Abstract
In this study, lipid accumulation and biodiesel production potentials of novel yeast isolates grown in pumpkin peel were investigated. Effect of some critical parameters on lipid production such as initial biomass loading (5–20%), type and concentration of nitrogen source (cheese whey and (NH4)2SO4, 0.25–2 gL−1), and incubation time (0–96 h) were also determined. Furthermore, the effect of biomass loading (0.5–2 gL−1) and catalyst concentration (NaOH 1–3%) with methanol on fatty acid methyl ester (FAME) yields were optimized with response surface methodology (RSM). One yeast isolate showed the highest lipid production, and this strain was identified as Candida boidinii. The maximum lipid accumulation of the novel isolate was observed as 45.6% in the presence of 10% initial pumpkin peel loading, 0.5 gL−1 cheese whey, and 72-h incubation time. The highest FAME (C16-C18) yield was 92.3% when 2 gL−1 biomass and 3% catalyst (NaOH) were used. These results showed that Candida boidinii is a promising microorganism for biodiesel production and pumpkin peel supports the microbial growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fossil-based fuels have been used for many years at the fuel transportation primarily, and this situation causes to increase environmental problems such as greenhouse gas emissions (GHG), climate change, and global warming. Transportation sector consists nearly 30% of fossil fuel consumption, and non-maturated renewable energy sources cannot be replaced directly with fossil based fuels. Moreover, existing renewable technologies such as wind or solar are relatively long-term solutions instead of mid- or short-term options. Therefore, renewable alternative fuel sources such as biodiesel, bioethanol, or biohydrogen are the one of the most promising alternatives because of their ready availability, eco-friendly, and cheap properties. Moreover, biodiesel or bioethanol which exploited the microorganisms for production has several advantages over other sources since microorganisms can be cultivated in cost-effective and abundant carbon sources such as lignocellulosic feedstocks. Biorefinery can be identified as transforming the biomass into value-added products and renewable energy sources such as biofuels or fine chemicals via sustainable way. Therefore, this concept can be easily applied during the biodiesel production process, and this situation is also beneficial for the process in terms of preventing the disposal problems of lignocellulosic feedstocks and providing cheap growth environment for microorganisms. Efficiency of the processes can also be increased with the usage of the techniques such as exergy, techno-economic analyses, and life cycle assessment [1, 2].
Among the renewable energy sources, biodiesel (monoalkyl esters of long-chain fatty acids) is considered one of the most promising ones [3, 4]. Biodiesel is mainly produced by renewable lipid sources such as plant, animal, or microbial fats, and it has several advantages compared to fossil fuels such as biodegradability or renewability [5].
Biodiesel can be classified into various generations according to the type of the raw material. For instance, biodiesel obtained from edible raw materials such as cattle fat or soybean oil is called as first generation. Despite its high productivity, usage of edible fuels in biodiesel production has caused increasing food prices [6]. On the other hand, biodiesel from non-edible raw materials such as food waste or Jatropha seeds is called as second generation. Second-generation biodiesel is produced from cost-effective raw materials; however, 70–80% of the total production cost is related to processing and harvesting steps. Third-generation biodiesel is derived from microorganisms such as yeasts, bacteria, fungi, or algae and has several advantages over other generations. For instance, microorganisms do not compete with food sources for biodiesel production, they have high growth rate, they are not affected by climate conditions, and they can use different carbon sources. Disadvantages of these generations are large investment requirements, problems for larger scale productions, and oil extraction issues. Additionally, photobiological solar fuels, electro-fuels, synthetic cells, or genetically engineered microorganisms are the source of the fourth-generation biodiesel. This generation provides more lipid accumulation from different sources via eco-friendly way. However, main disadvantages of the fourth-generation biodiesel can be stated as high initial investment costs and immature technologies for industrial scale [7,8,9].
However, high operating costs or usage of edible sources such as plant oil are generated ethical concerns about the biodiesel production [10]. Therefore, lipids from microorganisms are promising alternatives to conventional biodiesel sources.
Microbial oils that obtain from yeasts have some significant advantages over other lipid sources. For instance, cultivation of the yeasts is easy. In general, they do not need to complex or expensive media for growth, they can utilize a broad range of fermentable sugars from lignocellulosic wastes without competing with edible sources and lipids of the yeasts have similar properties and energy values to animal and plant oils. Moreover, yeasts have very rapid growth rate, and some oleaginous strains, i.e., Rhodotorula sp., Lipomyces sp., and Cryptococcus sp., can accumulate lipids up to 60–70%. On the other hand, it was reported that the only 5% yeasts have lipid content above the 25%. Therefore, investigations about the lipid accumulated yeasts have importance in terms of sustainability [11, 12]. For these reasons, comprehensive studies have been carried out with microbial lipids [13, 14].
