Abstract
This study assesses whether the use of Tessaria absinthioides (TA) pellets as a bioenergy resource is feasible or not. Its fuel properties were characterized and its combustion behavior assessed via a traditional log wood stove with a basket of wood pellets. Combustion of pinewood (Pinus radiata) commercial pellets (CP) was used as a reference in order to analyze experimental results. TA has a high ash content of 8.1% and an HHV of 17.47 MJ kg−1. Alkaline index on the TA pellets was of 1.46 kg alkali GJ−1, a relatively high value, which resulted from high sodium content in ash. Oven and exhaust temperatures during the combustion of TA and CP present similar behavior. The combustion chamber’s highest temperature for CP was 841.15 °C, while for TA, the highest temperature was 685.65 °C. Furthermore, CO and PM emission was superior for TA pellets, averaging about 4943.6 ± 235.2 mg Nm−3 and 32.45 ± 6.49 mg MJ−1, respectively at 13% O2. Results obtained have shown that there were no apparent hindrances for an extensive use of TA as a bioenergy resource and use of pellet baskets to combust pellets in traditional wood log stoves.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global energy demand will certainly rise in upcoming years, with regional differences. Whereas the energy demand of industrialized countries will remain relatively stable, the one for emerging economies and developed countries will significantly increase. Thus, it is imperative to develop alternative energy production methods which are technically mature, economically feasible, and environmentally sound. Fuels derived from biomass can substantially contribute to the global energetic matrix, reducing environmental impact of energy generation.
Weeds are undesirable and highly diversified plants which can affect crops worldwide. They are the most omnipresent class of crop pests, infesting crop fields every year due to high growth rates and broad adaptation to different agroclimatic conditions [1]. These species can grow in croplands and degraded lands, increasing fire hazards, reducing pasture production, and harming local ecosystems by negatively affecting local biodiversity [2]. In the current scenario, in which world population (7700 million) keeps rising, they are considered a substantial global food production hindrance. For instance, weed-crop competition can cause losses in crop yield and quality close to 30%, leading to a gross economical loss higher than 100 billion dollars per year [3]. Concurrently, they occupy important fertile land space, which prevents development of new crops. Many of these species have become resistant to herbicides; therefore, novel agronomic strategies are required to eliminate them or reduce their pressure over crops [2].
In Chile, the Atacama Region is known for the Atacama Desert, one of the Earth’s driest areas. In the last 5 years, croplands (> 1000 ha) have been abandoned due to water scarcity in semiarid regions. In contrast, there are also fertile valleys which surround rivers where various crops such as olives, vines, grapes, and vegetables grow. Currently, weeds have partially occupied those areas, decreasing their productivity. The Atacama Region drought negatively affected weed management, reduced the herbicide treatment efficacy, and favored weeds over desirable species. Analogous problems have previously been observed in other places with similar weather conditions [4]. Furthermore, it is necessary to find novel alternative weed uses to reverse such hindrances. For instance, new value-added products could be developed from highly available biomass. Hence, they could change their role as pests into further alternative crops for semiarid regions.
There are several reports about weed use in order to obtain commercial products such as leather preservatives, essential oils, pharmaceuticals, and agrochemicals [5, 6]. Other studies focus on soil phytoremediation application, heavy metal removal, and biosorbents for different pollutants [7, 8]. In addition, biochar for soil amendment has also been prepared from several weed species. One of the most relevant weed applications is as a feedstock for bioenergy generation [9]. In this regard, several weeds such as switchgrass (Panicum virgatum) in the USA and miscanthus (Miscanthus spp.) in Europe are studied as promising energy crops [10, 11].
Tessaria absinthioides (Hook. And Arn.) DC (Asteraceae) is a shrubby perennial weed native from southern Brazil, Paraguay, Uruguay, Bolivia, Argentina, and Chile (Regions I to VIII) [12]. It is commonly known by different names such as Pluchea, Soroma, Chilquilla, Callacoco, Sorona, Peril, Black or Pink Suncho, and Silly Bird. In Chile, this plant is commonly known as Brea. These plants reach sizes ranging from 1 to 1.5 m in height and grow in arid and sandy soils from the sea coast, as well as humid and salty soils (Fig. 1). The plant stem is a little branched and has a grayish-green color, like its foliage, the leaves (5–8 cm) are lanceolate and narrow [13]. It is common to find it along roadsides, waterways, and rural areas. Their habitat encompasses the coast to the Andes Mountains in the desert, high-Andean steppe, sclerophyllous forest, and scrub. They can grow at altitudes up to 3000 m.a.s.l (meters above sea level).
Due to its extensive habitat and its high adaptability to extreme environmental conditions (drought, high temperature, and salinity), this weed became a severe problem for farmers in Chilean valleys in semiarid regions. The olive crop is one of the most affected by this weed in the Atacama Region (Figure S1A). Approximate estimation of brea availability is based on practical field observations. This weed usually occupies about 25% of the land used for olive cultivation (1917.3 ha). Thus, the total area covered by TA in olive fields would be about 479.3 ha. Hence, if a use for this weed is found, it can be treated as a second crop for olive farmers, which would improve their income. Moreover, it is important to notice that brea can also grow wild in other open spaces without being associated to any crop (Figure S1B). The Atacama Region has a surface area of 75,176.2 km2, which is equivalent to 9.9% of Chilean territory. It has 29,780 ha of farmlands, but around 10,000 ha are still unexploited. Due to the global water crisis, such lands have low probabilities of being used soon. Nevertheless, they are full of wild weeds, including Tessaria species, which can be collected from nature.
There are previous reports on the biological activity of alcoholic extracts and essential oils from Tessaria absinthioides [13]. However, no previous attempts to use it as feedstock for clean energy production have been reported.
With these facts in mind, this study aims to evaluate fuel properties and combustion behavior of T. absinthioides, arriving at some conclusion regarding the possibilities of using this biomass as an energy source.
