Abstract
Cellulase production by solid-state fermentation (SSF) is a promising pre-treatment strategy for optimizing methane generation from passion fruit and orange peel. This study employed an experimental design the enzymatic process for cellulase production using orange peel and passion fruit peel as substrates. The biochemical methane potential (BMP) test was used to assess the effectiveness of the SSF pre-treatment in methane generation from waste products. The study results showed that passion fruit peel had a higher cellulase activity than orange peel. Moreover, the experimental design successfully optimized the enzymatic process, with a maximum cellulase activity of 13.91 and 14.46 U/mL for FPase e CMCase of passion fruit peel and 2.21 and 5.67 U/mL for FPase e CMCase of orange peel. BMP assays revealed that SSF pre-treatment increased methane production, with the most significant increases observed in orange peel waste with both granular and flocculent sludge (17 and 25 NmL/gVS) compared to passion fruit peel waste (13 and 14 NmL/gVS). Furthermore, configurations obtained a high percentage of methane (63 to 71%). The cone and logistic models exhibited superior performance in terms of coefficient of determination (0.891 to 0.991) and minimized residual squares (7.3 to 247.7 NmL/gVS). Overall, this study demonstrates SSF potential as an efficient pre-treatment method for cellulase production and methane generation from agro-industrial waste (passion fruit peel and orange). This sustainable, cost-effective approach not only reclaims waste products but also contributes to renewable energy production, offering significant implications for agro-industrial waste management and innovative biotechnological solutions.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agro-industrial fruit waste such as peels, seeds, and peel have great potential to be used to produce enzymes, biopolymers, chemical fertilizers, animal feed, and biofuels (biogas, ethanol, biodiesel) [1,2,3,4]. The use of these wastes as a source of biomass for biogas production and energy use has been gaining prominence due to the large generation of agro-industrial waste and economic and environmental gains. However, its use as a source of biomass for anaerobic digestion is constrained by its recalcitrant nature (lignocellulosic), acidic pH, high moisture content, and seasonal nature of production [3,4,5], requiring the application of pre-treatment (chemical, physical, biological) to improve the accessibility and biodegradability of this material during anaerobic digestion, through action on lignin, cellulose, and hemicellulose [6, 7]. These fractions are responsible for 97–99% of the dry mass of agro-industrial waste [8]. Cellulose is the most abundant component, representing approximately 35 to 50% of the dry weight of biomass [9]. Cellulose, a complex polysaccharide found in plant cell walls, represents one of the main sources of renewable carbon on Earth, but its complex structure makes it difficult to degrade.
Enzymatic pre-treatment of lignocellulosic waste using cellulolytic enzymes is a highly recommended strategy [10, 11]. Cellulase plays a fundamental role in this process, as it breaks the glycosidic bonds of cellulose, releasing simpler fermentable sugars (such as glucose) that can be substrates for microorganisms (bacteria and fungi) and produce by-products of biotechnological interest [12, 13]. It also works by facilitating the degradation of hemicellulose (another potential source of fermentable sugars), making these sugars also bioavailable. Although cellulase does not act directly on the degradation of lignin, its action on cellulose facilitates its indirect removal.
Cellulase is a complex multicomponent enzyme consisting of three types of enzymes in synergisms, the endo-β-1,4-glucanase, exo-β-1,4-glucanase (cellobiohydrolase), and β-glucosidase. These components act synergistically, converting cellulose into glucose [14, 15]. Each of these enzymes has a specific role in the cellulose degradation process. Endoglucanases act on the internal bonds of cellulose, exoglucanases on the ends of cellulose chains, while beta-glucosidases convert cellodextrins into glucose [8, 16].
The biochemical mechanism of cellulose degradation begins with the adsorption of cellulase on the surface of cellulose molecules through specific active sites in their structures [9]. Once bound to cellulose, cellulase acts on the glycosidic bonds between the glucose residues that make up the cellulose, using water molecules to break these bonds (hydrolysis). The insertion of water molecules between glucose residues (through hydrolases) creates free OH− groupings at the ends of glucose molecules, resulting in the breakdown of the cellulose chain into smaller fragments (cellodextrins). Cellodextrins are released from the cellulose surface and can be further reduced by cellulases into individual glucose fragments.
Cellulase can be applied for pre-treatment of agro-industrial waste and any organic substrates with similar characteristics [17]. Facilitating the breakdown of the hydrolytic step of cellulose degradation significantly accelerates anaerobic digestion resulting in greater biogas production. The efficiency of enzymes in the pre-treatment stage of hemicellulosic waste can improve methane production by up to 50%, which justifies its application in various applications. Enzymatic pre-treatment for agro-industrial waste can contribute as an additional source of renewable energy to replace fossil fuels, contributing to the diversification of the energy matrix and better use of the energy potential of these wastes. Economic viability, however, will depend on several factors, including the type and amount of waste available, the cost of the cellulase enzyme, the efficiency of converting cellulose to methane, and market prices for methane [17]. Furthermore, environmental and regulatory considerations also play an important role.
Among the existing pre-treatment methods, using enzyme-producing microorganisms through solid-state fermentation (SSF) has shown promise in facilitating hydrolysis [12, 18].
SSF applies to the growth process of aerobic microorganisms on organic substrates in the absence of free water, with controlled moisture, to obtain a desired product [12]. SSF is a widely used fermentation technique for producing proteases, pectinases, and cellulases [13, 19]. The SSF process has several advantages, such as higher concentration and productivity, high yield of the required product, low energy cost, lower operating costs, the use of simpler machines for fermentation, shorter fermentation time, and decrease or absence of degradation of enzymes by undesirable proteases, in addition to the use of low-cost substrates [20, 21]. SSF has been used as a suitable method for producing cellulases from lignocellulosic substrates due to the low operational cost and high yield of the fermented product [12, 22].