Lignocellulosic raw materials are abundant and zero-cost feedstocks for lipid accumulation and biodiesel production. They also have high-energy return of investment values (EROI) compared to algae or plant-derived oil-based biodiesel production [15,16,17].
By-products of the food industry are caused by severe environmental problems. Therefore, evaluation of these by-products in renewable energy production is a reasonable approach for sustainability [18]. Pumpkin peel (PR) is an essential by-product of the food industry since it is abundant in carbohydrate and β-carotene content as well as cellulose and hemicellulose. For these reasons, PR was evaluated as the production medium of lignocellulolytic enzymes [19] or the fiber source [20] in some previous reports.
Because of its biotechnological potential, high inhibitor tolerance, and xylose assimilation capacity, Candida boidinii is an attractive yeast [21]. For these reasons, C. boidinii has been used in many studies including biofuel production [22] or lipid production [23]. Despite its potential, studies about the biodiesel production from lipids of C. boidinii are very limited in the literature. Therefore, the main motivation behind the current study was to investigate the biodiesel production capacities of novel yeast isolates which were cultivated in low-cost PR medium for the first time.
Materials and Methods
Isolation and Sequencing of Yeast Cells
The yeast samples were collected from soil samples which contain sugar factory wastes/Ankara. Samples were centrifuged, and 0.1 mL cell was spread on Petri plates containing potato dextrose agar (Merck-Germany). In order to prevent bacterial contamination, 600.000 IU penicillin was also added to Petri plates. The incubation temperature was set as 30 °C. These steps were repeated, and cells were kept in slant PDA at + 4 °C.
ITS1 and ITS4 primers were used to amplify the ITS regions [24]. PCR conditions were as follows (each steps were 30 cycles): denaturation, 94 °C for 1 min; annealing, 55 °C for 45 s; and elongation, 72 °C for 45 s. ABI 3100 Genetic Analyzer device was used for sequencing of the DNA in an external laboratory namely (REFGEN, Ankara, Turkey). Strains were identified according to NCBI/BLAST results of DNA, 18S rDNA gene sequence, and PCR analyses. According to the BLAST results, the isolate was confirmed as C. boidinii. The ITS sequence of the C. boidinii was deposited in the GenBank database (Accession number: ON409985).
Pretreatment of Pumpkin Peel
PR was supplied from Local Markets Ankara/Turkey and was kept at − 20 °C until experiments. For pretreatment, H2SO4 (1%, vv−1) was used, and PR was autoclaved at 121 °C for 15 min immediately after acid addition. Pretreated PR hydrolysate was filtered through Whatman No.1 paper, and supernatant of the hydrolysate was used for experiments as a carbon source.
Cultivation and Growth Conditions of Yeasts for Lipid Accumulation
Yeasts were pre-cultured in the YEPD medium (yeast extract 3.0 gL−1, peptone 10 gL−1, and glucose 20 gL−1) for activation. Twenty-four-hour-old pre-cultures were inoculated to 250-mL Erlenmeyer flasks working volume with 100-mL contained PR medium. Incubation of the yeast was performed at pH 5. Batch experiments were carried out in the orbital shaker (Gerhardt, Thermoshake, THO500/1, Germany) at 100 rpm, 30 ± 1 °C.
Lipid Accumulation Assays
The effects of some vital parameters for lipid accumulation such as initial biomass loading (5%, 10%, and 20%), cultivation time (24, 48, 72, and 96 h), type (cheese whey and (NH4)2SO4), and concentration (0.25–2 gL−1) of nitrogen sources were examined in the study.
Lipid Extraction
To determine the lipid accumulation of the yeasts, lipids were extracted by chloroform–methanol. Yeasts were harvested at 6000 rpm. for 10 min. To calculate the yeasts’ lipid content, cells were dried in an oven at 80 °C for overnight. Lipids were extracted with 2:1 chloroform–methanol solution. The total lipid was estimated gravimetrically (Bligh and Dyer 1954).
Transesterification Assays
C. boidinii, which showed the highest lipid accumulation among the tested yeasts, was used for transesterification experiments. For optimization of transesterification, response surface methodology was used, and combined effects of biomass loading (0.5–2 gL−1) and catalyst concentration (1–3% vv−1) on transesterification were investigated in the current study. Transesterification was carried out at 25 °C for 30 min. Alkali (NaOH) catalyst and methanol were used for this procedure. Oil to alcohol ratio was adjusted as 1:5 (wv−1). After transesterification, the supernatant was filtered with 0.45-µM membrane filter, and FAME analysis was performed at gas chromatography.
Analytical Methods
The value of dry yeast weight was used in a calculation which corresponds to the weight of wet biomass, and the lipid amount of the yeast cells was calculated as mg lipid/dry cell weight in % [25]. Reducing sugar concentration was measured according to DNS method [26].
