar ratio. Energy 152:144–153. https://doi.org/10.1016/j.energy.2018.03.125
Kookos IK (2018) Technoeconomic and environmental assessment of a process for biodiesel production from spent coffee grounds (SCGs). Resour Conserv Recycl 134:156–164. https://doi.org/10.1016/j.resconrec.2018.02.002
Acknowledgements
The authors express their sincere appreciation to Starbucks coffee house, Siam Square One, and Samyan Mitrtown Branches, Bangkok, Thailand, for kindly donating of spent coffee ground samples. The authors are also thankful for comments and questions of the audiences from The 4th International Conference on Bioresource Technology for Bioenergy Bioproducts and Environmental Sustainability, 14–17 May 2023, Biorender and Flaticon for visually appealing vectors to create the process diagram and graphical abstract.
Funding
This research is funded by Thailand Science research and Innovation Fund Chulalongkorn University (IND66610028) and partially supported by Asahi Glass Foundation (2022).
Author information
Authors and Affiliations
Contributions
Wirasinee Supang: methodology, investigation, and writing—original draft. Nutthakit Charoendee: methodology and investigation. Somkiat Ngamprasertsith: writing—review and editing and supervision. Winatta Sakdasri: validation and writing—review and editing. Ruengwit Sawangkeaw: conceptualization, writing—review and editing, and visualization.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Supang, W., Ngamprasertsith, S., Sakdasri, W. et al. Biodiesel Production from Spent Coffee Grounds by Using Ethanolic Extraction and Supercritical Transesterification. Bioenerg. Res. (2024). https://doi.org/10.1007/s12155-024-10782-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12155-024-10782-z