Abstract
Cassava pulp (CP) is considered as a major and low-cost agro-industrial solid byproduct of cassava starch processing, where 48% of starch granules was still entrapped in its complex lignocellulosic matrix of cell wall structure with 21% cellulose and 8% hemicellulose. In this study, the liquid hot water (LHW) pretreatment was aimed to disrupt the cell wall structure of CP and solubilized hemicellulose and starch into saccharides to enhance biomethane production. Reaction conditions of LHW pretreatment, temperature (160–200 °C), and time (0–30 min) were optimized by response surface methodology. LHW pretreatment at 187 °C for 7 min boosted the highest level of starch and hemicellulose solubilization into the liquid phase, resulting in the maximized glucose yield of 444 mg/gCP. Starch and hemicellulose were mostly removed from CP at 99 and 79%, respectively, while 68% cellulose and 10% lignin particularly remained in solid residue. The disrupted cell wall structure and crystalline cellulose after LHW pretreatment were also observed. Microbial inhibitors such as furfural and hydroxymethylfurfural were found in low concentration at 31 and 32 μg/mL, respectively. In addition, there were no lag phase and inhibitory effect during anaerobic digestion of the pretreated CP. Biochemical methane potential of the LHW-pretreated CP showed the methane production rate and methane yield at 1.6 times and 35% higher than that of non-pretreated CP, respectively, by shortening anaerobic digestion time from 22 d to 10 d. It was shown that LHW was an efficient chemical-free method for enhancing biodegradability of CP in conversion to biomethane.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thailand has been recorded as the global highest cassava starch exporter. Within the year of 2015–2019, Thailand exported cassava starch about 3.8–4.2 million tons per year, thus a large amount of cassava pulp (CP) as a major agroindustrial byproduct from cassava starch manufacturing was massively generated [1]. CP is generated during the step of fiber and pulp separation after starch extraction in cassava starch processing [2]. The production of one ton cassava starch generates approximately 2.5 ton of CP with 70–80% moisture content [3]. By calculation from these data, a huge amount of CP (9.5–10.5 million tons) was annually generated. Improper CP treatment and management can cause air pollution from the unpleasant odor of decayed CP and the contamination of CP leachate to the environment around the factory [4]. Starch is the main polysaccharide in CP, consisting of 55–75% starch. Non-starch polysaccharide contains with approximately 15–17% cellulose, 4–6% hemicellulose, 1–4% lignin, and a number of minor organic compounds such as crude fat and crude protein [5,6,7,8,9,10,11]. Considering to its high organic content, CP has gained increasing interest to be utilized as a low-cost raw material for various valuable products, such as bioethanol [5, 6], biogas [12], hydrogen [9], aromatic compounds, pullulan [13] and organic acids [14].
Biogas production through anaerobic digestion (AD) was considered as an interesting choice for CP utilization in Thailand, more than 90% of cassava starch factories in Thailand used AD for their wastewater treatment system and biogas production [15]. In addition, the factories’ owner requires a strategy to increase biomethane production from CP. Biomethane production through AD consists of four main steps. The first step is hydrolysis which breakdowns insoluble carbohydrate, protein, or lipid into their soluble oligomers or monomers. These products are used as substrates in the second step or acidogenesis to produce H2, CO2, volatile fatty acids (VFAs) and alcohols. Then, acetogenesis as the third step converts VFAs and alcohol to H2 and acetic acid which further be utilized in methanogenesis as final step to produce methane and CO2 [16]. Biomethane, renewable energy, can be utilized to generate heat and electricity which supplied to the factory for replacement of fossil fuels utilization [17]. By using biomethane, the operating cost in factories can be reduced as well as mitigation of greenhouse gas (GHG) emissions [18]. However, high recalcitrant starchy-lignocellulosic content in CP led to limit hydrolysis rate [8] and biomethane production due to its entrapped starch granules in the complex lignocellulosic matrix of the cell wall. One challenge of CP utilization as feedstock for biomethane production is to disrupt the cellular matrix and release the entrapped starch granules from the complex lignocellulosic structure for easy access by anaerobic microbial digestion and improvement of hydrolytic enzyme. To solve this problem, pretreatment is generally used to accelerate and increase the rate of lignocellulosic hydrolysis for enhancing methane production [19].
Biological pretreatment, especially using an enzyme, was considered as the most applicable way to hydrolyze polysaccharides into monosaccharides [7]. Since starch was the main component in CP, starch-degrading enzymes were widely used. Liquefaction and saccharification processes involved two enzymes, alpha-amylase and amyloglucosidase [20, 21]. In addition, cellulase, hemicellulase, and cell wall-degrading enzymes were also used to increase the efficiency of starch hydrolysis and improve fermentable sugar yields by breaking down the cell wall structure [7, 22]. The enzymatic process was an environmental-friendly process, low-energy consumption, high-substrate specificity, and no inhibitor substance production. However, this process has limitations such as long reaction time, high cost, and large space requirement for application in the industrial scale [23]. Pretreatment using liquid hot water (LHW), also known as a hydrothermal or autohydrolysis process, had gained attention due to no chemical used, with no or minor erosion to the equipment, and short reaction time. Water in LHW can maintain in a liquid phase by operating under the temperature and pressure at 150–230 °C and 4.9–20 bar, respectively. Hydronium ion occurs by the autoionization of water and acts as weak acid. Raising the operating conditions in theses range, the concentration of hydronium ion will increase and effect to breakdown glycosidic bond of carbohydrate polymer into small compounds like oligosaccharides and monosaccharides. The breakdown of acid compounds in side-chain of hemicellulose structure represents as second sources of acid in reaction [24]. LHW pretreatment resulted in high removal of hemicellulose, high recovery of hemicellulose-derivative sugar, and low amount of inhibitors production, such as furfural (FF) and hydroxymethylfurfural (HMF), comparing to other pretreatment methods [25]. It also showed a great disruption of cell wall component especially hemicellulose for other lignocellulosic materials, such as sugarcane bagasse [26], wheat straw [27], Eucalyptus grandis [28], and soybean straw [29]. The products resulted from LHW pretreatment can be directly used in the fermentation process without detoxification process due to the low concentration of inhibitors. Moreover, LHW pretreatment has been considered as one of the most potential technologies to apply in the pilot and industrial scale for lignocelluloses fractionation and breaking down cell wall structure [24]. Thus, LHW pretreatment is a promising method on cell wall disruption and starch solubilization with result in high total sugar recovery and low inhibitor production to enhance degradation and methane production in AD. Furthermore, no information from literature search has been reported for CP pretreatment by LHW prior AD for methane production.
In this present study, the optimization of LHW pretreatment in CP to disrupt the cell wall structure, solubilize starch, and recover produced-sugar using response surface methodology (RSM) approach was carried out. The temperature and reaction time of process was considered as the major factors used in the process optimization, which inscribed central composite design (CCD) and was used for experimental design. After obtaining the optimum conditions, chemical composition and morphology of pretreated CP were analyzed and observed. This research work was intended to investigate the effectiveness of LHW pretreatment in CP. Furthermore, biochemical methane potential (BMP) assay of LHW-pretreated CP at optimum condition and non-pretreated CP was also studied.