Filamentous fungi are the main source of hydrolases that produce multienzymes such as endo- and exo-enzymes, including cellulase, xylanase, and pectinase, which degrade biopolymers such as cellulose, hemicellulose, and pectin, respectively [23]. Fungi commonly used in enzyme production include the genera Aspergillus, Trichoderma, Penicillium, Fusarium, Humicola, and Phanerochaete [18, 23, 24]. The genus Aspergillus has stood out as an excellent cellulase producer, representing an advantage in the biomass fermentation process [12, 14, 15, 24].
Therefore, this work aimed to determine the production of cellulase by Aspergillus japonicus through SSF as a pre-treatment strategy for orange peel and passion fruit peel to contribute to increased methane generation.
Material and Methods
Standardization of Substrates
In the experimental tests, two substrates were used: orange peel (OP) and passion fruit peel (PF). Initially, the substrates were manually chopped into pieces of approximately 2 × 2 cm. The material was dried in an oven at 65 °C until moisture stabilization. After drying, the material was crushed in a Willye knife mill (SPLabor brand) with 2.0-mm sieve attached. After this first separation, it was manually sieved through a 0.5-mm sieve. The substrate retained in this last sieve was used for SSF.
Obtaining the Fungus and Anaerobic Inoculum
Aspergillus japonicus URM5620 was obtained from the culture collection of the URM mycology collection at the Federal University of Pernambuco (UFPE). The fungus was selected because it has proven cellulolytic activity in the literature for lignocellulosic substrates [12, 14, 15, 24, 25]. The microorganism was reactivated in a nutrient solution (10 g of peptone, 3 g of meat hydrolyzed, and 20 g of glucose for 100 mL of distilled water) and placed in a 125-mL Erlenmeyer flask containing PDA medium (potato, dextrose, agar), sterilized (121 °C/20 min), and incubated at 30 °C for 7 days in a microbiological oven. The spore suspension was obtained by adding 10 mL of a saline solution NaCl (0.9%) with Tween 80 (0.01%) previously sterilized. The spore count was performed using a Neubauer chamber, and the inoculum was standardized to a concentration of 107 spores/mL.
Two anaerobic inoculums (sludge) were used: a granular sludge obtained from an industrial UASB reactor used in the treatment of vinasse and a flocculent sludge obtained from a UASB reactor used in the treatment of sanitary sewage. UASB reactors operate at full scale. Samples of 5 L of the two sludges were obtained directly from the sludge disposal valves of the UASB reactors. The sludge was stored under refrigeration at 4 ± 1 °C until the experiments were carried out.
Characterization of Dry Substrates and Inoculum
The dry substrates (OP e PF) and inoculum (granular sludge (GS), floccular sludge (FS)) were characterized by pH, moisture, total solids (TS), and volatile solids (VS) [26]. The elemental analysis (carbon/nitrogen ratio) was performed by direct combustion in an elemental analyzer (EC EA 111 instruments).
Experimental Design
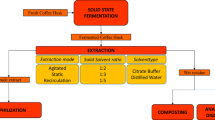
After the characterization of substrates, sludge and fungus, a methodological sequence in 4 steps was proposed, which is briefly presented in Fig. 1.
Step 1: Enzymatic Hydrolysis
SSF was used as a strategy for pre-treatment of substrates through enzymatic hydrolysis. The SSF was performed in a 250-mL Erlenmeyer flask containing the amount of substrate to be used in grams, according to the experimental design (Table 1), with granulometry between 0.5 and 2.0 mm. Then, the spore solution 107 spores/mL (orange and passion fruit peel) was added to the nutrient solution (citrate buffer at pH 6.0 containing 0.5% yeast hydrolyzed and 1.0% glucose) until it reached the desired moisture according to the experimental design. The substrates and nutrient solution were sterilized in an autoclave at 121 °C/1 atm for 20 min.
The enzyme extraction was performed with 7.5 mL of citrate buffer per gram of substrate added to each Erlenmeyer flask and shaken in an incubator with temperature control and orbital agitation—shaker (Tecnal, TE-424) at 150 rpm per 90 min [27]. Then, the enzymatic hydrolyzed was filtered and centrifuged to quantify the cellulolytic activity.
The enzymatic hydrolyzed of orange peel (OPH) and passion fruit peel (PFH) substrates were characterized through analysis of hydrogen ion potential (pH) using the methodology of the NBR 10006 standard [28] and chemical oxygen demand (COD) by the method of SMEWW [29].
Step 2: Enzymatic Analysis
The determination of cellulolytic activity was determined according to Ghose (1987), through the determination of the total activity in paper (FPase) and endoglucanase (CMcase).
Total cellulase activity on filter paper (FPase) was determined by incubating 1 mL of diluted enzyme solution (0.5 mL of enzyme extract and 0.5 mL of 0.05 mol/L citrate buffer and pH 4.8) containing paper filter n° 1 (50 mg, 1 × 6 cm) and incubated at 50 °C in water bath for 60 min. Endoglucanase activity (CMCase) was performed in 1 mL mixture, containing 0.5 mL of enzyme extract and 0.5 mL of 1% carboxymethylcellulose (CMC) solution in citrate buffer (0.05 mol/L pH 4.8) incubated at 50 °C in water bath for 30 min [30]. One unit of enzyme activity was defined as the amount of enzyme required to release 1 µmol of glucose or p-nitrophenol from appropriate substrates per minute, under assay conditions and was expressed as units per milliliter, determined by 3,5-dinitrosalicylic acid method (DNS) [31]. The amount of glucose released was measured, also by reaction with DNS. In both activities, we used enzyme blank and reaction blank controls, and we converted absorbance into glucose using a standard curve previously established. One international unit (IU) was defined as 1 µmol of glucose released per minute, equivalent to 0.18 mg of glucose per minute.