Materials and Methods
Collection of Tessaria absinthioides Samples
The sampling process of Tessaria absinthioides (TA) specimens was done in five areas of the Atacama Region (Chile) in 2018. Selected locations and their georeferencing are presented in Tables S1 and Figures S2, and S3. Those locations were selected because they have different climatic conditions (Table S2), geomorphological characteristics, and soil conditions. Sampling area was divided into five different sectors (1 ha each), and 20 different brea plants were randomly selected per sector as proposed by [6]. Once collected, brea samples were dried in an oven and stored hermetically in the dark for further analysis. For pellet preparation, brea plants from different locations were sampled.
Pellet Preparation
The main objective was to validate the viability of TA as a solid fuel. Considering the shrubby’s properties, its best option for combustion is in the form of pellets. Biomass (200 kg) was ground to a particle size in the range of 2–4 mm using an AMIS S-20/20 3661 (AMIS Maschinen-Vertriebs GmbH, Zuzenhausen, Germany) cutting mill with a coupled sieve. Biomass was further dried in a IKA Oven 125 (IKA Works Inc., Staufen, Germany) tray dryer for 2 days at 50 °C until reaching humidity which ranged from 15 to 20 wt% (recommended humidity from pelletizer supplier). The TA powder was pelletized in a bench-scale pelletizer machine (South American Pellet MPSP 100, Chile), and fallows the requirements of ENplus [14]. The pelletizer’s processing capacity was set to 100 ± 3 kg/h and was fed continuously at room temperature. Five consecutive samples were collected, each containing pellets (6 mm diameter). Samples were stored in airtight bags at room temperature for further processing and characterization.
Commercial Pellets
As control feedstock, ENPLUS A1 quality pellets were used [15], commercialized by ECOMAS SA., which is the leading pellet producer from Pinus radiata in Chile.
Sample Characterization
Samples were characterized by employing the standard methodology for proximate analysis of solid biofuels, i.e., sample preparation (UNE-EN ISO 14780:2018/A1:2020), moisture content (UNE-EN ISO 18134-3:2016), ash content (UNE-EN ISO 18122:2016), and volatile content (UNE-EN ISO 18123:2016). Elemental analysis was performed in a Leco TruSpec Micro CHNS LECO elemental analyzer (LECO Europe B.V., Geleen, The Netherlands) [16].
Compositional Analysis
TA biomass was characterized according to lignin, cellulose, hemicellulose, ash, and extractive content. Plant samples were characterized following NREL, LAP [17].
Thermogravimetric and Differential Thermogravimetric Analysis
Thermal decomposition of TA was assessed by employing thermogravimetric and differential thermogravimetric analysis (TGA-DTA) [18] with a TG/DSC NETZSCH STA 409 PC (NETZSCH Holding, Selb, Germany) differential thermal analyzer. Characterization was performed at an air atmosphere with a heating rate of 10 °C/min.
Scanning Electron Microscopy Analysis
Scarce information is available regarding the TA pellet microstructure. Samples were analyzed by scanning electron microscopy (SEM) using a Jeol JSM-6380LV (JEOL Ltd, Tokyo, Japan) to study pellet morphology from both surface and inner parts [19]. Moreover, the SEM-EDS technique allows element mapping in small sample spaces. SEM with a Jeol JSM-6380LV under low vacuum (100 Pa) operating with the gaseous solid–state detector (GAD); collecting secondary electrons which were used for the study. Samples were mounted on aluminum stubs using carbon tape and sputter-coated with a gold layer for 3 min using an Edwards S150 sputter coater (BOC Edwards, São Paulo, Brazil) operated at a voltage at about 1 kV.
Heating Values
The highest heating value (HHV) was obtained using an Automatic Isoperibol Calorimeter Parr 6400 (Parr Instrument Company, Moline, USA) according to the UNE-EN ISO 18125:2018. The lowest heating value (LHV) was determined from the experimental value of HHV and the vaporization heat of the water content in the fuel, according to equation 1 in MJ kg−1, as follows [17]:
Variables H, M, and O are mass fractions of hydrogen, moisture, and oxygen in the sample.
Alkali Index
Alkali index (AI) is a threshold indicator for fouling and slagging. All biomass fuels exhibit fouling behavior, but at different rates which depend on composition and ash content. Woody biomass tends not to foul at higher rates as straws because they possess lower ash contents and, at the same fuel firing rate, there is less ash entering the combustor. Woody biomasses have more favorable ash compositions. The AI expresses the number of alkali oxides present in the fuel per unit of fuel energy (kg alkali GJ−1). The AI was determined according to Equation 2, which was developed by Jenkins et al. [20]:
In which Q is the fuel HHV, \( {Y}_f^a \) is the mass fraction of ash in the fuel, \( {Y}_{{\mathrm{K}}_2\mathrm{o}}^a \) and \( {Y}_{{\mathrm{Na}}_2\mathrm{o}}^a \) are the mass fractions of K2O and Na2O in the ash. Miles et al. [21] suggested that, for AI values above 0.17 kg, alkali GJ−1 fouling is probable, and above 0.34 kg, alkali GJ−1 fouling is virtually certain to occur. However, the AI is incomplete as a specifier for fouling behavior and is primarily useful as a general guiding tool.
These threshold levels are determined based on experience; thus, they may vary considerably. Values determined for TA will be compared with the values of AI reported for different feedstocks by [20] and [22] and determined, according to Equation 2, using the ash composition reported by [20].
Combustion procedure
TA’s combustion behavior was essential for designs in which combustion is applied and are fed with this feedstock to achieve high overall conversion efficiency. Comprehension of the process is also required to reduce combustion-related pollutant emissions, especially in large-scale applications [23,24,25].
Prior to the combustion test, biomass properties were characterized. The experimental design consisted of two sets of combustion experiments in order to compare the TA pellet combustion behavior with dry pine wood (Pinus radiata) commercial pellets (CP). Pinewood pellet is a highly demanded commercial fuel for industrial and domestic appliances, with known fuel properties. The TA pellets selected for the combustion test were randomly sampled from the pellets previously produced. For CP, the experimental test was performed with pellets from three different commercial lots. The experimental design included four replicates for TA’s experimental combustion tests and three replicates for CP. This study compared the mean experimental results with results reported for the combustion of Dichrostachys cinerea and German beech wood (Fagus sylvatica) in a traditional long wood stove under similar conditions (λ = 1.9).