Materials and Methods
Cassava Pulp and Sample Preparation
Fresh CP was collected from Cholcharoen Co., Ltd., Chonburi Province, Thailand. The sample was preserved in the dry form without starch gelatinization by drying fresh CP under low temperature at 50 °C for 48 h by convection oven until its moisture content was lower than 10%. Then, dried CP was ground by ultracentrifuge mill and sieved with mesh no.10 (2.00 mm). Dry and ground CP was kept in sealed storage bags at room temperature prior using.
Moisture, total solid (TS), volatile solid (VS), and ash contents of fresh CP were determined based on the standard method [30]. Crude fat and protein concentrations were determined by Soxhlet extraction (Soxtherm®) and Kjeldahl method, respectively, according to AOAC official method [31, 32]. Starch content was determined by the total starch assay kit (Megazyme, Ireland) [33]. Cellulose, hemicellulose, and lignin contents were analyzed using calculation of neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) from Van Soest and McQueen and Nicholson method by using Fibreterm® [34]. Cellulose crystallinity index was analyzed by BRUKER D8 Discover X-ray diffractometer. Scan was started from 5 to 50 degrees 2θ and with 0.02 degrees per step [35]. The calculation of cellulose crystallinity index (CrI) used the intensity of crystalline peak and amorphous peak, which are approximately occurred at 22° and 18°, respectively [36]. The CrI was calculated using the following formula in Eq. 1.
Morphology of CP structure was observed by a scanning electron microscope (SEM). The sample was gold-coated using Cressington Sputter Coater 108 Auto, and then it was observed and photographed by SEM (JEOL JSM-6610LV). Sugar composition was analyzed by hydrolyzing sample using laboratory analytical procedure (LAP) of NREL [37] in which sugar were detected and measured by high-performance liquid chromatography (HPLC) with refractive index detector and Biorad Aminex HPX-87P column. Deionized water was used as mobile phase at a flow rate of 0.6 mL/min, with column temperature at 85 °C.
Optimization of Liquid Hot Water Pretreatment
Temperature and reaction time were used as the main factors for optimization of reaction conditions in liquid hot water (LHW) pretreatment of CP. Inscribed central composite design (CCD) was used to design the conditions in optimization. Temperature and reaction time range were selected at 160–200 °C and 0–30 min, respectively, by base on the beginning of breaking down hemicellulose and minimized inhibitors generation. Twelve experimental runs from CCD were designed as shown in Table 1. The highest amount of total saccharides in the clear hydrolysate was used as a response in optimization. LHW pretreatment with loading 5% TS of CP was conducted in 600-mL mini benchtop reactor (Parr Instruments, Moline, USA). The pressure was set as saturated vapor pressure at a specific temperature by N2 gas with a vertical shaking system at 500 rpm. The heating jacket elevated the temperature inside the reactor with a heating rate of 10 °C/min. After reaching the targeted temperature and time of reactions, the cooling coil was applied to instantly terminate the heating process and rapidly decrease the temperature. The control experiment (non-pretreated) was done as the same procedure but controlled at room temperature. Solid residue and clear hydrolysate fractions of the pretreated and non-pretreated CP were separated by filter paper (Whatman No.1). The solid residue was dried at 50 °C and then measured for its weight. For clear hydrolysate, it was collected to analyze its sugar compositions. The concentrations of monosaccharides, cellobiose, and maltose in the clear hydrolysate were also measured. In addition, oligo- and di-saccharides were converted into monosaccharides by H2SO4 before HPLC analysis for sugar composition, and it was reported as total sugar [38].
Experimental Validation of LHW Pretreatment at the Predicted Optimum Condition
The predicted optimum condition obtained from the statistical analysis of CCD was used to experiment with the validation step. The experiment was set in triplicate and carried out the same procedure of LHW pretreatment but controlled at the predicted temperature and reaction time. The clear hydrolysate was collected for sugar composition analysis. The presences of inhibitors, such as furfural (FF) and hydroxymethylfurfural (HMF), which were probably generated during the pretreatment, were also observed. The inhibitors in the clear hydrolysate were measured according to LAP protocol, which used Biorad Aminex HPX-87H as column and coupled with refractive index detector. 5 mM of H2SO4 was used as mobile phase at a flow rate of 0.6 mL/min and 50 °C of column temperature. All the samples were filtrated with 0.22-μm syringe filter before injection [38]. The solid residual fraction was analyzed for its starch content, lignocellulosic composition, cellulose crystallinity index, and morphology of biomass structure. Scanning electron microscope (SEM) was used to reveal morphology and the disruption of the intact biomass structure. The analyzed results would be compared with non-pretreated CP.
Biochemical Methane Potential (BMP) Analysis
The pretreated CP at the optimum condition was carried out to determine BMP. In addition, two fractions such as clear hydrolysate and solid residual fraction from pretreated CP were also analyzed for their BMP. Anaerobic inoculum for BMP assay was obtained from biogas reactor treating cassava starch wastewater (CholCharoen Co., Ltd.). Non-pretreated CP was used as a control treatment. BMP assay was conducted in 120 mL-serum vials with the working volume of 80 mL and the inoculum (VSS) to substrate (VS) ratio of 3:1 [39]. Each vial was flushed with 99.99% of N2 to remove the oxygen in the vial headspace before being closed and sealed with the rubber stopper and aluminum cap. All the vials were kept in the incubator (37 °C) without agitation. Biogas production and composition were determined by liquid displacement method and gas chromatography (GC) with a thermal conductivity detector (TCD) [40]. The data of methane accumulation were used in curve-fitting on the modified Gompertz equation (Eq. 2) using Solver add-in on Microsoft Excel version 2019 [41];
where G is the cumulative methane production at digestion time (NmL); Gm is the maximum methane production (NmL); Rm is the maximum methane production rate (NmL/d); e is the Euler’s number (2.7183); λ is the lag phase time (d); and t is the digestion time (d).
Statistical Analysis
Minitab® 18.1 and Design-Expert® version 10 software were used for all statistical analysis, wherein LHW pretreatment optimization, experimental design, analysis of variance (ANOVA) response surface plot, and contour plot of the optimum point were performed. One-way ANOVA was carried out for kinetic data in BMP test. Turkey’s test was used in multiple comparisons with the significant level at 95% (α = 0.05).