Step 3: Anaerobic Fermentation
For the anaerobic fermentation step, the biochemical methane potential (BMP) test was used. The BMP test followed the methodology by Silva et al. [25] and Santos et al. [3].
The hydrolyzed of orange peel (OPH) and passion fruit peel (PFH) obtained from the SSF in the best condition obtained by the experimental design were used in the BMP test. Borosilicate flasks (total volume of 250 mL) were used, consisting of a threaded nylon lid and sealing rings. The top of the flask had two needle valves attached, one for releasing the biogas-generated pressure or inserting N2 (purge of O2 at the beginning of the assay) and another, where a mechanical manometer (100 kPa) is installed for monitoring the biogas pressure inside the reactor.
The test was carried out to evaluate the increase in biogas production of orange peel and passion fruit peel substrates after pre-treatment by SSF using A. japonicus. Tests were conducted using enzymatic hydrolysates of orange peel (OPH) and passion fruit peel (PFH) with inoculum (GS, FS).
The extract inserted to OPH and PFH into the reactors were 13 mL and 30 mL, respectively (Table 2). The inoculum (GS, 26.5 mL, and FS, 72.0 mL) added to each flask was 5 g VSS/L. The sodium bicarbonate used was 1 g, according to Santos et al. [3]. Distilled water was added to the reactor to maintain COD of 2 g/L and a useful volume of 200 mL for each assay. A headspace of 50 mL was maintained in all reactors, representing 20% of the reactor volume. The test was performed in triplicate, also considering a blank (distilled water and inoculum). A total of 18 reactors with 6 configurations were evaluated over 60 days.
The filling of each reactor followed the sequence performed by Santos et al. [3]: (1) addition of inoculum, (2) addition of enzymatic hydrolyzed, (3) volume complementation with distilled water (calculated), (4) addition of sodium bicarbonate, (5) initial measurement of electrical conductivity and pH, (6) closing of the reactor and valves, and (7) wrapping of the reactor in aluminum foil. After the reactors were filled and closed, the pressure gauges were removed, and N2 was introduced with the needle valves open for 2 min (to provide ideal anaerobic conditions for substrate degradation). Then, the gas outlet and inlet valves were closed, and the manometers were replaced in the reactors, maintaining a pressure of 20 kPa in all the flasks. Then, the reactors were kept in an incubator table (Tecnal, TE-424) with temperature control (37 °C) and orbital agitation (60 rpm) for 60 days. The accumulated biogas production was analyzed by measuring the accumulated pressure of biogas obtained in the daily monitoring following the methodology of Ivanova et al. [32].
The characterization of the biogas was analyzed weekly, keeping the headspace of the flasks (reactors) accumulated for 2 days before measuring the biogas, without relief, to increase the volume of biogas for injection into the gas chromatograph [3]. The composition of the biogas (CH4, CO2) was analyzed using a gas chromatograph (APPA GOLD), with a column (Porapak “N”) that uses H2 as a carrier gas, at an oven temperature of 60 °C, with a thermal conductivity detector (TCD). The chromatograph was calibrated using a standardized gas consisting of 60% CH4 and 40% CO2. The N2000 Chromatostation Chromatographic Data Acquisition System was used for data processing.
The initial and final contents of the BMP test through pH, COD, COD removal, electrical conductivity (EC), total alkalinity (TA), volatile fatty acids (VFA), and VFA/TA ratio [33].
Step 4: Analysis of Results
Biogas and Methane Potential Analysis
The biogas or methane potential (Ym) was calculated by subtracting the accumulated volume of biogas/methane from the substrate + inoculum by the accumulated volume of the respective inoculum blank, divided by the mass (volatile gram solids) of the substrate according to Eq. 1.
where Ym = biogas or methane potential (NmL/VS), VAs = accumulated biogas/methane volume of the BMP test setup (NmL), VAi = accumulated biogas/methane volume of the inoculum blank (NmL), and VSg = initial VS concentration of the dry substrate (g/VS).
Models and Kinetic Parameters
The methane potential obtained from BMP assays was adjusted using modified Gompertz (Eq. 2), first-order (Eq. 3), cone (Eq. 4), modified logistic (Eq. 5), and Fitzhugh (Eq. 6) models, commonly used for batch tests [3, 4, 34, 35]. The kinetic parameters were also evaluated for each model studied to compare the most suitable for this type of substrate. Kinetic models were obtained using the OriginPro 8.0 software, based on exponential curve and non-linear regression.
where y(t) is the methane cumulative production (NmL/gVS), t is the experimental execution time (d), k is the hydrolysis constant (d−1); \({y}_{m}\) refers to the methane maximum production (NmL/g VS), \(\mu\) is the maximum methane production rate (NmL/d), and \(\lambda\) is the lag phase (d).
Statistical Analysis
The results obtained in the BMP test were evaluated through analysis of variance (ANOVA) and Tukey’s test with a confidence level of 95% (p ≤ 0.05) using the Statistica® 7.0 program (StatSoft, Tulsa, USA). To evaluate the performance of the kinetic models, the determination factor (R2) (Eq. 7) and the residual sum of squares (RSS) (Eq. 8) were calculated, where \({Z}_{{f}_{i}}\) is the predicted value and \({Z}_{{o}_{i}}\) is the observed value.
Results and Discussion
Production of Cellulolytic Enzymes by A. japonicus URM5620
Table 3 presents the results of the cellulolytic activity of the waste studied. The maximum FPase activity according to factorial design for passion fruit peel was 13.91 U/mL, while the maximum CMCase activity was 14.46 U/mL. Pareto charts show the influence of conditions on the total cellulase (FPase) and endoglucanase (CMCase) activity (Fig. 2).