Lipids extracted at optimal conditions obtained from the C. boidinii were solved in hexane and transesterified to biodiesel in NaOH as a catalyst with methanol. After transesterification, 1 μL sample was taken from the supernatant, and the methylated fatty acids were analyzed by the GC-2010 gas chromatograph (Shimadzu, Japan). The GC analysis condition was as follows: flame ionization detector (FID) 240 °C; column TR-CN100, 60 m, 0.25 mm, and 0.20 mm (Teknokroma). Nitrogen (N2) was used as the carrier gas. Peaks obtained from analyses were identified against the chromatogram of a mixed fatty acid methyl ester standard (37 Comp. FAME Mix 10 mg/mL in CH2Cl2; Supelco, USA). Experiments and measurements were performed in triplicates.
Statistical Analysis
Statistical analyses were performed with SPSS 25.0 to determine the significance of the difference of tested groups with Analysis of Variance (ANOVA).
Design Expert Software program was used for RSM, and central composite design with two factors with three levels was used to evaluate the effects of independent variables on FAME yield of C. boidinii. Total 10 runs were generated for RSM. Catalyst concentration (1–3% NaOH) and biomass loading (0.5–2 gL−1) were selected as independent factors for RSM experiments.
Results and Discussions
Determination of Lipid Accumulated Yeast in PR Medium
Microorganisms are suitable bioagents for lipid and biodiesel production. Furthermore, lignocellulosic wastes create serious advantages for sustainable energy production. For these reasons, lipid accumulation capacities of four different yeasts grown in PR medium were determined in the current study. Furthermore, one of the main objectives of the present work was to investigate the lipid accumulation and biodiesel production potential of different yeasts. By this context, 4 different yeasts were selected for the experiments. The internal transcribed spacer (ITS) is considered as powerful tool for the examination of yeast diversity, and numerous studies in the literature use the ITS regions for the identification of various yeasts species [27]. Therefore, ITS regions belong to yeast cells which showed the highest lipid accumulation capacity were sequenced. Basic Local Alignment Search Tool (BLAST) program of NCBI was used for the identification and verification of the results. According to the BLAST, the isolate exhibited high identities to C. boidinii. Therefore, this strain was identified as Candida boidinii. The ITS sequence of the yeast was deposited into the GenBank sequence database of NCBI under the accession number of ON409985. According to Fig. 1, PR hydrolysate supports all the tested yeasts’ growth, and the highest lipid accumulation is determined as 0.46 gL−1 day−1 in isolate 3. Lipid accumulation of isolates 1, 2, and 4 were observed as 0.26, 0.19, and 0.39 gL−1 day−1, respectively. These results show the novel isolate has the lipid accumulation potential which able to turn into biodiesel effectively. By this context, since the determination of oleaginous yeast is important for biotechnological applications, lipid accumulation potentials of novel yeast isolates are frequently investigated. For instance, lipid yield of C. podzolicus which was cultivated in glucose as a sole carbon source was found as 31.8%, and volumetric productivity was observed as 0.09 g L−1 h−1 in the same study [28]. Liu et al. [29] reported that the dual culture system which contained C. vulgaris and different yeast strains was dominated by the yeasts and the maximum total fatty acid productivity was obtained as 1.75 g L−1 day−1. Because the highest lipid amount was found in isolate 3, this microorganism was selected for further experiments and identified as C. boidinii according to sequencing techniques.
The Effect of Initial PR Loading on Lipid Amount of C. boidinii
Initial biomass loading is an essential parameter for microbial growth and lipid accumulation [30]. To determine the effect of initial biomass loading, three different PR loadings (5%, 10%, and 20%, wv−1) were prepared. Sugar concentrations of PR hydrolysates were 23.72 ± 1.3 gL−1, 39.71 ± 2.9 gL−1, and 65.03 ± 2.1 gL−1, respectively, for 5, 10, and 20% (wv−1) PR loading. According to the data in Fig. 2, 10% PR loading is favorable for the lipid accumulation of C. boidinii, and the maximum lipid amount is detected as 0.46 gL−1 day−1 in the same PR loading. Some previous studies in the literature showed that 10% initial biomass loading is the optimal choice for lipid accumulation or microbial growth of various yeasts. At higher biomass loadings, mass transfer limitation and mixing problems can inhibit the microbial growth and lipid accumulation [31]. On the other hand, in the presence of lower biomass loadings, insufficient sugar concentrations resulted in lower lipid accumulations. For these reasons, lipid accumulation of C. boidinii were observed as 0.28 and 0.36 gL−1 day−1 when initial PR loading was adjusted to 5% and 20%. Lower lipid amount obtained from 5% PR loading can be explained by lower sugar concentration of relevant biomass loading. On the other hand, decreased lipid accumulation of 20% PR loading may be related to inhibitory compounds derived from dilute acid pretreatment. Because the highest lipid accumulation was obtained from 10% PR loading, this biomass loading was used for further experiments. Similar findings were also reported by Intasit et al. [16]. Researchers found the optimal palm biomass waste loading as 10% for lipid accumulation of various yeast and fungi such as C. tropicalis X37 and A. tubingensis TSIP 9. In another study, Thangevalu et al. [32] showed that the lipid accumulation of C. tropicalis ASY2 was 49% when sago processing wastewater was used as a feedstock. The highest biomass and lipid productivity were also observed as 0.021 g L−1 h−1 and 0.010 g L−1 h−1, respectively.