During the combustion process, the most important variables were the temperature variations of the combustion chamber and flue gas, the latter’s composition behavior, the mass variation of the combusting sample, and the total PM emitted [26] .
Experimental Setup
Figure 2 shows the experimental setup schematic for the combustion study. This study took place in a 7.5 kW traditional Bosca Scan 350 (Bosca, Huechuraba, Chile) log wood stove with a combustion chamber which had 0.023 m3 capacity and a rated power output of 7.5 kW. The stove was equipped with air controllers that regulated primary air amount during combustion to control the combustion rate. Flue gas temperature and composition were recorded within 1-min intervals. Oven temperature was measured at the center of the combustion chamber within 10-min intervals. Temperatures were obtained using type K thermocouples and registered in a Omega OM-DAQXL-2-NA (Omega Engineering Limited, Connecticut, USA) data logger. Total particulate matter emission (PM) was also assessed during the combustion process through the Standard Method 5G (determination of particulate matter emissions from wood heaters [dilution tunnel sampling location]) [27]. The dilution tunnel was made of a stainless-steel cover with 0.30 m diameter, which guarantees the stove’s exhaust gases’ dilution from atmospheric air. PM, exhaust temperature, and velocity sampling measurements were made in transversal ports on a straight stainless-steel duct with a diameter of 0.15 m in which the mixture is baffled and a blower maintains the velocity and dilution rate within specifications (3.66 m/s). Exhaust gases were vented outside the laboratory. Figure 2 shows the simplified installation schematic for PM measurements.
For each experiment, a gas analyzer (ProTech V-Gas; Melton Mowbray, UK) simultaneously measured the carbon dioxide (CO2), carbon monoxide (CO), and oxygen (O2) concentrations. The analyzer’s measurement range was 0–15% for CO, 0–20% for CO2, and 0–25% for O2, with the same resolution as 0.01% for all gases. Particulate matter (PM) was sampled under isokinetic conditions using cassettes with Teflon filters (Whatman Inc., Maidstone, UK) which had 47 mm diameter, were 40 mm thick and had a pore size of 2 μm, connected parallel to a sampling manifold for the continuous collecting of PM during the experimental test.
The stove was heated up before the experiment began, by the combustion of wood (Pinus radiata), until a temperature of 476.85 °C was reached in the combustion chamber center. The rest of the combusted wood was removed and the pellet samples that were going to be analyzed were placed in the oven. This sample was then wholly combusted. The test ended when the CO2 concentration in the exhaust dropped below 3%. Total duration of the experimental procedure was between 110 and 120 min. Effects of different feedstock properties were tested at nominal load conditions (1.3 kg of commercial and TA pellets in each run). Pellets were loaded to the stove using a pellet basket, an accessory which allows uniform pellet combustion, enabling the use of this fuel in wood stoves. The duration of the test was around 55–60 min. Experiments were carried out at the same combustion regimes (λ = 1.9). Results achieved with this experimental setup are discussed in detail below.
Statistical Analysis
Experimental data were subjected to a variance analysis using a Statistical Analysis Software SPSS 11.0. The Student’s t test was used to compare the differences among means at the level of p < 0.05.
Results and Discussion
During the preparation of TA pellets, plant biomass had a moisture content of around 20 wt%, which is an important parameter that affects pellet quality. Moisture can act as a binder during the biomass pelletization process by promoting molecular interactions (hydrogen bonds and Van der Walls’ forces) among their chemical components. Therefore, low and controlled moisture content allows pellets to remain compact, maintaining their dimensional stability and preventing cracking due to further water absorption during storage. In order to develop biofuel applications for TA pellets, it is necessary to first know their physicochemical properties, and, thus, a comprehensive characterization of the material was carried out.
Fuel Properties
Proximate Analysis
The fractions of volatiles, ash, and fixed carbon were determined by proximate analysis. Table 1 shows the results of TA, CP, and other woody and non-woody biomasses. For TA, 18.5% consisted of fixed carbon and 73.4% was volatile matter. The overall ash content reached 8.1%. The mass distribution was very similar to that of wheat straw.
Information on the proximate composition of the studied biomass is scarce. The ash content values obtained are higher compared to the figures published in [29] (i.e., 8.1% (Table 1) vs. 3.0% [29]). One reason for this difference may be that the characteristics of the soil influence to some extent the composition of the ash in the plant material.
It can be seen that the TA volatile content is similar to those of Dichrostachys cinerea and wheat straw, but higher than that of rice straw. It is smaller than the other herbaceous or woody biomass, although within the same order of magnitude. However, the TA ash content is comparable to that of wheat straw and it is about half of that of rice straw. Furthermore, it is higher than those reported for other biomasses. The content of non-combustible compounds in brea is within the range typically found on this type of feedstocks.
The amount of fixed carbon in the brea plant was higher than in some woody biomasses, showing a fundamental difference in the expected behavior of this component in non-woody biomasses. The moisture content was 10.87%, which is within the expected range of this material.
Ultimate Analysis
The TA elemental composition was determined by ultimate analysis. Results are also reported in Table 1, and show that the material contains 40.8% of carbon (C) and 42.8% of oxygen (O). Hydrogen (H) amounts to 6.0%. All other elements investigated here (N, S, Cl) are clearly below 3%. The C content is relatively low, but within the order of magnitude usually found in this type of biomass. The H content is comparable to that of sugarcane bagasse and woody biomasses, and it is higher than that for wheat and rice straw. This value is slightly higher than the mean value typically reported in non-woody biomasses. Similar behavior can be observed in sulfur content, as the expected pollutant formation is increased during combustion of this biomass, possibly caused by the combination of S with H and O. Nevertheless, these values are within the range found in herbaceous and non-woody biomasses [20]. The O content is relatively low compared to other biomasses presented in the table.
Compositional Analysis
An additional analysis was performed for the TA, which is its chemical characterization in terms of lignin, cellulose, and hemicellulose contents. Results, in comparison to other biomasses, are presented in Table 2.