Analyzing the Pareto chart of passion fruit hydrolysate (Fig. 2a, b), it is possible to observe that FPase and CMCase had very similar results, where only moisture had a significant effect on the process, which with negative values, i.e., a lower moisture condition positively influences the evaluated cellulolytic activities. This can be confirmed when considering the response in Table 3, where the highest activities occurred when moisture was 40% (Assay 1 and 3). Assay 1 was the best result, with values of 13.91 and 14.46 U/mL for FPase and CMCase, respectively.
The analyzed variables (substrate and moisture) nothing had a significant effect on the production of FPase for the orange peel substrate (Fig. 2c). Still, they would escape the conditions of the fermentative method since fermentation under moisture conditions greater than 60% corresponds to a semi-solid fermentation. For CMCase production, all variables were significant (Fig. 2d), with positive values, so that the greater the amount of substrate, the most favored will be the production of endoglucanase. The moisture had a positive and synergistic effect with the amount of substrate. High moisture prevents this stress during fermentation, keeping water available until the end of the fermentative process. The best result for FPase in orange peel occurred under the central point conditions with a production of 2.21 U/mL. In contrast, for CMCase, the best results were obtained in 60% moisture and 10 g substrate, levels higher of conditions, with the production of 5.63 U/mL.
The cellulase enzyme acts in the degradation of cellulose and hemicellulose during anaerobic digestion. It has the function to increase the digestibility of complex lignocellulosic substrates [36]. This process can be used industrially, without the need to use expensive commercial enzymes, which sometimes make this alternative unfeasible. It is also noteworthy that the production of the enzyme through fungal pre-treatment such as SSF is an efficient and low-cost method. It has a lower energy consumption and is environmentally safer (no toxic by-products are formed during the process) compared to other pre-treatment methods such as physical and chemical [36].
Several lignocellulosic wastes (mango and passion fruit waste, orange peel) used to produce cellulase. Santos et al. [37] studied the mango waste SSF using the Aspergillus niger and obtained lower enzymatic activity for CMCase (7.26 U/mL) and FPase (2.55 U/mL) after 74.5 h and 98.5 h, respectively. Mrudula and Murugammal [13] studied cellulase production from coconut waste through SSF using A. niger and too obtained lower enzymatic activity for CMCase and FPase with 3.42 and 1.77 U/mL, respectively, in 96 h. Junqueira et al. [38] found lower results for the enzymatic activity of 0.57 U/mL using passion fruit waste as substrate and A. niger.
Delabona et al. [39] studied cellular production from orange peel through SSF using the A. niger and obtained lower enzymatic activities for CMCase with 0.9 U/mL at 120 h with 70% moisture. Mamma et al. [40] too investigated cellulase production from orange peel using A. niger by solid-state fermentation after 1 day to 70% moisture and pH 5.0, obtained a higher result for CMCase activity (12.9 U/mL). According to the results, the A. japonicus was promising in cellulase production using passion fruit peel and orange peel substrates.
Characterization of Substrates, Enzymatic hydrolyzed, and Inoculum
The pH of the enzymatic hydrolyzed, OP and PF were acidic, typical of substrates obtained by hydrolytic processes and waste fruit (Table 4). Similar pH (3.5 to 4.6) was obtained by Silva et al. [25] and Marín et al. [12] when working with orange peel and passion fruit pre-treated with fungi (Penicillium digitatum, P. italicum, A. japonicus).
The subtract OP (18,508 mg/L) and enzymatic hydrolyzed OPH (30,337 mg/L) showed a high initial concentration of COD, indicating a greater organic load to be degraded compared to PF (16,279 mg/L) and PFH (12,526 mg/L). In the literature, a lower COD value (1075 to 10,777 mg/L) was found for orange peel [41, 42].
The PF and OP substrates showed high moisture content (> 81%), corroborating the results found by Zhao et al. (2016) and Siles et al. (2016) for citrus waste (80%) and passion fruit peel (85%). Moisture is a crucial parameter in anaerobic digestion, as it facilitates the transport of nutrients and enzymes and the distribution of microorganisms in the digester, in addition to reducing the concentration of dilution of toxic compounds in the medium [3, 43]. The high VS content for PF (94%) and OP (95%) was characteristic for fruit residues due to the high fraction of organic matter favorable for methane production [3]. Similar VS values were found by other authors for orange peel (97%) and passion fruit (94%) [5, 25]. The inoculum (FS, GS) showed high levels of VS (56.8%, 75.9%) and moisture (89.4%, 94.7%), corroborating with other authors who used flocculent and granular sludge as inoculum [44,45,46].
Accumulated Volume of Biogas and Methane from the BMP Test
In terms of accumulated biogas production, considering the average value of the experimental of passion fruit peel and orange peel hydrolyzed by SSF, the configurations PFH + FS and PFH + GS presented the highest accumulated volume of biogas (266 NmL, 237 NmL, respectively), indicating that culture present with greater enzymatic activity produced it increased the production of biogas (passion fruit hydrolyzate = FPase 13.91 U/mL and CMCase 14.46 U/mL). The greater enzymatic activity of the PFH hydrolyzed indicates greater degradation of cellulose, that is, a greater concentration of free glucose usable by FS, GS inoculum rich in methanogenic microorganisms. The configuration OPH + GS obtained the lowest accumulated volume of biogas (144 NmL), being 45% lower compared to PFH + FS.
Regarding the accumulated volume of CH4, the configuration PFH + FS (118.9 NmL of CH4) obtained a superior result, followed by OPH + FS (97.5 NmL of CH4) and PFH + GS (73.5 NmL of CH4), respectively (Fig. 3b). The OPH + GS configuration showed the lowest accumulated volume of CH4 (62.3 NmL).