The Effect of Different Nitrogen Types and Concentrations on Lipid Accumulation
Nitrogen type and concentration are vital parameters for lipid production. For these reason, effects of inorganic (NH4)2SO4) and organic (cheese whey) nitrogen sources and their different concentrations (0.25–2 gL−1) on lipid accumulation were examined in this part of the study. The data in Table 1 depict that the usage of cheese whey (organic nitrogen source) resulted in more microbial lipid accumulation than (NH4)2SO4 (inorganic). This positive effect on lipid accumulation can be explained by the presence of some additional substances in cheese whey such as vitamins or amino acids [33]. The highest lipid amount was observed as 0.61 gL−1 day−1 in the 0.5 gL−1 cheese whey. On the other hand, lipid percentage was obtained as 0.56 gL−1 day−1 when 1 gL−1 (NH4)2SO4 was used as the nitrogen source. Positive effects of organic nitrogen sources were shown previously. For instance, Poontawee et al. [34] found the lipid accumulation of R. fluvialis as 29.4% in the presence of (NH4)2SO4. On the other hand, the same value increased to 42.9% when organic (yeast extract) and inorganic (NH4)2SO4 nitrogen sources were used together.
Lipid accumulation of microorganism can be affected by excessive or insufficient nitrogen usage. Therefore, the determination of optimal nitrogen concentration is an essential step for microbial lipid production. For this reason, the effects of increasing nitrogen concentrations on lipid accumulation were investigated in this part of the study. According to the results in Table 1, increased nitrogen concentrations result in lower lipid amounts. Lipid accumulation of C. boidinii significantly increased from 0.32 to 0.61 gL−1 day−1 when cheese whey concentration decreased from 2 to 0.5 gL−1 (p < 0.05). Similarly, in the presence of 1 gL−1 (NH4)2SO4, lipid amount of C. boidinii was detected as 0.56 gL−1 day−1. This value was observed as only 0.38 gL−1 day−1 when (NH4)2SO4 concentration was adjusted to 2 gL−1 which was found as significantly lower (p < 0.05) when compared with 1 gL−1 (NH4)2SO4 loading.
The negative effect of increased nitrogen sources on lipid accumulation was reported in the literature previously. For example, the lipid amount of C. albicans increased from 22 to 36% when (NH4)2SO4 concentration decreased to 50 to 10 mgL−1 [35]. Because the highest lipid was found in 0.5 gL−1 cheese whey, further experiments were carried out with this nitrogen source.
Previous reports in the literature also demonstrated that PR is rich in nitrogen and protein contents. In a study, for instance, PR has the highest crude protein amount (16.5 g/100 g dry matter) among the tested agricultural by-products including pea husk, banana, and potato peel [36]. In another study, Mala and Kurian [37] found 12.5% total protein content in pumpkin peel. Therefore, it can be concluded that PR hydrolysate has sufficient nitrogen concentrations for the C. boidinii growth and lipid accumulation, and nitrogen supplementation is not essential for PR medium.
Effects of Incubation Time on Lipid Accumulation
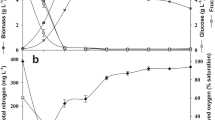
Incubation time is an important element for microbial growth and lipid accumulation. Therefore, C. boidinii was incubated for 96 h to observe the effect of cultivation time on lipid production. The data in Fig. 3 depicts that the maximum lipid accumulation was observed at the end of 72 h as 2.38 g L−1. Moreover, the lipid concentration of C. boidinii decreased to 2.30 g L−1 at the end of the 96 h. One of the advantageous features of the yeasts for biodiesel production is rapid lipid accumulation when lignocellulosic raw materials are used as a carbon source. For instance, it was previously reported that C. curvatus cells produced 12.4 gL−1 lipid which was the maximum lipid concentration at the end of the 72 h in the corn stover medium [38]. Moreover, Intasit et al. [16] showed that lipid content of the Y. lipolytica reached its highest level at the end of the 96-h incubation time when palm biomass was used as feedstock. Lipid content of the same yeast decreased from 52.1 to approximately 38% after 96 h. Because of the highest lipid accumulation was observed at 72 h, this incubation time was selected for further experiments.