Cellulose content of TA biomass is within the same range as switchgrass and miscanthus, but lower than in pine and poplar. Moreover, the hemicellulose content is higher than the one found in hybrid poplar but similar to other species. Cellulose and hemicellulose contents are similar to the ones recently reported for cardoon (37.65% and 16.7%) and Turkey oat (35.56% and 15.19%, respectively) [31]. Both polysaccharide contents are typical of biomass used as feedstock for bioenergy production. It is interesting to note that TA showed a similar lignin content of pine’s; however, it was higher than the other species’ values. The extractive content found in this plant was 5.2%, which compares with other species [31].
Heating Values
The TA HHV was determined following the method outlined in section 2.3. The LHV was determined according to Equation 1. Table 1 also shows the results for TA and other woody and non-woody biomasses. The heating values measured and calculated for TA pellets are similar to those of some non-woody biomass’ calorific values and are slightly lower than those for woody biomasses like fir and Pinus radiata, commonly used as renewable energy sources. Notwithstanding, an HHV of 17.47 MJ/kg shows the viability of using this biomass as a renewable energy feedstock.
Ash Composition
Table 3 shows the obtained elemental composition of TA ash in mg/kg of dry fuel, along with values that allow comparison between different biomass feedstock pellets. It can be concluded that TA pellet ash content shows higher similarity to that of Dichrostachys cinerea (woody biomass) than to that of Miscanthus giganteus or switchgrass, which are non-woody species.
Additionally, it was found that 25.67 wt% of TA ash consists of sodium (Na), an element with considerable influence in reducing ash fusion temperature. Compared to this element, all other components are found in lower amounts. The other elements which contribute to the ash composition are calcium (Ca), potassium (K), magnesium (Mg), and phosphorus (P). In addition to Na, these elements are responsible for more than 90% of the ash content. These values are within the same order of magnitude reported for other types of non-woody biomass [20]. Therefore, the TA ash composition does not show any significant irregularities.
Alkali Index
The AI of TA pellets shows a high value (1.46 kg alkali GJ−1) in comparison to woody biomasses (fir: 0.06 kg alkali GJ−1[20]; Pinus radiata: 0.02 kg alkali GJ −1 [20]; Pinus radiata with bark: 0.02 kg alkali GJ−1 [20]; willow wood: 0.14 kg alkali GJ−1 [20]; Dichrostachys cinerea: 0.08 kg alkali GJ−1[22]). The AI is also higher than the one found in several non-woody biomasses, like Alfalfa stems (0.82 kg alkali GJ−1 [20]), wheat straw (1.07 kg alkali GJ−1 [20]), and Miscanthus giganteus (0.06 kg alkali GJ−1 [33]. Conversely, the AI of TA is surpassed by the value of AI obtained for rice straw and almond hulls (1.64 and 1.75 kg alkali GJ−1, respectively [20]). Low ash fusion temperature is expected, with probable slagging and fouling behavior during the combustion of this fuel in systems in which large residues of feedstock occur.
Thermogravimetric and Derivative Thermogravimetric Analysis
Thermogravimetric and derivative thermogravimetric analysis (TG/DTG) provides direct and instantantaneous information like moisture loss, decarboxylation, pyrolysis, oxidation, and decomposition for solid fuels. It also finds useful applications when comparing combustion behavior. TG/DTG analysis has been a widely used technique for characterizing biomass fuel pellets.
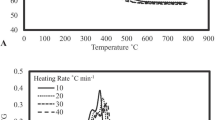
Figure 3 shows the mass loss (TG) and derivative mass loss (DTG) curves of TA pellets, which were obtained through a non-isothermal run performed at a heating rate of 10 °C/min in atmospheric air. The weight loss was recorded as a function of temperature from 20 to 950 °C.
The results suggest that the thermal degradation of the TA pellets occurs in several phases, as evidenced by the presence of three peaks of decomposition and the final baseline gently tilted on the TG curve. This could be associated with the complex chemical composition of this feedstock, which includes cell wall components like cellulose, pectin, hemicellulose and lignin, as well as other minor components such as sugars, proteins, waxes, and fats [34]. Similar results have been found during the thermal degradation of other biomass feedstocks [35, 36].
Thermogravimetric (TG–DTG) curves could be observed in four stages associated with feedstock combustion. Stage I occurs from room temperature to 168.3 °C, showing maximum decomposition rate temperature (TPeak) at 86.2 °C, accounting for 5.8% of the feedstock mass loss. This process is mainly associated with water evaporation due to pellet-remaining moisture. This value agrees with the ones obtained from different biomasses such as olive pomace (3.7%), spent mushroom (8.8%), and flax sieves (8.0%) [35, 36]. The primary combustion stage (II) occurs in the range 168.4–400.4 °C, with maximum decomposition rate at 299.3 °C, triggering a mass loss between 52.1 and 59.41% of pellet thermal decomposition. This stage is characterized by the release and combustion of volatiles [36]. It has been associated with the devolatilization of the cell wall polysaccharide fraction (hemicellulose and cellulose) and the initial decomposition of proteins and lignin [35, 37]. Hemicellulose has a lower molecular weight and crystallinity than cellulose; therefore, its thermal decomposition starts first [38]. For TA pellets, the Tpeak value is close to the one for the cellulose thermal degradation temperature and it is similar to what has been found in previous biomass combustion studies [35]. Thermal degradation of polysaccharides is a complex process which involves chain depolymerization, sugar dehydration and oxidation. Whereas for lignin, initial degradation is associated with oxidation processes. Stage III (400.5–543 °C) shows a 25.5% mass loss that accounts for 29.08% of biomass combustion, which corresponds to the combustion of fixed carbon, where the volatilization of remaining volatiles takes place [35]. Such compounds are mainly associated with lignin aromatic degradation products. The final combustion stage (IV) starts at temperatures higher than 540 °C, which involves a mass loss of 4.3%. Some authors suggest that this stage is associated with decomposing of the remaining char and degradation of inorganic compounds [39]. Finally, a residual 12.3% mass at 935 °C is formed by ashes.
Scanning Electron Microscopy Analysis
In the pellet surface micrographs obtained via the SEM-EDS analysis (Figure S3A and B), an irregular, rough, and fibrous morphology with some longitudinal configuration was observed. The fibrous aspect is typical of plant biomass due to the cell wall composition, which is composed of fiber (lignins, celluloses, hemicelluloses, and pectins) [40].