The accumulated volume of biogas (128 NmL) and CH4 (70 NmL) in the FS blank was higher than that obtained by the GS blank (47 NmL, 23 NmL). That is, there was a significant difference between the inoculum alone. The FS inoculum produced more methane in methane volume production levels in the digestion with the hydrolyzates. The FS was 2.98 times greater than the GS compared to the best digestion with the passion fruit hydrolyzate (PFH).
It should be noted that, despite the possibility of improvement via fungal hydrolysis, this process represents an additional step that must be considered in the scale-up, costs, and economic evaluation of gains not performed by this work.
Composition and Potential for Biogas and Methane Generation
Figure 4a, b shows the BMP test setups’ biogas and methane potential composition (CO2, CH4). The PFH + FS and OPH + GS configurations showed the highest biogas generation potential (55 NmL/g VS, and 52 NmL/g VS, respectively). In contrast, the OPH + FS showed the best methane potential (25 NmL/g VS).
Jos et al. [47] obtained a similar biogas generation potential (58 NmL/g VS) when studying the fungal pre-treatment (SSF) of palm fruit waste using a consortium of microorganisms with the inoculum (bovine rumen) in batch reactors (2 L) under mesophilic conditions. Other studies have reported higher biogas potential (500 NmL/g VS) when evaluating the effect of different mixed strains of fungi (Sporotrichum sp., Aspergillus spp., Fusarium sp.) cultivated under SSF from orange waste (8% TS) using as inoculum cow dung in semi-continuous reactors (1.5 L) under mesophilic conditions in 25 days was found by Srilatha et al. [48]. Ruiz et al. [49] found a higher methane generation potential (359 NmL/g VS) when using orange peel pre-treated with fungi (P. digitatum and P. italicum) with digestate (cow manure with vegetable waste) in BMP test under mesophilic conditions. Marín et al. [12] reported a production of 552 NmL/g VS at 37 °C for 25 days of incubation for orange peel with digested, higher than the value obtained in this study.
No studies were found that used SSF using Aspergillus with passion fruit peel to optimize the methane production.
Figure 4a shows that all configurations achieved a high average percentage of methane, ranging from 63 to 71%, demonstrating that SSF increased the effectiveness of the hydrolysis step in anaerobic digestion, facilitating the breakdown of cellulose by the cellulase enzyme into simpler fermentable sugars. This pre-treatment allows microorganisms to degrade lignocellulosic materials more effectively, producing more methane.
The PFH + GS had the lowest methane percentage (63%) compared to the other configurations. The percentage of CH4 for the configurations studied was higher than the work by Srilatha et al. [48]. The authors reported methane production of 45–50% of the biogas produced for orange residues pre-treated by SSF (Sporotrichum sp., Aspergillus sp., Fusarium sp., Penicillium sp.), with the addition of cow manure in semi-continuous reactors (1500 L) in mesophilic conditions for 25 days.
The GS and FS inoculum without interaction with hydrolyzed substrate showed methane percentages above 71%, indicating that both can be used as process accelerators for the studied substrates.
Kinetic Parameters of the Configurations
Figure 5 and Table 5 show the kinetic curves of methane production and the parameters obtained through the kinetic models of modified Gompertz, cone, first order, logistic, and Fitzhugh.
In general, the studied configurations showed a good R2 (0.901 to 0.991) for the five kinetic models, except for the OPH + FS configuration (R2 < 0.9). The configurations studied in the batch tests showed a better fit to the kinetic logistic (PFH + FS, OPH + FS) and cone (PFH + GS, OPH + GS) models with determination coefficient (R2) ranging from 0.891 to 0.991, being confirmed through the smallest residual sum of squares (RSS) of the models. The OPH + GS configuration obtained the best R2 (0.991) and OPH + FS the lowest (0.891) fit.
The ym value of all kinetic models was similar to the methane potential of the experimental data, indicating that the models can be used in future scale-up studies of reactor sizing and predictions and full-scale simulations.
Regarding the kinetic parameters, the value of k ranged from 0.077 to 0.152 d, 0.077 to 0.154 d, and 0.086 to 0.128 d for the first-order models, cone, and Fitzhugh, respectively. The OPH + FS configuration obtained the highest k (0.128–0.154 d) in all kinetic models. The k value obtained in this study was within the range (0.02 to 0.66 d) reported by other authors for fruit substrates [4, 25, 34, 50]. Li et al. [51] obtained similar degradation constant (k) values for fruit and vegetable waste of 0.07 d for the configurations with OPH + GS and PFH + GS. In contrast, Santos et al. [4] reported a higher k (0.10–0.11 d) when studying the anaerobic digestion of ensiled orange peel (14 and 21 days) with the addition of granular sludge.
The methane µ parameter ranged from 0.28 to 2858 NmL/d (logistic) and 0.33 to 2989 NmL/d (modified Gompertz), respectively. OPH + FS showed the highest µ (2858–2989 NmL/d) of methane for the two models used. In contrast, PFH + GS obtained the smallest µ (0.28–0.33 NmL/d).
The OPH + GS configuration presented a faster λ for methane production for the logistic model (− 11,991 d) and modified Gompertz (4.031 d). The slow λ phase was reported by Santos et al. [4] when studying orange peel using another pre-treated (silage at 14 and 21 days) ranging from 2334 to 4371 d (logistic) and 2031 to 3764 (modified Gompertz), respectively. In contrast, λ (3361 d, 5279 d) was slower for PFH + FS for both kinetic models than the other configurations, indicating that microorganisms took longer to adapt and convert organic matter into methane. Other authors reported a faster λ phase (1.96 d) when studying dried passion fruit peel anaerobic digestion by adding granular sludge using the modified Gompertz model [3]. However, Zhao et al. [50], when studying passion fruit peel with acclimatized anaerobic inoculum, obtained a longer λ phase (6.9 d).