Results and Discussions
Characterization of Cassava Pulp
Fresh CP resembles starchy biomass with 21.4% TS and 78.6% moisture, it was characterized by high organic content (98.3% VS) and a starch-rich composition (47.9% starch) with 20.9% cellulose, 8.1% hemicellulose, and 3.4% lignin as shown in Table 2. Less amount of crude fat, crude protein, and ash was found. Lower starch content of CP in this present study compared with the other studies (approximately 55.5–75.1%) was due to this factory (Cholcharoen Co., Ltd., Chonburi Province, Thailand) has the policy of waste minimization and cleaner production [42]. Thus, the process efficiency of starch extraction from the cassava root was improved. High efficiency of starch extraction resulted in a high yield of cassava starch production and few starch granules trapping in the cell wall structure, while cellulose and hemicellulose contents were slightly high compared with other studies. The variation of the lignocellulosic component was probably caused by the cultivar and the cultivation method of the cassava plant. It was also observed that CP mainly contained 76.9% (dry basis) of carbohydrate polymer, especially starch and lignocellulosic components.
To consider total monosaccharide constituents in CP, carbohydrate polymer was breakdown into monosaccharides by H2SO4 before measurement and refers as monosaccharide sugar composition. Glucose was observed as the main monosaccharide in CP (56.8%), which was followed with 11.5% galactose, 9.8% mannose, 7.6% L-arabinose, and 6.9% xylose. These monosaccharides might be derived from starch, hemicellulose, or other cell wall components. Chaikaew et al. [10] reported similar results for the monosaccharides composition in CP, in which glucose was also found as the major monosaccharides along with the small amounts of xylose, galactose L-arabinose, and mannose.
Liquid Hot Water (LHW) Pretreatment
The amounts of solid residue (as %TS) and clear hydrolysate (as mL) resulted from 12-runs of LHW pretreatment at various operating conditions was shown in Fig. 1. The amounts of solid residue from those runs ranged from 21.4 to 44.6%. The experiment run no. 6 (200 °C, 15 min) showed the lowest amount of solid residue at 21.4%, while the highest amount of solid residue (44.6%) was observed in the experiment run no. 1 (166 °C, 4 min). Low amounts of solid residue were observed for the operating conditions at temperature and reaction time higher than 180 °C and 15 min, respectively (run no. 6 and 8). Contrarily, the temperature and reaction time at ≤ 180 °C and 15 min resulted in high amounts of solid residue, as observed in runs no. 1 and 7. More than 50% of solids in pretreated CP was solubilized into clear hydrolysate, while control experiment showed low solid solubilization with 97.2% of solid residue remained. In addition, temperature and reaction time also affected the volume of clear hydrolysate due to the starch gel was formed during the gelatinization process. Low volumes of clear hydrolysate were observed in the experiment of low temperature (≤ 180 °C) and short reaction time (< 15 min) at runs 1, 5, and 7. On the other hand, under higher temperature (≥ 180 °C) and longer reaction time (≥ 15 min), large molecules like polysaccharides and starch gel were auto-hydrolyzed by hydronium ion into small molecules, mono- and oligo-saccharides resulted in a large volume of clear hydrolysate and low solid residue [25].
Clear hydrolysate contained monosaccharides, disaccharides, and oligosaccharides, which were auto-hydrolyzed and solubilized during LHW pretreatment of CP. Maltose and cellobiose were detected, derived from the hydrolysis of starch and cellulose. The amount of cellobiose and maltose in the clear hydrolysate was demonstrated in Fig. 2. Maltose was detected at the temperature of higher than 180 °C or reaction time of longer than 15 min. The amount of maltose ranged from 4 to 49 mg/gCP. The highest amount was observed in run no. 4 (194 °C, 26 min) followed by no.6 (200 °C, 15 min) and 8 (180 °C, 30 min) at 48, 36, and 13 mg/gCP, respectively. Most of the experimental runs contained maltose in the clear hydrolysate. While cellobiose was detected in only three operating runs (no. 4, 6 and 8). High temperature and long reaction time conditions were considered as the factors resulted in a higher amount of maltose and cellobiose since these conditions could hydrolyze more starch and cellulose structure. That assumption was supported by the absences of maltose and cellobiose in low temperature or short reaction time conditions, as shown in run nos. 1 and 7. The detection of cellobiose in clear hydrolysate at high-temperature condition was also reported by Yu, Wu [43], which reported that glucose and cellobiose were initially detected at 180 °C for LHW pretreatment of microcrystalline cellulose, and those amounts increased along with the increases of reaction temperature to 230 °C.
Figure 2 also illustrated the amount of monosaccharides in clear hydrolysate as the result of LHW pretreatment. Not only for maltose and cellobiose results but run no. 4 also found in the highest numbers of monosaccharides. The concentrations of glucose, xylose, galactose, L-arabinose, and mannose in run no. 4 were approximately 54, 6, 13, 10, and 13 mg/gCP, respectively. Glucose generated the highest proportion than the other monosaccharides because of the major component of starch and cellulose in CP. The increases in monosaccharides after pretreatment showed similar patterns to those in maltose and cellobiose, which increased along with the increase in the temperature and reaction time. It was shown by the experiment run nos. 3 and 4, which had a different temperature at 166 and 194 °C, respectively. The enormous amount of glucose and other sugar were shown in run no. 4, which were 14 and 3–4 times higher than those in run no. 3, respectively. Longer reaction time applied in run no. 8 (30 min) also resulted in a higher amount of monosaccharides, compared with that in the run no. 7 (0 min). L-arabinose was a sugar attached on the side chain of hemicellulose structure which is easy to breakdown and it contained in a small amount. The amounts of disaccharides and monosaccharides after pretreatment increased along with the increase in the temperature and reaction time. The glycosidic bonds were broken down by the increase in the hydronium ion generated from the auto-ionization under high temperature. In addition, other acids in the hemicellulose component may also contribute as another source of hydronium ion which were also able to break down the glycosidic bonds and hydrolyze polysaccharides into oligosaccharides or monosaccharides [44].
Oligosaccharides compounds were also detected in chromatogram during the determination of mono- and disaccharides by HPLC (data not shown). However, it was difficult to estimate the amount of oligosaccharides in the sample due to the limitation of the analysis method. In this study, the di- and oligosaccharides were converted into monosaccharides for HPLC detection and reported as the total sugar as shown in Fig. 3. The high amounts of glucose, xylose, galactose, and mannose were detected, while no L-arabinose was observed. It indicated that most of the sugar in the clear hydrolysate were in the form of oligosaccharides, where the amount of glucose was the highest (398–482 mg/gCP) than the other sugar. The highest amount of glucose (482 mg/gCP) was observed in run no. 2 (194 °C, 4 min) and the lowest (398 mg/gCP) in run no. 5 (160 °C, 15 min). The high amount of glucose in the clear hydrolysate was mainly produced because starch was the main component in CP and easily to be solubilized. Cellulose also contributed as the other source for the amount of glucose [43]. The total amount of monosaccharides, disaccharides, and oligosaccharides in the form of xylose, galactose, L-arabinose, and mannose increased as approximately 6, 10, 2 and 6 times higher than monosaccharide portion. Among these four sugar, the amount of galactose was the highest (52–73 mg/gCP) followed by mannose (26–35 mg/gCP), L-arabinose (12–21 mg/gCP), and xylose (7–8 mg/gCP). The LHW pretreatment was assumed to disrupt the cell wall component, particularly hemicellulose, and result in oligosaccharides. Similar results of oligosaccharides production from hemicellulose were reported by other studies, Yu et al. [45]. Yu et al. [28] also reported that the yields of xylose oligosaccharides from sorghum bagasse and eucalyptus wood chips were 185 and 74 mg/g substrate, respectively.