However, the apparent configuration should be observed in the same direction in which the pressure was applied during pellet preparation. Nevertheless, the inner pellet part micrographs (Figure S3C and D) were obtained via cryofracture, where an internal highly fibrillar structure and the lack of order are observed. The EDS analysis allows the determination of several majority and minority elements present in the TA pellet and their relative abundance percentage by weight (Figure S3E). It is important to note that determining the abundance from those elements falls within a range close to the one obtained for whole pellet samples (Table 3). A new element determined by this analysis was chlorine, which appears in fractions smaller than 1 wt%, representing low content, which is in agreement with findings from other biomasses of plant species used as biofuels [20].
Combustion Behavior
TA pellet combustion behavior can be described by the time profiles of temperature and concentrations of flue gas components during thermochemical conversion. Both aspects are discussed below.
Temperature
Figure 4 shows values of the oven and exhaust gas temperatures measured during the third and second replications of the TA and CP pellet combustion experiments, respectively. The maximum oven temperature achieved during the combustion of CP was at an average of 844 ± 17 °C. In contrast, the highest mean temperature during TA combustion was 685 ± 15 °C under similar experimental conditions. It is important to remark that such a difference was statistically significant (p < 0.05). The highest temperatures for both feedstocks have been reached after similar combustion periods, indicating that the studied fuels have similar combustion rates. TA pellets reached an average exhaust gas temperature of 239 ± 16 °C, which is lower than what was observed for the CP average combustion temperature (267 ± 13 °C). However, this difference was not statistically significant (p > 0.05), which indicates that their fuel properties are comparable.
CO2 and CO Emission
In spite of CO2 being the most important greenhouse gas, its formation behavior is a fundamental indicator of combustion process quality. CO2 emission on stoichiometric combustion of TA can be estimated by Equation 3.
With the equation above, it is possible to estimate that for the combustion of 1 gram of TA, there will be an emission of 1.62 g of CO2 and 0.025 g of SO2.
Estimated theoretical CO2 emission on stoichiometric combustion of the CP is higher than the estimated for TA. Equation 4 describes the stoichiometric combustion of CP with air as an oxidant.
The stoichiometric combustion of CP theoretically emits 1.838 g of CO2 per gram of combusted CP, although low SO2 emission is expected.
Figure 5 I indicates that TA (third replication) and CP (second replication) have similar combustion behaviors based on CO2 formation rate. The CO2 formation behavior is also compared with German beech wood (Fagus sylvatica) and Dichrostachys cinerea under similar experimental conditions [22]. All values are referred at 13% of O2.
Maximum formation of CO2 is observed simultaneously in both cases (i.e., close to when maximum temperature is reached in the oven). When comparing CO2 emission behavior of TA and CP with woody biomass, the two first ones emit more CO2 per kg of feedstock. Non-woody biomass, when compared to Dichrostachys cinerea, emits 100% higher values of CO2 concentrations.
Even taking the differences between TA and CP outlined above into consideration, these two non-woody biomasses exhibit the same behavior as per CO2 emissions. Their comparable fuel properties can explain these similarities.
The conversion of the carbon content over to CO2 takes place through intermediate steps at different reaction rates. Carbon monoxide (CO) is the most critical intermediate species from these conversion processes.
CO is harmful to humans because it hinders the oxygen transportation to the blood and can cause death. This is one of the reasons for strict CO emission regulation in most countries. Furthermore, high CO emissions indicate a low overall process combustion efficiency, as CO oxidation to CO2 releases energy. Due to thermodynamic constraints, complete conversion of the fuel—C to CO2 is rarely achieved.
The rate at which CO is oxidized to CO2 relies primarily on the temperature level that the intermediate oxidative reaction of CO to CO2 takes place, on the quantity and method of supplying oxygen and on the residence time of the flue gas inside the combustion chamber. CO production reaches its lowest value at a specific oxygen to fuel ratio for a given combustion device. Higher excess oxygen ratios decrease the combustion flame temperature, while lower excess oxygen ratios result in inadequate mixing conditions. Consequently, to diminish CO emissions, the following aspects must be assessed: enough residence time, elevated temperatures, and proper oxidizer-fuel mixing within the combustion chamber. Fuel characteristics define CO content within the flue gas to a minor degree.
CO emissions obtained for the third and second replications of TA and CP, respectively, are also shown in Fig. 5 II. CO formation behaviors are also compared to the one obtained in the combustion of Dichrostachys cinerea under similar experimental conditions [9]. Results indicated relatively higher CO emission values for TA when compared to CP. Maximum CO emission value detected in the combustion occurred before the instant CO2 reached its maximum value. This indicates that conditions within the combustion chamber are not optimal for the full oxidation of CO into CO2. In both cases, maximum CO2 value was observed when the minimum value of CO was measured within the flue gas.
Furthermore, experimental conditions for both experiments have been the same; CO emissions in both cases showed different behaviors. As for CP, the formation of CO was stable throughout all replications under 2706.5 mg Nm−3 at 13% O2 (averaging 1700.52 ± 203.7 mg MJ−1). After reaching the maximum value, however, for TA pellets, the value of CO oscillated during all the experiments with an average value of 4943.6 ± 235.2 mg Nm−3 at 13% O2. Experimental values observed were higher than the ones reported by other authors for similar feedstock types [33, 41], primarily due to differences in the experimental procedure and the combustion devices employed; which were automated loading pellet stoves. Relatively high CO values obtained for both feedstocks can be related to the combustion stove air control, which is not fully adapted to pelletized fuel burning conditions.
Although these values are higher than expected for woody fuels, the use of the pellet basket induces the emission values to be lower than expected for a traditional wood stove [42]. The CO emission has a similar behavior to the one observed in combustion of Dichrostachys cinerea [9], as presented in Fig. 5. However, as expected, the woody fuel produces lower emissions under these combustion conditions, with an average value of 1196.76 ± 177.12 mg Nm−3 at 13% O2 (approximately 755.03 ± 112.10 mg MJ−1). Analysis of the measured results suggests that there are no reasons regarding CO emissions that prevent the use of TA pellets as a renewable energy source in combustion appliances.