Initial and Final Characterization of the Contents of the BMP Test
Table 6 presents the characteristics of pH, EC, COD, total alkalinity, and VFA of the BMP test, before and after the tests.
The initial and final mean pH values of the enzymatic hydrolyzed and inocula were within the ideal range (6.7 to 7.5) for methane production [52]. The amount of sodium bicarbonate (1 g of NaHCO3/g COD) used had a beneficial effect, ensuring the necessary pH control for the development of microorganisms in all reactors during the 60 days [4].
The EC in all configurations increased from the initial to the final condition in the BMP tests, indicating that the waste was degraded [53]. The final EC (5468 to 7871 µs/cm) of most configurations was close to the range (5328 to 7969 µs/cm) found by Santos et al. [3] for orange and passion fruit waste.
The initial COD concentration of all configurations was standardized, according to the methodology of Field et al. [54], for 2000 mg/L. It can be observed that there was an average reduction in COD (298 to 586 mg/L), satisfactory, indicating the conversion of the organic substrate into biogas by anaerobic microorganisms. The OPH enzymatic hydrolyzed, with the addition of GS and FG inocula, obtained the best COD removal efficiency, whose value ranged from 83 to 85%. In contrast, PFH + FS showed the lowest COD removal (70.7%).
Regarding total alkalinity, the experimental OPH + GS, OPH + FS, and PFH + GS configurations were close to the recommended total alkalinity range of 2500 to 5000 mg CaCO3/L which is necessary for process stability. That is, the addition of sodium bicarbonate at the beginning of the BMP test had a positive effect in maintaining the pH within the ideal range for methane production for 60 days. Srilatha et al. [48], when evaluating the effect of different strains of fungi (Sporotrichum sp., Aspergillus sp., Fusarium sp., Penicillium sp.) cultivated under SSF of orange residues, obtained lower final alkalinity ranging from 2200 to 3000 mg CaCO3/ L.
The VFA/TA ratio of the OPH + GS (0.013) and OPH + FS (0.024) configurations was within the range (0.5 to 1.0) recommended by Liu et al. [55] and Poggi-Varaldo and Oleszkiewicz [56] so that there is no predisposition to the accumulation of acids. The PFH + GS (0.63) and PFH + FS (0.59) configurations were within the ratio recommended by these authors.
Conclusions
This study demonstrates the potential of solid-state fermentation as an effective pre-treatment strategy to produce cellulase, which can then be used to optimize methane generation from agro-industrial waste such as orange peel and passion fruit peel. The results suggest that this approach could offer a sustainable solution for valorizing these waste products while also contributing to producing renewable energy. Further research is needed to optimize the process parameters and evaluate the economic feasibility of this approach at a larger scale. Overall, the findings of this study highlight the importance of exploring innovative biotechnological solutions for the sustainable management of agro-industrial waste.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper. Should raw data files be needed in another format, they are available from the corresponding author upon request.
References
Kalyani D, Lee K-M, Kim T-S et al (2013) Microbial consortia for saccharification of woody biomass and ethanol fermentation. Fuel 107:815–822. https://doi.org/10.1016/j.fuel.2013.01.037
Cui Y, Dong X, Tong J, Liu S (2015) Degradation of lignocellulosic components in un-pretreated vinegar residue using an artificially constructed fungal consortium. Bioresources 10. https://doi.org/10.15376/biores.10.2.3434-3450
dos Santos LA, Valença RB, da Silva LCS et al (2020) Methane generation potential through anaerobic digestion of fruit waste. J Clean Prod 256:120389. https://doi.org/10.1016/j.jclepro.2020.120389
dos Santos LA, Silva THL, de M Oliveira CR et al (2022) Silage as a pre-treatment of orange bagasse waste to increase the potential for methane generation. Sci Total Environ 823:153613. https://doi.org/10.1016/J.SCITOTENV.2022.153613
Calabrò PS, Fazzino F, Sidari R, Zema DA (2020) Optimization of orange peel waste ensiling for sustainable anaerobic digestion. Renew Energy 154:849–862. https://doi.org/10.1016/j.renene.2020.03.047
Tantayotai P, Pornwongthong P, Muenmuang C et al (2017) Effect of cellulase-producing microbial consortium on biogas production from lignocellulosic biomass. Energy Procedia 141:180–183. https://doi.org/10.1016/j.egypro.2017.11.034
Wagner A, Lackner N, Mutschlechner M et al (2018) Biological pretreatment strategies for second-generation lignocellulosic resources to enhance biogas production. Energies (Basel) 11:1797. https://doi.org/10.3390/en11071797
de Castro AM, Pereira N Jr (2010) Produção, propriedades e aplicação de celulases na hidrólise de resíduos agroindustriais. Quim Nova 33:181–188. https://doi.org/10.1590/S0100-40422010000100031
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577. https://doi.org/10.1128/MMBR.66.3.506-577.2002
Singhania RR, Sukumaran RK, Patel AK et al (2010) Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb Technol 46:541–549. https://doi.org/10.1016/j.enzmictec.2010.03.010
Sipos B, Benkő Z, Dienes D et al (2010) Characterisation of specific activities and hydrolytic properties of cell-wall-degrading enzymes produced by Trichoderma reesei Rut C30 on different carbon sources. Appl Biochem Biotechnol 161:347–364. https://doi.org/10.1007/s12010-009-8824-4
Marín M, Sánchez A, Artola A (2019) Production and recovery of cellulases through solid-state fermentation of selected lignocellulosic wastes. J Clean Prod 209:937–946. https://doi.org/10.1016/j.jclepro.2018.10.264