The increases in the amount of total sugar showed similar patterns with the results of disaccharides and monosaccharides. For instance, run nos.1 and 2, the amount of glucose highly increased from 418 to 482 mg/gCP, whereas xylose, galactose, L-arabinose, and mannose slightly increased. Other examples were run nos.1 and 3, which the amount of all sugar increased. However, elevated temperature and prolonged reaction time were not always increased the amount of sugar as shown in run nos. 2 and 4 which demonstrated the amount of glucose of 482 and 403 mg/gCP, respectively. With the same temperature of 180 °C, the different reaction time of 0, 15, and 30 min in run nos. 7, 8, and 9 illustrated the increased trends for the amount of glucose from 0 to 15 min, while it decreased at 30 min of reaction time. This result implied that sugar increased at a certain point of temperature and reaction time, while further increases in these parameters could not enhance the amount of sugar in the clear hydrolysate. The decreases in the amount of sugar under high temperature or long reaction time were caused by the degradation of sugar into by-products. C5-sugar (xylose and L-arabinose) and C6-sugar (glucose, galactose and mannose) were degraded into furfural (FF) and hydroxymethylfurfural (HMF), respectively. In addition, further degradations of FF and HMF can occur which converted them into levulinic acid or formic acid, respectively [25]. The conversion of sugar into FF and HMF was an unpleasant process due to loss of sugar yield. Moreover, these compounds had inhibitory and harmful effects on the microorganisms [46].
Optimization of LHW Pretreatment
The amount of total sugar in clear hydrolysate after LHW pretreatment of CP was used as a response in statistical analysis. Prior to the analysis of variance (ANOVA), the normality of data was tested (Table S1). Xylose data was not demonstrated the normal distribution, while other sugar was normally distributed. Since ANOVA was tested based on normal data distribution, xylose was transformed using Johnson transformation prior to the analysis. Formula and transformed data of xylose were shown in Table S2. Analysis of variance (ANOVA) of sugar was shown in Table S3-S7. Fisher’s statistical analysis (F-test) was used. The model of all sugar data explained all the variation in the response. From the statistical analysis, the temperature had a significant effect on the amount of sugar (especially sugar from hemicellulose structure) higher than the reaction time. Only xylose and mannose had a significant effect on reaction time. The interaction between temperature and reaction time had a significant effect on the amount of sugar except mannose. The breakdown of hemicellulose into sugar was affected by temperature and reaction time.
Lack-of-fit is an important term for data prediction, in which glucose and mannose passed and qualified for predicted optimum condition. The mathematical models of glucose and mannose in Eqs. 3 and 4 were shown as followed, where T is the temperature and t is the reaction time. R2 of glucose and mannose were 87 and 97%, respectively, where high R2 means obtained data highly fitted mathematical model, while adjust R2 were 77 and 94%, respectively, which adjust R2 mostly used to compare other mathematical models with a different number of factors.
Figures 4a–b show the contour plot and 3D surface plot between the temperature and the reaction time of glucose production under the optimum condition. The optimum conditions for the maximum glucose production were at the temperature of 187 °C and the reaction time of 7 min, which showed the predicted glucose value of 474 mg/gCP. The contour plot and 3D surface plot of mannose production were shown in Figs. 5 a–b. The optimum condition for mannose production was at the temperature of 200 °C and the reaction time of 23 min, in which the predicted value of mannose was 36 mg/gCP. The optimum temperature and reaction time for the mannose production was higher than those for glucose production. High temperature and long reaction time were required to completely breakdown hemicellulose into soluble forms. To release all starch from the cell wall, hemicellulose must be started to be cracking at the temperature of 160 °C [47]. Several studies also reported that high temperature and long reaction time were required for breaking down the hemicellulose from wheat straw (184 °C for 24 min) [27] and sugarcane bagasse (180 °C for 20 min) [26].
Experimental Validation
Since the optimum condition (the temperature and the reaction time) for LHW pretreatment of CP to produce glucose and mannose were completely different, glucose was selected. Because glucose was the main monosaccharide found in all experimental runs and also the simple carbon source for all kinds of microbial growth and production of valuable products. The optimization of glucose production was chosen for the further study of BMP. The validation of the predicted optimum condition for CP pretreatment was carried out at 187 °C for 7 min.
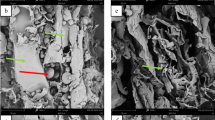
The remained solid residue after LHW pretreatment was approximately 22% TS. SEM revealed the structural morphologies of pretreated CP compared with non- pretreated CP as shown in Fig. 6. For non-pretreated CP, starch granules were seen and trapped inside the cell wall structure. Smooth surface without cracking or disruption of the cell wall was observed (Fig. 6a-b). However, no entrapping starch granules were observed in the cell wall of pretreated CP due to the gelatinization of starch. SEM illustrated the appearance of disruption and breakdown of an intact cell wall structure of pretreated CP (Fig. 6c). The hydrolysis of hemicellulose and cellulose by LHW pretreatment might cause the cracking appearances on the cell wall structure. Starch, cellulose, hemicellulose, and lignin contents in solid residue before and after CP pretreatment were shown in Fig. 7. Starch and hemicellulose were mostly removed from pretreated CP residue compared with non-pretreated CP, in which it was reduced from 47.9 to 0.1% and 8.1 to 1.7% (based on TS of non-pretreated CP), respectively. Starch and hemicellulose highly hydrolyzed from pretreated CP into aqueous phase and results will discuss in part of hydrolysate fraction. However, cellulose and lignin were partially hydrolyzed from pretreated CP. Cellulose and lignin were slightly reduced from 20.9 to 14.2% and 3.4 to 2.2%, respectively.
Along with the increases in the temperature and the reaction time of LHW pretreatment, starch was firstly hydrolyzed from CP followed by the hydrolysis of hemicellulose and cellulose. It was considered by the complexity of their structures. Cellulose remained as the main component in solid residue. The form of amorphous and crystalline cellulose was determined by X-ray diffraction and represented in term of the crystallinity index (CrI). The X-ray diffraction pattern of pretreated CP showed a spike at 22°, which was higher than that of non-pretreated CP (Fig. S1). Both samples showed a similar intensity of the amorphous region. CrI value of non-pretreated CP and pretreated CP were approximately 21.2 and 46.3%, respectively. The crystalline region of cellulose in pretreated CP was higher twofold than that in non-pretreated CP. Increase in CrI after LHW pretreatment was caused by the removal of starch, hemicellulose, amorphous cellulose, and lignin components leaving the high amount of crystalline cellulose in the solid residue. The increases in CrI after LHW pretreatment were also observed in other LHW-pretreated biomass, such as sugarcane bagasse [26, 45]. Due to the complexity and the strong interaction of chemical bonds of cellulose structure, which required a high temperature of 200 °C and long reaction time of 30 min to break it down [43], the temperature range of 160–200 °C and reaction time range of 0–30 min could partially or completely breakdown hemicellulose into soluble saccharides due to the baffling structures of hemicellulose. This result was similar to another study [45]. They reported that 86.5% of hemicellulose were removed from sugarcane bagasse at elevated temperature, while high content of cellulose remained in the solid residue [48]. The disruption of intact cell wall structure (Fig. 6c) and removals of organic compounds (starch, hemicellulose, amorphous cellulose, and lignin components) caused the increase of enzyme accessibility to the cellulose structure resulting in the enhancement of the enzymatic hydrolysis of cellulose [45].