Particulate Matter Emissions
Limitations on PM emissions in global and national regulations are becoming more restrictive [43]. Within the framework defined in the EU Ecodesign Directive, the PM emission limit for pellet stoves will be reduced to 20.0 mg Nm−3 starting January 01, 2022. According to Chilean regulations, the PM emissions limit for wood or pellet stoves with a power of up to 25 kW has a similar value, which represents a significant reduction, according to the global policy.
The formation of PM from biomass combustion depends on several factors, such as combustion conditions, feedstock properties, combustion appliances, equivalence ratio, fuel ash content, burning rate, and appliance load [44, 45].
The study determined the total particulate matter emissions using Method 5G [27]. Figure 6a) shows the average between the results obtained for TA and CP. CP presented a higher average burn rate of 1.52 ± 0.15 kg/h and a PM emission of 25.27 ± 4.55 mg MJ−1. TA emitted of 32.45 ± 6.49 mg MJ−1, with a lower burn rate of 1.15 ± 0.16 kg/h, at the same equivalence ratio. It is important to note that the emission difference was not statistically significant (p > 0.05). The results are comparable to those reported for the combustion of wood pellets in pellet stoves, where an automatic control system regulates the flow of air and fuel, ensuring efficient and relatively clean combustion with low PM emissions. Kistler et al. [48] reported PM emissions from combustion of several feedstocks. Emissions of wood pellet combustion in pellet stoves range between 16 and 31 mg MJ−1 for similar burn rates (1.4 and 1.9 kg/h). Besides, emissions for logwood combustion in traditional wood log stoves with the same capacity were notably higher, ranging between 32 and 100 mg MJ−1.
Total PM emissions a) for Tessaria absinthioides and commercial pellets obtained in the study and b) compared with PM emissions reported by other authors. TA, Tessaria absinthioides; CP, commercial pellets assessed in this work; MP, Miscanthus pellets [33]; CP 1–3, commercial pellets 1–3 [46, 47]; EB, European beech [48]
Figure 6b) shows the results of comparison between this experimental study and the values reported by other authors using a similar quality of commercial wood pellets (CP-1 and CP-2) [53], with pellets from non-woody biomass, which is the case of Miscanthus giganteus (MP) [35], as well as with standard wood pellets CP-3 [46].
The value for PM emissions obtained for the CP was higher in this experiment than the results reported for CP with similar ENPLUS quality, i.e., A1 (CP-1 and CP-2). These results occurred because the combustion artifact used in the experiments of CP-1 and CP-2 was a pellet stove. The artifact does not optimize the combustion performance of this type of feedstock.
In addition, CP-1 and CP-2 combustion processes were developed in a modified combustion chamber to reduce PM emissions in a commercial pellet stove. Under such conditions, the PM emitted by CP combustion was 25% higher than the one obtained for CP-1 and CP-2, which can be acceptable. According to this baseline, PM emission with TA combustion was higher than with CP, as expected. This is basically due to the difference in ash content of the fuels.
Another factor also influences PM emissions which is the quality of pellets. Non-woody biomass such as Miscanthus giganteus [33] has lower total PM emissions despite having higher ash content than CP, CP-1, and CP-2. Tessaria absinthioides emissions were lower than those emitted during the combustion of standard wood pellets CP-3 [46]. This can be attributed to the lower quality of pellets used in the reported experiments. European beech (EB) log wood combustion in traditional log wood stoves, under similar combustion conditions, also accounts for larger PM emission (66 ± 10 mg MJ−1 [48]) than TA.
Combustion of TA pellets in a log wood stove using a pellet basket presented similar emissions to those reported for wood pellet combustion in a pellet stove. In pellet stoves, air and fuel streams are regulated automatically, which guarantees efficient and relatively clean combustion with low PM emissions, significantly lower than the ones emitted during the combustion of log wood in traditional log stoves. The latter is the heating appliance used for the experimental tests in this research.
As stated by Mitchell et al. and Polonini et al. [49, 50], PM generation depends on combustion quality and fuel composition, particularly of the ash content. The ash-derived PM results mainly from the release of Na, Mg, K, and Ca contained in the fuel. Recent studies [43] have found that the ash-derived PM process is enhanced at higher combustion temperatures, suggesting that reducing this parameter is a means of decreasing PM generation from high-ash biomass fuels. A combination of both factors (ash content and combustion temperature) explains the total PM emission behavior on TA pellet combustion. Experiments showed that the combustion chamber temperatures for the TA pellets were always lower than the temperature reached in the combustion chamber for the rest of the reported experiments in Fig. 6, except for the Miscanthus giganteus pellets. This explains the low PM emission from combustion of TA, resulting in the expected PM emission for the combustion of high alkaline biomass with high ash content.
It is expected that PM emissions obtained from TA combustion in a pellet stove do not exceed the limits set for this parameter in global regulations, allowing the use of its pellets as feedstock in combustion appliances for the current energy scenario.
Conclusion
Compared to other woody and non-woody biomasses, TA shows, as expected, fuel properties closer to non-woody biomass than to woody raw materials, with a high ash content and AI. The combustion emissions of TA pellets were slightly higher than those of CP when used as baseline feedstock. Some higher values for airborne emissions can result from many trace elements within this biomass. Nevertheless, the TA combustion performance can be improved if it is burned in pellet stoves. The study evidence that a pellet basket’s use allows uniform combustion of TA pellets in a traditional wood stove, obtaining environmentally sound combustion. Results have shown that no apparent reason hinders an extensive use of TA as a biofuel as a substitute for wood pellets, which is socially and economically significant considering that this bush is an invasive species in vast areas of the Chilean territory.
Data Availability
Datasets generated and analyzed during the current study are available from the corresponding author upon a reasonable request.