Optimization of Transesterification Using RSM
Response surface methodology is a useful tool for optimizing different variables and their interactions with fewer experiments [39]. Thus, RSM is frequently used in biodiesel production studies [40,41,42]. Therefore, the effects of independent variables (catalyst concentration and biomass loading) on fatty acid methyl ester (FAME) yield were investigated using RSM. NaOH (1–3%) was used as a catalyst with methanol, and biomass loadings were tested between 0.5 and 2 gL−1. According to the ANOVA results, the p value of catalyst concentration and biomass loading was found significant (0.0006 and 0.0082, respectively). Moreover, the equation used to predict the correlation between FAME (C16-C18) and independent variables was given below in Eq. (1).
It is imperative to determine optimal reactant concentration since it can enhance transesterification efficiency significantly. On the other hand, excessive amounts of these molecules have negative impact on FAME formation [43]. Moreover, it is possible to obtain higher FAME yields by employing higher biomass concentrations. Therefore, catalyst concentration and biomass loading were selected as independent variables in this part of the study. Moreover, according to the results, the effect of these variables on transesterification was significant (p < 0.05).
NaOH is a commonly used catalyst for transesterification because of its high effectivity and cost-effectiveness [44]. Therefore, numerous studies in the literature used NaOH for transesterification purpose. For instance, in a study, the highest FAME content of the R. toruloides which was cultivated in enzymatically hydrolyzed rapeseed meal was found as 97.7% when 0.4% (wv−1) NaOH was used as a catalyst [45]. In another study conducted with biodiesel production from rapeseed oil, usage of NaOH resulted in the highest FAME yields and shortest reaction duration [46].
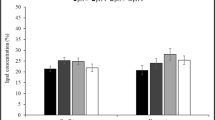
FAME yields and response surface plots of C. boidinii are also presented in Table 2, Figs. 4 and 5. According to the results, increased catalyst concentration and biomass loading caused higher FAME (C16-C18) yields. The maximum FAME (C16-C18) was 92.3% when 3% NaOH/methanol and 2 gL−1 biomass were used. The same yield decreased to 66.2%, which was the lowest FAME yield obtained in the presence of 1% NaOH/methanol and 0.5 gL−1 biomass. Moreover, C8:0, C16:0, C17:1, C18:0, C18:1, C18:2n1, and C20:0 percentages of the C. boidinii were found as 1.1, 38.9, 6.4, 43.2 5.9, 4.3, and 2.0%, respectively. There are also reports which showed that the lignocellulosic feedstocks are suitable for lipid accumulation of the yeasts. For instance, Siwina and Leesing [47] found the C16-C18 percentages of R. mucilaginosa which grown in durian peel hydrolysate as 19.2, 4.5, 9.5, and 51.2% for C16:0, C16:1, C18:0, and C18:1, respectively. In another study, FAME profile of R. taiwanensis was obtained as 24.4, 1.4, 2.9, 46.8, and 6.5% for C16:0, C16:1, C18:0, C18:1, and C18:2, respectively, when the yeasts were cultivated in corncob hydrolysate as a carbon and nitrogen source [48].
It was also previously reported that FAME yields of R. glutinis rapidly decreased when NaOH concentration excess 1 gL [49]. A possible explanation of this phenomenon may be related to catalyst concentration which did not trigger the saponification reaction. Excess usage of alkaline catalyst (NaOH) can cause more lipid-NaOH interaction by saponification reaction leading to reduced ester yields [50]. Moreover, in the current study, transesterification was carried out 25 °C to avoid the excess saponification which mainly derived from higher transesterification temperatures [51].
Similar to catalyst concentration, increased biomass loadings caused higher FAME yield in the current study. Our results are consistent with the report of Katre et al. [52] who showed that 2 g Y. lipolytica loading resulted in 0.45 g FAME yield, while this value was only 0.21 g when 1.05 g biomass loading was used. In the current study, FAME (C16-C18) yield increased by 39% and reached from 66.2 to 92.3% when biomass loading increased from 0.5 to 2 gL−1.
Biodiesel Properties of C. boidinii Lipids
Biodiesel properties are strongly depended on distribution of FAME types. Among them, kinematic viscosity, cetane number, and density have crucial importance. For these reasons, biodiesel features of total FAMEs that obtained from C. boidinii lipids were compared with EN 14,214 and ASTM D6751 standards in this part of the study. Estimation of biodiesel properties was performed in Biodiesel Analyzer® software [53]. Kinematic viscosity is one of the vital parameters that affects the fuel atomization in engine [54, 55]. Kinematic viscosity value of C. boidinii FAMEs was 4.25 mm2/s which exhibited a good accordance with EN 14,214 (3.5–5 mm2/s) and ASTM D6751 (1.9–6 mm2/s). Cetane number is directly related to ignition performance of biodiesel. According to EN 14,214 and ASTM D6751, the lowest acceptable value of cetane number is 51 and 47, respectively. In consistency with the standards, cetane number of C. boidinii was obtained as 69.54. Moreover, density of the C. boidinii biodiesel was calculated as 866 kg/m3 which is acceptable value for EN 14,214 standard (860–900 kg/m3).