In clear hydrolysate fraction after pretreatment of CP, sugar in form of disaccharides were observed maltose at 4 mg/gCP, while no cellobiose was detected, in which monosaccharides form as glucose, xylose, galactose, L-arabinose, and mannose were found at 4, 2, 4, 9 and 2 mg/gCP, respectively. In addition, total sugar in form of monosaccharides was determined (Fig. S2). The total amount of glucose was 444 mg/gCP, which was 6% lower than the predicted value. The amount of solubilized glucose in the clear hydrolysate was calculated as 78% of total glucose in CP. Glucose in the clear hydrolysate was mostly hydrolyzed from starch (99.8% reduction, calculated from Fig. 7), which was released from the cell wall structure after LHW pretreatment as revealed by Fig. 6c and that from partially hydrolysis from hemicellulose and cellulose at 79.0 and 32.1% reduction (calculated from Fig. 7) were determined. It was mainly derived from starch, hemicellulose, and cellulose which referred to the hydrolysis of those compounds from the solid residues and solubilization into hydrolysate. Furthermore, the amount of xylose, galactose, L-arabinose, and mannose in the clear hydrolysate was also observed at 28, 113, 35 and 19 mg/gCP, respectively. High total sugar recovery from LHW pretreatment was observed comparing with dilute acid pretreatment (DAP) on CP [9]. Sugar composition in hydrolysate after DAP contained glucose, xylose, and L-arabinose at 201, 175 and 35 mg/gCP, respectively, which glucose was twice lower than this study, because DAP can cause further degradation of sugar in to furan derivatives and lead to loss of sugar yield.
The amounts of furfural (FF) and hydroxymethylfurfural (HMF) at optimum condition were 31 and 32 μg/mL, representing to 0.53 and 0.52 mg of FF and HMF per 1 g of pretreated CP, respectively. Optimum conditions of high temperature and long reaction time for LHW pretreatment did not only increase the number of organic compounds in clear hydrolysate but also increased the concentrations of inhibitors as found in the study of López González et al. [49]. It was reported that the highest concentration of sCOD was observed at 200 °C for 5 min of reaction time, followed by 150 °C for 20 min condition. Even though higher sCOD concentration was obtained, lower yield of methane at pretreatment condition of 200°C for 5 min was mirrored by the presence of FF and HMF at 1.21 and 0.20 mg/mL, respectively. The concentration of inhibitors was considered as the important criteria for the utilization of clear hydrolysate. The high concentration of inhibitors prolonged the lag phase of microorganism growth and was toxic to the microorganisms by inhibiting the microbial enzyme, damage the cell membrane, or disrupt the cell osmolarity, which then led to cell death and reduced the maximum methane production [46]. An inhibitory effect of FF and HMF on fermentative microorganisms was presented at a concentration of more than 1 mg/mL [50,51,52]. To overcome that problem, the detoxification step must be applied before the utilization of clear hydrolysate as the substrate in the fermentation process. However, with a low concentration of inhibitors, clear hydrolysate could be directly used in the fermentation process with no or minor adverse effects on the microorganisms.
Biogas and Methane Production
Pretreated CP contained two fractions of solid residue (SR) and clear hydrolysate (CH). In this study, pretreated CP named mixture (MX), SR, and CH at optimum condition were observed for their biochemical methane potential (BMP). The cumulative biogas and methane production obtained from the AD of those pretreated CP and non-pretreated CP were monitored during the 60 d experimental period as illustrated in Fig.8. No lag times of non-pretreated CP and three samples of pretreated CP were observed. To determine the effect of pretreatment on biogas and methane production, MX (pretreated CP) was compared with non-pretreated CP. No inhibitory effects of FF and HMF (31–32 μg/mL in CH) after LHW pretreatment was found and observed high methane production rate without lag time. During 10 d of the initial stage (Fig. 8a), higher cumulative biogas production from MX was observed (220 NmL) compared with non-pretreated CP (179 NmL). The methane content of MX was sharply increased up to 61.2%, while non-pretreated CP was to 52.2%. The rapid increase of cumulative methane production in MX was reflected by increased biogas production and methane content compared with non-pretreated CP. The cumulative of methane production of MX and non-pretreated CP were 112 and 87 NmL, respectively (Fig. 8b). After 10 d of incubation time, biogas and methane production from MX was slightly increased and reached to steady state, while that from non-pretreated CP still sharply increase until to d 22 and then slightly increased. The methane content of MX and non-pretreated CP were up to 62.1 and 54.4%, respectively. The cumulative biogas and methane production at d 60 of MX were 247 and 133 NmL, while that of non-pretreated CP were 238 and 123 NmL, respectively. The accumulated maximum biogas production from MX was observed within 10 d (220 NmL) compared with non-pretreated CP within 22 d (220 NmL) (Fig. 8a), which corresponded to the accumulated methane production of 112 NmL and 110 NmL, respectively (Fig. 8b). The AD times for maximum production of methane from MX and non-pretreated CP were approximately 10 and 22 d, respectively. It was illustrated that pretreatment of CP accelerated higher production of methane within a shorter time compared with non-pretreated CP.
Different trends of biogas and methane production from two fractional forms of LHW pretreated CP (CH and SR) were observed (Fig. 8). Biogas and methane production from CH were rapidly increased at the beginning of AD, while that from SR were gradually increased as shown in Figs. 8a and b, respectively. Considering AD of pretreated CP (MX) at d 10, it was demonstrated that total biogas and methane production were mainly from CH (72.6 and 70.7%) compared with SR (18.7 and 17.7%) due to the high availability of soluble sugar in CH. The higher methane production from CH than that from SR could be caused by the soluble organic compounds in those samples. CH contained high amounts of soluble saccharides, which microorganisms could easily degrade and consume. On the other hand, lignocellulose was the main component in solid residue, which was recalcitrant to be digested. A similar profile of biogas and methane production of MX was according to CH and SR. After 10 d until the end of AD (d 60), cumulative biogas and methane production of SR were slightly higher shared (18.7 to 22.4% and 17.7 to 21.3%) in MX, while that of CH were stable after d 8–10.