References
Pimentel D (2001) Economic and environmental impacts of invasive species and their management. Pestic You 21:10–11
Adeux G, Vieren E, Carlesi S et al (2019) Mitigating crop yield losses through weed diversity. Nat Sustain 2:1018–1026. https://doi.org/10.1038/s41893-019-0415-y
Matloob A, Ehsan Safdar M, Abbas T et al (2020) Challenges and prospects for weed management in Pakistan: a review. Crop Prot 134:104724. https://doi.org/10.1016/j.cropro.2019.01.030
Yang Z, Liu Y, Bao G et al (2006) Rehabilitation and sustainable use pattern of rocky-desertified land in Southwest China’s poverty-stricken karst mountainous areas. J Mt Sci 3:237–246. https://doi.org/10.1007/s11629-006-0237-z
Ternelli M, Brighenti V, Anceschi L et al (2020) Innovative methods for the preparation of medical Cannabis oils with a high content of both cannabinoids and terpenes. J Pharm Biomed Anal 186:113296. https://doi.org/10.1016/j.jpba.2020.113296
Ma J, Feng X, Yang X et al (2020) The leaf extract of crofton weed (Eupatorium adenophorum) inhibits primary root growth by inducing cell death in maize root border cells. Plant Divers 42:174–180. https://doi.org/10.1016/j.pld.2020.02.001
Kamaraj M, Srinivasan NR, Assefa G et al (2020) Facile development of sunlit ZnO nanoparticles-activated carbon hybrid from pernicious weed as an operative nano-adsorbent for removal of methylene blue and chromium from aqueous solution: extended application in tannery industrial wastewater. Environ Technol Innov 17:100540. https://doi.org/10.1016/j.eti.2019.100540
Saini VK, Suthar S, Karmveer C, Kumar K (2017) Valorization of toxic weed Lantana camara L. biomass for adsorptive removal of lead. J Chem 2017:1–12. https://doi.org/10.1155/2017/5612594
Olugbade TO, Ojo OT (2020) Biomass torrefaction for the production of high-grade solid biofuels: a review. BioEnergy Res 13:999–1015. https://doi.org/10.1007/s12155-020-10138-3
Atkinson CJ (2009) Establishing perennial grass energy crops in the UK: a review of current propagation options for Miscanthus. Biomass Bioenergy 33:752–759. https://doi.org/10.1016/j.biombioe.2009.01.005
Scordia D, Cosentino S (2019) Perennial Energy grasses: resilient crops in a changing European agriculture. Agriculture 9:169. https://doi.org/10.3390/agriculture9080169
Torres-Carro R, Isla MI, Thomas-Valdes S et al (2017) Inhibition of pro-inflammatory enzymes by medicinal plants from the Argentinean highlands (Puna). J Ethnopharmacol 205:57–68. https://doi.org/10.1016/j.jep.2017.04.013
Persia FA, Rinaldini E, Carrión A et al (2017) Evaluation of cytotoxic and antitumoral properties of Tessaria absinthioides (Hook & Arn) dc, “Pájaro Bobo”, aqueous extract. Medicine (B Aires) 77:1–15
ENplus (2015) Part 2-Quality certification scheme for wood pellets. In: ENplus Handbook, 3rd edn. European Pellet Council (EPC), Brussels, pp 1–50
ENplus (2011) Part 2: Certification procedure. In: ENplus handbook (UK Pellet Council-Handbook). European Pellet Council (EPC), London, pp 1–49
ISO-ISO/TC-238 ISO - ISO/TC 238 - Solid biofuels. https://www.iso.org/committee/554401/x/catalogue/. Accessed 31 Dec 2019
Sluiter JB, Ruiz RO, Scarlata CJ et al (2010) Compositional analysis of lignocellulosic feedstocks. Review and description of methods. J Agric Food Chem 58:9043–9053. https://doi.org/10.1021/jf1008023
Wunderlich B (2001) Thermal Analysis. In: Buschow KHJ, Flemings MC (eds) Encyclopedia of materials: science and technology, 1st edn. Elsevier, Amsterdam, pp 9134–9141
Yesilkir-Baydar S, Oztel ON, Cakir-Koc R, Candayan A (2017) Chapter 11: Evaluation techniques. In: Razavi M, Thakor A (eds) Nanobiomaterials science, development and evaluation. Woodhead Publishing, Duxford, pp 211–232
Jenkins B, Baxter L, Miles T, Miles T (1998) Combustion properties of biomass. Fuel Process Technol 54:17–46. https://doi.org/10.1016/S0378-3820(97)00059-3
Miles TR, Baxter LL, Bryers RW et al (1995) Boilers. Fuels and deposit formation. In: Alkali deposits found in biomass power plants, vol 1, 1st edn. National Techrucd Momation Service (NTIS), Golden, pp 1–122
Pedroso DT, Kaltschmitt M (2012) Dichrostachys cinerea as a possible energy crop—facts and figures. Biomass Convers Biorefin 2:41–51. https://doi.org/10.1007/s13399-011-0026-y
Bain RL, Overend RP, Craig KR (1998) Biomass-fired power generation. Fuel Process Technol 54:1–16. https://doi.org/10.1016/S0378-3820(97)00058-1
Demirbas A (2005) Potential applications of renewable energy sources, biomass combustion problems in boiler power systems and combustion related environmental issues. Prog Energy Combust Sci 31:171–192
Jenkins BM, Jones AD, Turn SQ, Williams RB (1996) Particle concentrations, gas-particle partitioning, and species intercorrelations for polycyclic aromatic hydrocarbons (PAH) emitted during biomass burning. Atmos Environ 30:3825–3835. https://doi.org/10.1016/1352-2310(96)00084-2
Jimenez J, Farias O, Quiroz R, Yañez J (2017) Emission factors of particulate matter, polycyclic aromatic hydrocarbons, and levoglucosan from wood combustion in south-central Chile. J Air Waste Manage Assoc 67:806–813. https://doi.org/10.1080/10962247.2017.1295114
Agency USEP (2017) Method 5G - Determination of particulate matter emissions from wood heaters (dilution tunnel sampling location). Patent Fed Regist 65:61867–61877
Arteaga-Pérez LE, Segura C, Espinoza D et al (2015) Torrefaction of Pinus radiata and Eucalyptus globulus: a combined experimental and modeling approach to process synthesis. Energy Sustain Dev 29:13–23. https://doi.org/10.1016/j.esd.2015.08.004