Conclusions
In the current study, the lipid accumulation potential of newly isolated oleaginous C. boidinii was optimized in the medium containing sustainable agricultural waste PR hydrolysate as a carbon source and cheese whey as a nitrogen source. The maximum lipid accumulation and total C16-C18, FAME yields, were found as 0.61 gL−1 and 92.3%, respectively, and the maximum lipid percentage was observed at the end of the 72-h incubation time. Those results indicated that lipid accumulation and FAME profile of C. boidinii are suitable for biodiesel production. Moreover, kinematic viscosity and cetane number of C. boidinii biodiesel matched well with international standards EN 14,214 and ASTM D6751. This work showed that usage of PR for oleaginous yeast growth is a promising and cost-effective approach for biodiesel production.
References
Tabatabaei M, Aghbashlo M (2020) The critical role of advanced sustainability assessment tools in enhancing the real-world application of biofuels. Acta Innov 37:67–73. https://doi.org/10.32933/ActaInnovations.37.6
Cherubini F (2010) The biorefinery concept: using biomass instead of oil for producing energy and chemicals. Energy Convers Manag 51:1412–1421. https://doi.org/10.1016/j.enconman.2010.01.015
Kakkad H, Khot M, Zinjarde S, RaviKumar A (2015) Biodiesel production by direct in situ transesterification of an oleaginous tropical mangrove fungus grown on untreated agro-residues and evaluation of its fuel properties. Bioenergy Res 8:1788–1799. https://doi.org/10.1007/s12155-015-9626-x
Mandari V, Devarai SK (2021) Biodiesel production using homogeneous, heterogeneous, and enzyme catalysts via transesterification and esterification reactions: a critical review. Bioenergy Res. https://doi.org/10.1007/s12155-021-10333-w
Festel G, Würmseher M, Rammer C (2014) Scaling and learning effects of biofuels conversion technologies. Energy Technol 2:612–617. https://doi.org/10.1002/ente.201400014
Morais ARC, Da Costa Lopes AM, Costa P et al (2014) Cattle fat valorisation through biofuel production by hydrogenation in supercritical carbon dioxide. RSC Adv 4:32081–32091. https://doi.org/10.1039/c4ra05225k
Singh D, Sharma D, Soni SL et al (2020) A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 262:116553. https://doi.org/10.1016/j.fuel.2019.116553
Koreti D, Kosre A, Jadhav SK, Chandrawanshi NK (2022) A comprehensive review on oleaginous bacteria: an alternative source for biodiesel production. Bioresour Bioprocess 9:1–19. https://doi.org/10.1186/s40643-022-00527-1
Escorsim AM, Cordeiro CS, Ramos LP et al (2015) Assessment of biodiesel purification using CO2 at high pressures. J Supercrit Fluids 96:68–76. https://doi.org/10.1016/j.supflu.2014.08.013
Bhuiya MMK, Rasul MG, Khan MMK et al (2016) Prospects of 2nd generation biodiesel as a sustainable fuel - Part: 1 selection of feedstocks, oil extraction techniques and conversion technologies. Renew Sustain Energy Rev 55:1109–1128. https://doi.org/10.1016/j.rser.2015.04.163
Leiva-Candia DE, Pinzi S, Redel-Macías MD et al (2014) The potential for agro-industrial waste utilization using oleaginous yeast for the production of biodiesel. Fuel 123:33–42. https://doi.org/10.1016/j.fuel.2014.01.054
Bhatia SK, Gurav R, Choi TR et al (2019) A clean and green approach for odd chain fatty acids production in Rhodococcus sp. YHY01 by medium engineering. Bioresour Technol 286:121383. https://doi.org/10.1016/j.biortech.2019.121383
Chebbi H, Leiva-Candia D, Carmona-Cabello M et al (2019) Biodiesel production from microbial oil provided by oleaginous yeasts from olive oil mill wastewater growing on industrial glycerol. Ind Crops Prod 139:111535. https://doi.org/10.1016/j.indcrop.2019.111535
Chopra J, Tiwari BR, Dubey BK, Sen R (2020) Environmental impact analysis of oleaginous yeast based biodiesel and bio-crude production by life cycle assessment. J Clean Prod 271:122349. https://doi.org/10.1016/j.jclepro.2020.122349
Fakas S, Papanikolaou S, Galiotou-Panayotou M et al (2008) Organic nitrogen of tomato waste hydrolysate enhances glucose uptake and lipid accumulation in Cunninghamella echinulata. J Appl Microbiol 105:1062–1070. https://doi.org/10.1111/j.1365-2672.2008.03839.x
Intasit R, Cheirsilp B, Louhasakul Y et al (2020) Valorization of palm biomass wastes for biodiesel feedstock and clean solid biofuel through non-sterile repeated solid-state fermentation. Bioresour Technol 298:122551. https://doi.org/10.1016/j.biortech.2019.122551