Mrudula S, Murugammal R (2011) Production of cellulase by Aspergillus niger under submerged and solid-state fermentation using coir waste as a substrate. Braz J Microbiol 42:1119–1127. https://doi.org/10.1590/S1517-83822011000300033
Singh A, Bajar S, Devi A, Bishnoi NR (2021) Adding value to agro-industrial waste for cellulase and xylanase production via solid-state bioconversion. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01503-z
Srivastava N, Mohammad A, Pal DB et al (2022) Enhancement of fungal cellulase production using pretreated orange peel waste and its application in improved bioconversion of rice husk under the influence of nickel cobaltite nanoparticles. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-03070-3
Menon V, Rao M (2012) Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog Energy Combust Sci 38:522–550. https://doi.org/10.1016/j.pecs.2012.02.002
Sanders JPM, Clark JH, Harmsen GJ et al (2012) Process intensification in the future production of base chemicals from biomass. Chem Eng Process 51:117–136. https://doi.org/10.1016/j.cep.2011.08.007
Caroca E, Elorrieta M, Palma C et al (2022) Lignocellulosic residue valorization in a sequential process of solid-state fermentation and solid substrate anaerobic digestion. J Chem Technol Biotechnol 97:1575–1584. https://doi.org/10.1002/jctb.6967
Pant G, Prakash A, Pavani JVP et al (2015) Production, optimization and partial purification of protease from Bacillus subtilis. J Taibah Univ Sci 9:50–55. https://doi.org/10.1016/j.jtusci.2014.04.010
Yegin S, Fernandez-Lahore M, Jose Gama Salgado A et al (2011) Aspartic proteinases from Mucor spp. in cheese manufacturing. Appl Microbiol Biotechnol 89:949–960. https://doi.org/10.1007/s00253-010-3020-6
Ravikumar G, Gomathi D, Kalaiselvi M, Uma C (2012) A protease from the medicinal mushroom Pleurotus sajor-caju; production, purification and partial characterization. Asian Pac J Trop Biomed 2:S411–S417. https://doi.org/10.1016/S2221-1691(12)60198-1
Dhillon GS, Kaur S, Brar SK, Verma M (2012) Potential of apple pomace as a solid substrate for fungal cellulase and hemicellulase bioproduction through solid-state fermentation. Ind Crops Prod 38:6–13. https://doi.org/10.1016/j.indcrop.2011.12.036
Ramos-Ibarra JR, Guatemala G, Miramontes C et al (2017) Production of hydrolytic enzymes by solid-state fermentation with new fungal strains using orange by-products. Rev Mex Ing Quim 1:19–31
Alabdalall AH, Almutari AA, Aldakeel SA et al (2023) Bioethanol production from lignocellulosic biomass using Aspergillus niger and Aspergillus flavus hydrolysis enzymes through immobilized S. cerevisiae. Energies (Basel) 16:823. https://doi.org/10.3390/en16020823
Silva AFV, Santos LA, Valença RB et al (2019) Cellulase production to obtain biogas from passion fruit (Passiflora edulis) peel waste hydrolysate. J Environ Chem Eng 103510. https://doi.org/10.1016/J.JECE.2019.103510
WHO (1978) International reference center for waste disposal. Methods of Analysis of Sew- age Sludge Solid Wastes and Compost Switzerland
Alves RO, de Oliveira RL, de MS Santos AF, Porto TS (2020) Produção de celulases por Aspergillus japonicus URM5620 e Aspergillus niger URM5741 por diferentes processos fermentativos utilizando bagaço de cana-de-açúcar como substrato. Revista Geama 6:44–50
ABNT, NBR 10.006 (2004) Procedimento para obtenção de extrato solubilizado de resíduos sólidos (Rio de Janeiro)
Standard Methods for the Examination of Water and Wastewater (1995) 19o edition. APWA; AWWA; WPCF, Washington
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Miller GL (1959) Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Ivanova LK, Richards DJ, Smallman DJ (2008) The long-term settlement of landfill waste. Proc Inst Civil Eng – Waste Resour Manag 161:121–133. https://doi.org/10.1680/warm.2008.161.3.121
Kapp H (1984) Schlammfaulung mit hohem Feststoffgehalt. Stuttgarter Berichte zur Siedlungswasserwirtschaft. German In: Oldenbourg Verlag 300
Edwiges T, Frare L, Mayer B et al (2018) Influence of chemical composition on biochemical methane potential of fruit and vegetable waste. Waste Manage 71:618–625. https://doi.org/10.1016/j.wasman.2017.05.030
Morais NWS, Coelho MMH, de Oliveira MG et al (2021) Kinetic study of methanization process through mathematical modeling in biochemical methane potential assays from four different inoculants. Water Air Soil Pollut 232:423. https://doi.org/10.1007/s11270-021-05387-7
Abraham A, Mathew AK, Park H et al (2020) Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour Technol 301:122725. https://doi.org/10.1016/j.biortech.2019.122725
dos Santos TC, Cavalcanti IS, Bonomo RCF et al (2011) Optimization of productions of cellulolytic enzymes by Aspergillus niger using residue of mango a substrate. Ciência Rural 41:2210–2216. https://doi.org/10.1590/S0103-84782011005000145
Junqueira LL (2019) Partial characterization and immobilization of carboxymethylcellulase from Aspergillus niger production by solid-state fermentation. Rev Mex Ing Quim 18:241–250. https://doi.org/10.24275/uam/izt/dcbi/revmexingquim/2019v18n1/Junqueira
da Silva Delabona P, Pirota RDPB, Codima CA et al (2013) Effect of initial moisture content on two Amazon rainforest Aspergillus strains cultivated on agro-industrial residues: Biomass-degrading enzymes production and characterization. Ind Crops Prod 42:236–242. https://doi.org/10.1016/j.indcrop.2012.05.035
Mamma D, Kourtoglou E, Christakopoulos P (2008) Fungal multienzyme production on industrial by-products of the citrus-processing industry. Bioresour Technol 99:2373–2383. https://doi.org/10.1016/j.biortech.2007.05.018