The kinetic parameters for batch-methane production of pretreated CP compared with non-pretreated CP during 60 d were shown in Table 3. A modified-Gompertz equation was used to fit the curve for the determination of methane production potential, the maximum methane production rate and methane yield with R2 greater than 98%. From the kinetic analysis, a close relationship between the experimental data and the Gompertz model can be seen, with a coefficient of determination (R2) of > 0.98. Results indicated that pretreatment (MX) significantly affected the cumulative methane production, methane production rate, and methane yield in comparison to non-pretreated CP without pretreatment. The AD of MX to methane was completed within 10 d, whereas non-pretreated CP took 22 d to reach steady state. In addition, fractions of CH and SR were completed within 10 and 22 d, respectively.
Significant methane production potentials from different samples were shown at 95% significant level (α = 0.05). LHW-pretreated CP (MX) showed the highest of the maximum cumulative methane production rate of 18 NmL/d compared with non-pretreated CP (11 NmL/d) which resulted to the methane production potential of MX (131 NmL within 10 d) and non-pretreated CP (123 NmL within 22 d). Methane production potential of CH (87 NmL within 8 d) was majorly shared in MX that boost total methane production of MX, whereas methane production from SR was only 28 NmL within 22 d. The CH contained easily digestible organic compounds, such as soluble oligo-, di-, and mono-saccharides, which could contribute to the higher methane production than SR fraction. On the other hand, SR contained difficult digestible organic compounds (lignocellulosic compounds). According to those characteristics, the maximum methane production rate of CH (13 NmL/d) was faster than that of SR (2 NmL/d). Methane yield from different samples also showed significant different (α = 0.05), where MX had the highest methane yield of 328 NmL/gVSadded within 10 d at maximum methane production rate 18 NmL/d. Non-pretreated CP showed the methane yield of 243 NmL/gVSadded within 22 d and the maximum methane production rate of 11 NmL/d, which were lower compared to that in MX. The results showed that the maximum rates, production potential and yield of methane from MX were 1.6-, 1.2-, and 1.4-fold higher than non-pretreated CP, respectively, with a shorter time for methane production. In addition, methane yields of CH and SR were 216 and 70 NmL/gVSadded, respectively. The pretreated CP (MX) could be directly used in AD after LHW pretreatment. These results indicated that LHW pretreatment of CP resulted in the breakdown of cell wall structure, autohydrolysis, and solubilization of polysaccharides in CP was able to increase the maximum methane production rate that resulted to accelerate the methane production and the maximum methane yield. The methane production from LHW pretreated CP while raising the reaction rate of methane production and shortening AD time to obtain high methane yield. There were some advantages of using LHW at 187 °C for 7 min as a pretreatment method for CP to produce methane: (i) with a less extra heat source, it can use excess heat from cassava starch drying processing and (ii) with less extra payment and saving operating cost for pretreatment process.
The methane production and yield from CP depend on its characteristics, especially the amounts of easily digestible compounds such as starch, might contribute to those different results. Panichnumsin et al. [40] reported methane yield of non-pretreated CP was 344 NmL/gVSadded. While this study was lower because of less amount of starch content in CP from the program of waste minimization and cleaner production for starch recovery. The application of LHW pretreatment for several types of biomass feedstock and conversion to methane by AD was also conducted by the other studies (Table 4). The methane yield from LHW pretreated CP at 187 °C for 7 min was 35% higher than non-pretreated CP. It was illustrated that LHW pretreatment of lignocellulosic biomass feedstock promotes to increase methane yield in the range of 23–63% compared with non-pretreated feedstock. Song et al. [53] reported that the highest reducing sugar and maximum methane yield were obtained from LHW pretreatment of corn stover (100 °C for 30 min). A similar finding was also reported by other studies that used LHW pretreatment for Sida hermaphrodita [54], sugarcane press mud [49], and poultry slaughterhouse waste sludge [55]. The increase in the soluble organic compounds (in term of soluble chemical oxygen demand (sCOD) or reducing sugar) in hydrolysate could increase the maximum methane yield compared to the non-pretreated feedstock.
Mass balance of non-pretreated CP and LHW pretreated CP were calculated by base on 1 kgVS of CP and illustrated in Fig. 9. After LHW pretreatment of 1 kgVS of CP, 0.965 kgVS of the mixture (0.718 kgVS of clear hydrolysate and 0.247 kgVS of solid residue) was obtained. By that, sugar derived from these components was mainly found. A small amount of cellulose and lignin were solubilized. The other 0.035 kgVS or 3.5% was loss during LHW pretreatment. Consequently, methane production of LHW pretreated CP was 317 NL/kgVS, which was higher than 30% compared with non-pretreated CP.
Conclusions
Lignocellulosic CP was pretreated by LHW with free chemical usage prior to bio-conversion into methane. The reaction temperature and time as the main factors for LHW conditions were studied. LHW pretreatment of CP enhanced the releasing of cell wall-entrapped starch, the solubilization of starch and hemicellulose, and the partial hydrolysis of the cell wall components (cellulose and lignin) in form of oligo- and mono-saccharides in clear hydrolysate. Glucose was found as a major sugar component, mostly derived from the hydrolysis of starch and a small amount from the lignocellulosic component. After LHW pretreatment, starch and hemicellulose were greatly removed from CP, while cellulose and lignin remained in the solid residue. The increasing of soluble sugar in hydrolysate and more disruption in the lignocellulosic matrix of a cell wall structure from pretreatment resulted in higher methane production yield and maximum methane production rate comparing to non-pretreated CP. With the low generation of inhibitors (FF and HMF) during pretreatment, no inhibitory effect and lag time during AD were observed.