Sathiyamoorthi E, Dikshit PK, Kumar P, Kim BS (2020) Co-fermentation of agricultural and industrial waste by Naganishia albida for microbial lipid production in fed-batch fermentation. J Chem Technol Biotechnol 95:813–821. https://doi.org/10.1002/jctb.6271
Schieber A, Stintzing FC, Carle R (2001) By-products of plant food processing as a source of valuable compounds-recent developments. Trends Food Sci Technol 12:401–413. https://doi.org/10.1016/b978-0-08-100596-5.21346-2
Yang R, Meng D, Hu X et al (2013) Saccharification of pumpkin residues by coculturing of trichoderma reesei RUT-C30 and phanerochaete chrysosporium burdsall with delayed inoculation timing. J Agric Food Chem 61:9192–9199. https://doi.org/10.1021/jf402199j
Fissore EN, Ponce NM, Stortz CA et al (2007) Characterisation of fiber obtained from pumpkin (cucumis moschata duch.) mesocarp through enzymatic treatment. Food Sci Technol Int 13:141–151. https://doi.org/10.1177/1082013207077914
Santana NB, Teixeira Dias JC, Rezende RP et al (2018) Production of xylitol and bio-detoxification of cocoa pod husk hemicellulose hydrolysate by Candida boidinii XM02G. PLoS ONE 13:1–15. https://doi.org/10.1371/journal.pone.0195206
Gonçalves DB, Batista AF, Rodrigues MQRB et al (2013) Ethanol production from macaúba (Acrocomia aculeata) presscake hemicellulosic hydrolysate by Candida boidinii UFMG14. Bioresour Technol 146:261–266. https://doi.org/10.1016/j.biortech.2013.07.075
Papanikolaou S, Rontou M, Belka A et al (2017) Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng Life Sci 17:262–281. https://doi.org/10.1002/elsc.201500191
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
Li Y, Zhao Z (Kent), Bai F (2007) High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb Technol 41:312–317. https://doi.org/10.1016/j.enzmictec.2007.02.008
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Yang RH, Su JH, Shang JJ et al (2018) Evaluation of the ribosomal DNA internal transcribed spacer (ITS), specifically ITS1 and ITS2, for the analysis of fungal diversity by deep sequencing. PLoS ONE 13:1–17. https://doi.org/10.1371/journal.pone.0206428
Schulze I, Hansen S, Großhans S et al (2014) Characterization of newly isolated oleaginous yeasts - Cryptococcus podzolicus, Trichosporon porosum and Pichia segobiensis. AMB Express 4:1–11. https://doi.org/10.1186/s13568-014-0024-0
Liu L, Chen J, Lim PE, Wei D (2018) Dual-species cultivation of microalgae and yeast for enhanced biomass and microbial lipid production. J Appl Phycol 30:2997–3007. https://doi.org/10.1007/s10811-018-1526-y
Jeevan Kumar SP, Banerjee R (2019) Enhanced lipid extraction from oleaginous yeast biomass using ultrasound assisted extraction: a greener and scalable process. Ultrason Sonochem 52:25–32. https://doi.org/10.1016/j.ultsonch.2018.08.003
Koppram R, Tomás-Pejó E, Xiros C, Olsson L (2014) Lignocellulosic ethanol production at high-gravity: challenges and perspectives. Trends Biotechnol 32:46–53. https://doi.org/10.1016/j.tibtech.2013.10.003
Thangavelu K, Sundararaju P, Srinivasan N et al (2020) Simultaneous lipid production for biodiesel feedstock and decontamination of sago processing wastewater using Candida tropicalis ASY2. Biotechnol Biofuels 13:1–14. https://doi.org/10.1186/s13068-020-01676-1
Kitcha S, Cheirsilp B (2011) Screening of oleaginous yeasts and optimization for lipid production using crude glycerol as a carbon source. Energy Procedia 9:274–282. https://doi.org/10.1016/j.egypro.2011.09.029
Poontawee R, Yongmanitchai W, Limtong S (2018) Lipid production from a mixture of sugarcane top hydrolysate and biodiesel-derived crude glycerol by the oleaginous red yeast, Rhodosporidiobolus fluvialis. Process Biochem 66:150–161. https://doi.org/10.1016/j.procbio.2017.11.020
Gohel HR, Ghosh SK, Braganza VJ (2013) Yeast as a viable and prolonged feedstock for biodiesel production. Int J Renew Energy Res 3:126–131. https://doi.org/10.20508/ijrer.93684
Hossain E, Sultana SA, Karim MH, Ahmed I (2015) Vegetable peels: a promising feed resource for livestock. J Anim Feed Res 5:33–39
Mala SK, Kurian AE (2016) Nutritional Composition and Antioxidant Activity of Pumpkin Wastes. Int J Pharm Chem Biol Sci 6:336–344