Siles JA, Vargas F, Gutiérrez MC et al (2016) Integral valorisation of waste orange peel using combustion, biomethanisation and co-composting technologies. Bioresour Technol 211:173–182. https://doi.org/10.1016/j.biortech.2016.03.056
Carvalho A, Fragoso R, Gominho J, Duarte E (2019) Effect of minimizing d-limonene compound on anaerobic co-digestion feeding mixtures to improve methane yield. Waste Biomass Valorization 10:75–83. https://doi.org/10.1007/s12649-017-0048-1
Schirmer WN, dos Santos LA, Martins KG et al (2023) The effect of alkaline pretreatment on the anaerobic digestion of fruit and vegetable wastes from a central food distribution market. J Mater Cycles Waste Manag 25:2887–2899. https://doi.org/10.1007/s10163-023-01722-8
Dos Santos Filho DA, de Oliveira LRG, Schirmer WN et al (2018) Evaluation of biogas production from anaerobic co- digestion of organic solid waste and residual glycerin. BIOFIX Scientific Journal 3:260. https://doi.org/10.5380/biofix.v3i2.59938
Valença RB, dos Santos LA, Firmo ALB et al (2021) Influence of sodium bicarbonate (NaHCO3) on the methane generation potential of organic food waste. J Clean Prod 317:128390. https://doi.org/10.1016/j.jclepro.2021.128390
Silva THL, dos Santos LA, de M Oliveira CR et al (2021) Determination of methane generation potential and evaluation of kinetic models in poultry wastes. Biocatal Agric Biotechnol 32:101936. https://doi.org/10.1016/j.bcab.2021.101936
Jos B, Farhan H, Ayu ND et al (2018) Biogas production from palm oil fruit bunch in anaerobic biodigester through liquid state (LS-AD) and solid state (SS-AD) method. MATEC Web of Conferences 156:03043. https://doi.org/10.1051/matecconf/201815603043
Srilatha HR, Nand K, Babu KS, Madhukara K (1995) Fungal pretreatment of orange processing waste by solid-state fermentation for improved production of methane. Process Biochem 30:327–331. https://doi.org/10.1016/0032-9592(95)87041-5
Ruiz B, de Benito A, Rivera JD, Flotats X (2016) Assessment of different pre-treatment methods for the removal of limonene in citrus waste and their effect on methane potential and methane production rate. Waste Manage Res 34:1249–1257. https://doi.org/10.1177/0734242X16661053
Zhao C, Yan H, Liu Y et al (2016) Bio-energy conversion performance, biodegradability, and kinetic analysis of different fruit residues during discontinuous anaerobic digestion. Waste Manage 52:295–301. https://doi.org/10.1016/j.wasman.2016.03.028
Li Y, Zhang R, Liu G et al (2013) Comparison of methane production potential, biodegradability, and kinetics of different organic substrates. Bioresour Technol 149:565–569. https://doi.org/10.1016/j.biortech.2013.09.063
Chernicharo CAL (1997) Princípios do tratamento biológico de águas residuárias- reatores anaeróbios. Segrac, 5. 2. DESA, UFMG, Belo Horizonte, p 246
Rocha L, Soares T, Araujo C, (2009) Avaliação de Biodigestor para uso domiciliar na reciclagem de resíduos semissólidos orgânicos. Encontro de Ensino (Pesquisa e Extensão, Presidente Prudente)
Field J, Alvarez RS, Lettinga G (1988) Ensayos anaerobios. 4 Seminario De Depuracion Anaerobia De Aguas ResiduaBL. Universidad de Valladolid, Spain, pp 52–81
Liu X, Gao X, Wang W et al (2012) Pilot-scale anaerobic co-digestion of municipal biomass waste: focusing on biogas production and GHG reduction. Renew Energy 44:463–468. https://doi.org/10.1016/j.renene.2012.01.092
Poggi-Varaldo HM, Oleszkiewicz JA (1992) Anaerobic co-composting of municipal solid waste and waste sludge at hlgh total solids levels. Environ Technol 13:409–421. https://doi.org/10.1080/09593339209385169
Acknowledgements
The authors are grateful to Federal Rural University of Pernambuco and Federal University of Pernambuco for the laboratory infrastructure.
Funding
The authors are grateful to Coordination for the Improvement of Higher Education Personnel – Brazil (CAPES), and National Council for Scientific and Technological Development, Brazil (CNPq) (financial code no APQ-1560–5.03/22) for financial funding.
Author information
Authors and Affiliations
Contributions
AFVdS: conceptualization, methodology, data curation, investigation, writing—original draft, and writing—review and editing; LAdS: conceptualization, methodology, data curation, investigation, writing—original draft, and writing—review and editing; AHFdM: conceptualization, methodology, data curation, investigation, writing—original draft, and writing—review and editing; JFTJ: conceptualization, methodology, data curation, investigation, writing—original draft, and writing—review and editing; AFMSS: conceptualization, methodology, data curation, investigation, writing—original draft, and writing—review and editing; and TSP: conceptualization, methodology, data curation, investigation, writing—original draft, and writing—review and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Solid-state fermentation (SSF) is promising for methane generation by fruit waste.
• Agro-industrial waste had higher cellulase activity, and the process was optimized.
• SSF pre-treatment increased methane production, especially from passion fruit peel.
• Study offers sustainable solution for waste valorization and energy generation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva, A.F.V., Santos, L.A.d., de Melo, A.H.F. et al. Use of Cellulase Obtained from Solid-State Fermentation of Orange and Passion Fruit Peels as an Enzymatic Pre-treatment Step for Anaerobic Digestion. Bioenerg. Res. 17, 1288–1301 (2024). https://doi.org/10.1007/s12155-023-10691-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10691-7