References
Thai tapioca development institute (2020) Trade statistics of tapioca starch export volume. https://www.tapiocathai.org/English/M2_e.html. Accessed 25 January 2020
Srinophakun T, Suriyapraphadilok U, Tia S (2000) Water-wastewater management of tapioca starch manufacturing using optimization technique. ScienceAsia 26:57–67. https://doi.org/10.2306/scienceasia1513-1874.2000.26.057
Trakulvichean S, Chaiprasert P, Otmakhova J, Songkasiri W (2017) Comparison of fermented animal feed and mushroom growth media as two value-added options for waste cassava pulp management. Waste Manag Res 35(12):1210–1219. https://doi.org/10.1177/0734242X17730135
Ubalua AO (2007) Cassava wastes: treatment options and value addition alternatives. Afr J Biotechnol 6(18):2065–2073. https://doi.org/10.5897/AJB2007.000-2319
Elemike EE, Oseghale OC, Okoye AC (2015) Utilization of cellulosic cassava waste for bio-ethanol production. J Environ Chem Eng 3(4, part a):2797–2800. https://doi.org/10.1016/j.jece.2015.10.021
Virunanon C, Ouephanit C, Burapatana V, Chulalaksananukul W (2013) Cassava pulp enzymatic hydrolysis process as a preliminary step in bio-alcohols production from waste starchy resources. J Clean Prod 39:273–279. https://doi.org/10.1016/j.jclepro.2012.07.055
Rattanachomsri U, Tanapongpipat S, Eurwilaichitr L, Champreda V (2009) Simultaneous non-thermal saccharification of cassava pulp by multi-enzyme activity and ethanol fermentation by Candida tropicalis. J Biosci Bioeng 107(5):488–493. https://doi.org/10.1016/j.jbiosc.2008.12.024
Sriroth K, Chollakup R, Chotineeranat S, Piyachomkwan K, Oates CG (2000) Processing of cassava waste for improved biomass utilization. Bioresour Technol 71(1):63–69. https://doi.org/10.1016/S0960-8524(99)00051-6
Phowan P, Danvirutai P (2014) Hydrogen production from cassava pulp hydrolysate by mixed seed cultures: effects of initial pH, substrate and biomass concentrations. Biomass Bioenergy 64:1–10. https://doi.org/10.1016/j.biombioe.2014.03.057
Chaikaew S, Maeno Y, Visessanguan W, Ogura K, Sugino G, Lee S-H, Ishikawa K (2012) Application of thermophilic enzymes and water jet system to cassava pulp. Bioresour Technol 126:87–91. https://doi.org/10.1016/j.biortech.2012.09.020
Poonsrisawat A, Wanlapatit S, Paemanee A, Eurwilaichitr L, Piyachomkwan K, Champreda V (2014) Viscosity reduction of cassava for very high gravity ethanol fermentation using cell wall degrading enzymes from Aspergillus aculeatus. Process Biochem 49(11):1950–1957. https://doi.org/10.1016/j.procbio.2014.07.016
Glanpracha N, Annachhatre AP (2016) Anaerobic co-digestion of cyanide containing cassava pulp with pig manure. Bioresour Technol 214:112–121. https://doi.org/10.1016/j.biortech.2016.04.079
Sugumaran KR, Jothi P, Ponnusami V (2014) Bioconversion of industrial solid waste—cassava bagasse for pullulan production in solid state fermentation. Carbohydr Polym 99:22–30. https://doi.org/10.1016/j.carbpol.2013.08.039
Shi X, Chen Y, Ren H, Liu D, Zhao T, Zhao N, Ying H (2014) Economically enhanced succinic acid fermentation from cassava bagasse hydrolysate using Corynebacterium glutamicum immobilized in porous polyurethane filler. Bioresour Technol 174:190–197. https://doi.org/10.1016/j.biortech.2014.09.137
Suwanasri K, Trakulvichean S, Grudloyma U, Songkasiri W, Commins T, Chaiprasert P, Tanticharoen M (2015) Biogas–key success factors for promotion in Thailand. JSEE 25(Special Issue):25–30
Li Y, Park SY, Zhu J (2011) Solid-state anaerobic digestion for methane production from organic waste. Renew Sust Energ Rev 15(1):821–826. https://doi.org/10.1016/j.rser.2010.07.042
Hansupalak N, Piromkraipak P, Tamthirat P, Manitsorasak A, Sriroth K, Tran T (2016) Biogas reduces the carbon footprint of cassava starch: a comparative assessment with fuel oil. J Clean Prod 134(part B):539–546. https://doi.org/10.1016/j.jclepro.2015.06.138
Tran T, Da G, Moreno-Santander MA, Vélez-Hernández GA, Giraldo-Toro A, Piyachomkwan K, Sriroth K, Dufour D (2015) A comparison of energy use, water use and carbon footprint of cassava starch production in Thailand, Vietnam and Colombia. Resour Conserv Recycl 100:31–40. https://doi.org/10.1016/j.resconrec.2015.04.007
Peral C (2016) Biomass pretreatment strategies (technologies, environmental performance, economic considerations, industrial implementation). In: Poltronieri P, D’Urso OF (eds) Biotransformation of Agricultural Waste and By-Products. Elsevier Inc., pp 125–160. https://doi.org/10.1016/B978-0-12-803622-8.00005-7
McMullen WH, Andino R (1977) Production of high purity glucose syrups. United States of America patent US4017363 a,
Hurst LS, Lloyd NE (1983) Process for producing glucose/fructose syrups from unrefined starch hydrolysates. United States of America patent US4376824 a,
Divya Nair MP, Padmaja G, Moorthy SN (2011) Biodegradation of cassava starch factory residue using a combination of cellulases, xylanases and hemicellulases. Biomass Bioenergy 35(3):1211–1218. https://doi.org/10.1016/j.biombioe.2010.12.009
Bhutto AW, Qureshi K, Harijan K, Abro R, Abbas T, Bazmi AA, Karim S, Yu G (2017) Insight into progress in pre-treatment of lignocellulosic biomass. Energy 122:724–745. https://doi.org/10.1016/j.energy.2017.01.005
Ruiz HA, Conrad M, Sun S-N, Sanchez A, Rocha GJM, Romaní A, Castro E, Torres A, Rodríguez-Jasso RM, Andrade LP, Smirnova I, Sun R-C, Meyer AS (2020) Engineering aspects of hydrothermal pretreatment: from batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour Technol 299:122685. https://doi.org/10.1016/j.biortech.2019.122685
Hu F, Ragauskas A (2012) Pretreatment and Lignocellulosic chemistry. Bioenergy Res 5(4):1043–1066. https://doi.org/10.1007/s12155-012-9208-0
Yu Q, Zhuang X, Lv S, He M, Zhang Y, Yuan Z, Qi W, Wang Q, Wang W, Tan X (2013) Liquid hot water pretreatment of sugarcane bagasse and its comparison with chemical pretreatment methods for the sugar recovery and structural changes. Bioresour Technol 129:592–598. https://doi.org/10.1016/j.biortech.2012.11.099
Pérez JA, Ballesteros I, Ballesteros M, Sáez F, Negro MJ, Manzanares P (2008) Optimizing liquid hot water pretreatment conditions to enhance sugar recovery from wheat straw for fuel-ethanol production. Fuel 87(17–18):3640–3647. https://doi.org/10.1016/j.fuel.2008.06.009
Yu Q, Zhuang X, Wang Q, Qi W, Tan X, Yuan Z (2012) Hydrolysis of sweet sorghum bagasse and eucalyptus wood chips with liquid hot water. Bioresour Technol 116:220–225. https://doi.org/10.1016/j.biortech.2012.04.031
Wan C, Zhou Y, Li Y (2011) Liquid hot water and alkaline pretreatment of soybean straw for improving cellulose digestibility. Bioresour Technol 102(10):6254–6259. https://doi.org/10.1016/j.biortech.2011.02.075