Gong Z, Shen H, Wang Q et al (2013) Efficient conversion of biomass into lipids by using the simultaneous saccharification and enhanced lipid production process. Biotechnol Biofuels 6.https://doi.org/10.1186/1754-6834-6-36
Nababan MYS, Fatriasari W, Wistara NJ (2020) Response surface methodology for enzymatic hydrolysis optimization of jabon alkaline pulp with Tween 80 surfactant addition. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00807-w
Yellapu SK, Bezawada J, Kaur R et al (2016) Detergent assisted lipid extraction from wet yeast biomass for biodiesel: a response surface methodology approach. Bioresour Technol 218:667–673. https://doi.org/10.1016/j.biortech.2016.07.011
Selvakumar P, Sivashanmugam P (2019) Ultrasound assisted oleaginous yeast lipid extraction and garbage lipase catalyzed transesterification for enhanced biodiesel production. Energy Convers Manag 179:141–151. https://doi.org/10.1016/j.enconman.2018.10.051
Tacias-Pascacio VG, Torrestiana-Sánchez B, Dal Magro L et al (2019) Comparison of acid, basic and enzymatic catalysis on the production of biodiesel after RSM optimization. Renew Energy 135:1–9. https://doi.org/10.1016/j.renene.2018.11.107
Verma P, Sharma MP, Dwivedi G (2016) Prospects of bio-based alcohols for Karanja biodiesel production: an optimisation study by response surface methodology. Fuel 183:185–194. https://doi.org/10.1016/j.fuel.2016.06.062
Ma F, Clements LD, Hanna MA (1998) The effects of catalyst, free fatty acids, and water on transesterification of beef tallow. Trans Am Soc Agric Eng 41:1261–1264. https://doi.org/10.13031/2013.17292
Thliveros P, Uçkun Kiran E, Webb C (2014) Microbial biodiesel production by direct methanolysis of oleaginous biomass. Bioresour Technol 157:181–187. https://doi.org/10.1016/j.biortech.2014.01.111
Pullen J, Saeed K (2015) Investigation of the factors affecting the progress of base-catalyzed transesterification of rapeseed oil to biodiesel FAME. Fuel Process Technol 130:127–135. https://doi.org/10.1016/j.fuproc.2014.09.013
Siwina S, Leesing R (2021) Bioconversion of durian (Durio zibethinus Murr.) peel hydrolysate into biodiesel by newly isolated oleaginous yeast Rhodotorula mucilaginosa KKUSY14. Renew Energy 163:237–245. https://doi.org/10.1016/j.renene.2020.08.138
Miao Z, Tian X, Liang W et al (2020) Bioconversion of corncob hydrolysate into microbial lipid by an oleaginous yeast Rhodotorula taiwanensis AM2352 for biodiesel production. Renew Energy 161:91–97. https://doi.org/10.1016/j.renene.2020.07.007
Kuan IC, Kao WC, Chen CL, Yu CY (2018) Microbial biodiesel production by direct transesterification of rhodotorula glutinis biomass. Energies 11. https://doi.org/10.3390/en11051036
Leung DYC, Guo Y (2006) Transesterification of neat and used frying oil: optimization for biodiesel production. Fuel Process Technol 87:883–890. https://doi.org/10.1016/j.fuproc.2006.06.003
Eevera T, Rajendran K, Saradha S (2009) Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions. Renew Energy 34:762–765. https://doi.org/10.1016/j.renene.2008.04.006
Katre G, Raskar S, Zinjarde S et al (2018) Optimization of the in situ transesterification step for biodiesel production using biomass of Yarrowia lipolytica NCIM 3589 grown on waste cooking oil. Energy 142:944–952. https://doi.org/10.1016/j.energy.2017.10.082
Talebi AF, Tabatabaei M, Chisti Y (2014) BiodieselAnalyzer: a user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res J 2:55–57. https://doi.org/10.18331/brj2015.1.2.4
Rozina AM, Zafar M et al (2017) Biodiesel synthesis from Saussurea heteromalla (D.Don) Hand-Mazz integrating ethanol production using biorefinery approach. Energy 141:1810–1818. https://doi.org/10.1016/j.energy.2017.11.074
Anwar F, Rashid U, Ashraf M, Nadeem M (2010) Okra (Hibiscus esculentus) seed oil for biodiesel production. Appl Energy 87:779–785. https://doi.org/10.1016/j.apenergy.2009.09.020
Funding
This study was supported by Ankara University Research Foundation. Project Number: 20L0430004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Demiray, E., Karatay, S.E. & Dönmez, G. Optimization Study for Enhanced Biodiesel Production by Novel Yeast Isolates Cultivated in Dilute Acid Pretreated Pumpkin Peel. Bioenerg. Res. 15, 1472–1481 (2022). https://doi.org/10.1007/s12155-022-10483-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10483-5