Joly D, Santoro CM, Gayo EM et al (2017) Late pleistocene fuel management and human colonization of the Atacama desert, northern Chile. Lat Am Antiq 28:144–160. https://doi.org/10.1017/laq.2016.8
Williams CL, Emerson RM, Tumuluru JS (2017) Biomass compositional analysis for conversion to renewable fuels and chemicals. In: Tumuluru JS (ed) Biomass volume estimation and valorization for energy, 1st edn. InTech, London, pp 1–21
Cavalaglio G, Cotana F, Nicolini A et al (2020) Characterization of various biomass feedstock suitable for small-scale energy plants as preliminary activity of biocheaper project. Sustain 12:2–20. https://doi.org/10.3390/su12166678
Zając G, Szyszlak-Bargłowicz J, Gołębiowski W, Szczepanik M (2018) Chemical characteristics of biomass ashes. Energies 11:2885–2900. https://doi.org/10.3390/en11112885
Carvalho L, Wopienka E, Pointner C et al (2013) Performance of a pellet boiler fired with agricultural fuels. Appl Energy 104:286–296. https://doi.org/10.1016/j.apenergy.2012.10.058
Kurina Sanz M, Hernandez XE, Tonn CE, Guerreiro E (2000) Enhancement of tessaric acid production Tessaria absinthioides cell suspension cultures. Plant Cell Rep 19:821–824. https://doi.org/10.1007/s002990000192
Huang J, Zhang J, Liu J et al (2019) Thermal conversion behaviors and products of spent mushroom substrate in CO2 and N2 atmospheres: kinetic, thermodynamic, TG and Py-GC/MS analyses. J Anal Appl Pyrolysis 139:177–186. https://doi.org/10.1016/j.jaap.2019.02.002
Abdelouahed L, Leveneur S, Vernieres-Hassimi L et al (2017) Comparative investigation for the determination of kinetic parameters for biomass pyrolysis by thermogravimetric analysis. J Therm Anal Calorim 129:1201–1213. https://doi.org/10.1007/s10973-017-6212-9
Brachi P, Miccio F, Miccio M, Ruoppolo G (2015) Isoconversional kinetic analysis of olive pomace decomposition under torrefaction operating conditions. Fuel Process Technol 130:147–154. https://doi.org/10.1016/j.fuproc.2014.09.043
Lin Y, Liao Y, Yu Z et al (2017) A study on co-pyrolysis of bagasse and sewage sludge using TG-FTIR and Py-GC/MS. Energy Convers Manag 151:190–198. https://doi.org/10.1016/j.enconman.2017.08.062
Chen Z, Zhu Q, Wang X et al (2015) Pyrolysis behaviors and kinetic studies on Eucalyptus residues using thermogravimetric analysis. Energy Convers Manag 105:251–259. https://doi.org/10.1016/j.enconman.2015.07.077
Marques G, Rencoret J, Gutiérrez A, del Río JC (2014) Evaluation of the chemical composition of different non-woody plant fibers used for pulp and paper manufacturing. Open Agric J 4:93–101. https://doi.org/10.2174/1874331501004010093
Vicente ED, Duarte MA, Tarelho LAC et al (2015) Particulate and gaseous emissions from the combustion of different biofuels in a pellet stove. Atmos Environ 120:15–27. https://doi.org/10.1016/j.atmosenv.2015.08.067
Koppejan J (2012) Environmental aspects of biomass combustion. In: The handbook of biomass combustion and co-firing, 1st edn. Routledge, London, pp 1–442
Itoh T, Fujiwara N, Iwabuchi K et al (2020) Effects of pyrolysis temperature and feedstock type on particulate matter emission characteristics during biochar combustion. Fuel Process Technol 204:106408. https://doi.org/10.1016/j.fuproc.2020.106408
Lamberg H, Tissari J, Jokiniemi J, Sippula O (2013) Fine particle and gaseous emissions from a small-scale boiler fueled by pellets of various raw materials. Energy Fuel 27:7044–7053. https://doi.org/10.1021/ef401267t
Sippula O (2010) Fine particle formation and emissions in biomass combustion. Dissertation. University of Eastern Finland
Carlsson S, Gustavsson L, Johansson M, et al (2018) Measurements of emissions of EC, OC and other pollutants from residential wood combustion in the Nordic countries. Project Report. Nordisk Ministerråd, Copenhagen
Polonini L, Petrocelli D, Parmigiani S, Lezzi A (2019) Influence on CO and PM emissions of an innovative burner pot for pellet stoves: an experimental study. Energies 12:590. https://doi.org/10.3390/en12040590
Kistler M, Schmidl C, Padouvas E et al (2012) Odor, gaseous and PM 10 emissions from small scale combustion of wood types indigenous to Central Europe. Atmos Environ 51:86–93. https://doi.org/10.1016/j.atmosenv.2012.01.044
Mitchell EJS, Lea-Langton AR, Jones JM et al (2016) The impact of fuel properties on the emissions from the combustion of biomass and other solid fuels in a fixed bed domestic stove. Fuel Process Technol 142:115–123. https://doi.org/10.1016/j.fuproc.2015.09.031
Polonini LF, Petrocelli D, Parmigiani SP, Lezzi AM (2019) Experimental study of PM emissions from wood pellet stoves with an innovative burning pot. J Phys Conf Ser 1224(8). https://doi.org/10.1088/1742-6596/1224/1/012018
Acknowledgments
Chilean National Commission for Scientific and Technological Research funded this paper through the projects: CONICYT PIA/APOYO CCTE AFB170007, FONDECYT 11180828, FIC Atacama 2016, BIP 30486479. The authors also would like to thank Carlos Salgado Riquelme for the experimental support provided on this research.
Funding
The National Commission for Scientific and Technological Research in Chile funded this paper through the projects CONICYT PIA/APOYO CCTE AFB170007, FAPESP-CONICYT 2018/04819-4, FONDECYT 11180828 as well as FIC Atacama 2016, BIP 30486479.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1370 kb).
Rights and permissions
About this article
Cite this article
Pedroso, D.T., Machin, E.B., Cabrera-Barjas, G. et al. A Study of Bioenergy Production from Chilean Tessaria absinthioides. Bioenerg. Res. 15, 385–398 (2022). https://doi.org/10.1007/s12155-021-10256-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10256-6