Sluiter A, Hames B, Hyman D, Payne C, Ruiz R, Scarlata C, Sluiter J, Templeton D, Wolfe J (2008) Determination of total solids in biomass and total dissolved solids in liquid process samples. National Renewable Energy Laboratory. https://www.nrel.gov/docs/gen/fy08/42621.pdf. Accessed 3 May 2018
Thiex NJ, Anderson S, Gildemeister B (2003) Crude fat, diethyl ether extraction, in feed, cereal grain, and forage (Randall/Soxtec/submersion method): collaborative study. J AOAC Int 86(5):888–898
Thiex NJ, Manson H, Anderson S, Persson J-Å (2002) Determination of crude protein in animal feed, forage, grain, and oilseeds by using block digestion with a copper catalyst and steam distillation into boric acid: collaborative study. J AOAC Int 85(2):309–317
McCleary BV, Gibson TS, Mugford DC (1997) Measurement of total starch in cereal products by amyloglucosidase-a-amylase method: collaborative study. J AOAC Int 80(3):571–579
Van Soest PV, Robertson J, Lewis B (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74(10):3583–3597
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3(1):10. https://doi.org/10.1186/1754-6834-3-10
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29(10):786–794. https://doi.org/10.1177/004051755902901003
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. Nat Renew Energy Lab 1617:1–16
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2006) Determination of sugars, byproducts, and degradation products in liquid fraction process samples. National Renewable Energy Laboratory. https://www.nrel.gov/docs/gen/fy08/42623.pdf. Accessed 3 May 2018
Paepatung N, Nopharatana A, Songkasiri W (2009) Bio-methane potential of biological solid materials and agricultural wastes. As J Energy Env 10(1):19–27
Panichnumsin P, Nopharatana A, Ahring B, Chaiprasert P (2010) Production of methane by co-digestion of cassava pulp with various concentrations of pig manure. Biomass Bioenergy 34(8):1117–1124. https://doi.org/10.1016/j.biombioe.2010.02.018
Lo H, Kurniawan T, Sillanpää M, Pai T, Chiang C, Chao K, Liu M, Chuang S, Banks C, Wang S, Lin K, Lin C, Liu W, Cheng P, Chen C, Chiu H, Wu H (2010) Modeling biogas production from organic fraction of MSW co-digested with MSWI ashes in anaerobic bioreactors. Bioresour Technol 101(16):6329–6335. https://doi.org/10.1016/j.biortech.2010.03.048
Saengchan K, Nopharatana M, Lerdlattaporn R, Songkasiri W (2015) Enhancement of starch-pulp separation in centrifugal-filtration process: effects of particle size and variety of cassava root on free starch granule separation. Food Bioprod Process 95:208–217. https://doi.org/10.1016/j.fbp.2015.05.008
Yu Y, Wu H (2010) Significant differences in the hydrolysis behavior of amorphous and crystalline portions within microcrystalline cellulose in hot-compressed water. Ind Eng Chem Res 49(8):3902–3909. https://doi.org/10.1021/ie901925g
Arenas-Cárdenas P, López-López A, Moeller-Chávez GE, León-Becerril E (2017) Current pretreatments of Lignocellulosic residues in the production of bioethanol. Waste Biomass Valoriz 8(1):161–181. https://doi.org/10.1007/s12649-016-9559-4
Yu Q, Zhuang X, Wang W, Qi W, Wang Q, Tan X, Kong X, Yuan Z (2016) Hemicellulose and lignin removal to improve the enzymatic digestibility and ethanol production. Biomass Bioenergy 94:105–109. https://doi.org/10.1016/j.biombioe.2016.08.005
Jung YH, Kim KH (2015) Acidic Pretreatment. In: Pandey A, Negi S, Binod P, Larroche C (eds) Pretreatment of biomass: processes and technologies. Elsevier B.V, Amsterdam, pp 26–50. https://doi.org/10.1016/B978-0-12-800080-9.00003-7
Tsoutsos T, Bethanis D (2011) Optimization of the dilute acid hydrolyzator for cellulose-to-bioethanol saccharification. Energies 4(10):1601–1623. https://doi.org/10.3390/en4101601
Jiang D, Ge X, Zhang Q, Li Y (2016) Comparison of liquid hot water and alkaline pretreatments of giant reed for improved enzymatic digestibility and biogas energy production. Bioresour Technol 216:60–68. https://doi.org/10.1016/j.biortech.2016.05.052
López González LM, Pereda Reyes I, Dewulf J, Budde J, Heiermann M, Vervaeren H (2014) Effect of liquid hot water pre-treatment on sugarcane press mud methane yield. Bioresour Technol 169:284–290. https://doi.org/10.1016/j.biortech.2014.06.107
Haroun BM, Nakhla G, Hafez H, Nasr FA (2016) Impact of furfural on biohydrogen production from glucose and xylose in continuous-flow systems. Renew Energy 93:302–311. https://doi.org/10.1016/j.renene.2016.02.072
van der Pol EC, Bakker RR, Baets P, Eggink G (2014) By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio)chemicals and fuels. Appl Microbiol Biotechnol 98(23):9579–9593. https://doi.org/10.1007/s00253-014-6158-9
Akobi C, Hafez H, Nakhla G (2017) Impact of furfural on biological hydrogen production kinetics from synthetic lignocellulosic hydrolysate using mesophilic and thermophilic mixed cultures. Int J Hydrog Energy 42(17):12159–12172. https://doi.org/10.1016/j.ijhydene.2017.03.173
Song X, Wachemo AC, Zhang L, Bai T, Li X, Zuo X, Yuan H (2019) Effect of hydrothermal pretreatment severity on the pretreatment characteristics and anaerobic digestion performance of corn Stover. Bioresour Technol 289:121646. https://doi.org/10.1016/j.biortech.2019.121646
Zieliński M, Kisielewska M, Dudek M, Rusanowska P, Nowicka A, Krzemieniewski M, Kazimierowicz J, Dębowski M (2019) Comparison of microwave thermohydrolysis and liquid hot water pretreatment of energy crop Sida hermaphrodita for enhanced methane production. Biomass Bioenergy 128:105324. https://doi.org/10.1016/j.biombioe.2019.105324
Park S, Yoon Y-M, Han SK, Kim D, Kim H (2017) Effect of hydrothermal pre-treatment (HTP) on poultry slaughterhouse waste (PSW) sludge for the enhancement of the solubilization, physical properties, and biogas production through anaerobic digestion. Waste Manag 64:327–332. https://doi.org/10.1016/j.wasman.2017.03.004
Acknowledgments
The author was supported by the “Scholarship for outstanding students” from School of Bioresources and Technology and KMUTT research funding. The author would like to thank Excellent Center of Waste Utilization and Management (ECoWaste), Integrative biorefinery laboratory (IBL) from Joint Graduate School of Energy and Environment (JGSEE), and Enzyme Laboratory from SBT, KMUTT for the equipment in the experiment and technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of Interests
All authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 65 kb)
Rights and permissions
About this article
Cite this article
Varongchayakul, S., Songkasiri, W. & Chaiprasert, P. Optimization of Cassava Pulp Pretreatment by Liquid Hot Water for Biomethane Production. Bioenerg. Res. 14, 1312–1327 (2021). https://doi.org/10.1007/s12155-020-10238-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10238-0