Abstract
The sugarcane bagasse is a heterogeneous material and needs a pretreatment to breakdown its complex structure to make cellulose accessible to enzyme action. This study aimed to evaluate pseudo-lignin formation, enzymatic hydrolysis of sugarcane fractions (leaf, external fraction, internode, and node), and bagasse after partial delignification and acid pretreatment. The leaf and external fraction presented the highest content of lignin, and external fraction was the most recalcitrant material resulting in lower glucose release. Pretreatment with diluted sulfuric acid (20% m/m or 2% m/v) generated 5 g/L of acetic acid and 2.07 g/L of 5-hydroxymethylfurfural (external fraction in natura and leaf extractive-free, respectively). Furfural ranged between 0.11 g/L (node delignified) and 0.06 g/L (leaf, external fraction, and node in natura). A decrease was observed in pseudo-lignin formed with extractive-free and delignified biomasses, with different structure compared with non-delignified samples. The biomass partial delignification and subsequent pretreatment with dilute acid generate a material with fragmented anatomical structure, with improved cellulose accessibility, favoring enzymatic hydrolysis achieving more than 90% of glucose yield (up to 12 g/L). This study has shown strategies to decrease material heterogeneity and avoid pseudo-lignin formation as it results in lower recalcitrance and better efficiency of the enzymatic hydrolysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The high demand for energy has led to the consumption of large quantities of fossil fuels, which have raised environmental concerns along with energy security issues. Global climate change caused by greenhouse gas emissions stimulated researchers to develop alternative fuels based on sustainable resources. Agro-energy crops and plant residues are the most promising, sustainable, low-cost feedstock for biofuel production and energy co-generation. Given the concern for sustainability and the need to maximize the use of natural resources, the use of sugarcane bagasse is receiving significant attention in biorefining applications as it is a promising resource for conversion into biofuels such as second-generation ethanol and co-generation [1]. However, the sugarcane biomass is heterogeneous with different tissue organization (anatomic fractions) and recalcitrance [2, 3].
Brazil is the main producer and consumer of sugarcane in the world. In 2018, Brazilian production was 746 million tons, while India, the world’s second largest producer, cultivated 376 million tons [4]. Large amounts of bagasse are generated in ethanol and sugar industries. One of the main challenges in the application of lignocellulosic biomass is the conversion of the complex polysaccharides into fermentable sugars, which can serve as a source of carbon for microbial fermentation process. Two steps are required to produce fermentable sugars: pretreatment and enzymatic hydrolysis. The purpose of some types of lignocellulosic material pretreatment is to remove hemicellulose and lignin, reducing recalcitrance (biomass resistance to fragmentation) and increasing the accessibility of cellulose for enzymatic hydrolysis, which is the most critical step in the biomass conversion process [5].
Lignin-like compounds (pseudo-lignin) can be formed under conditions of high severity in hydrothermal/acid pretreatments. This occurs due to the formation of carbohydrate degradation products that can condense with lignin fragments generating pseudo-lignin [6, 7]. These unwanted products such as sugars and lignin degradation compounds are inhibitors of the fermentation process [8]. High concentrations of inhibitors, like acetic acid, furfural, and 5-hydroxymethylfurfural (HMF), negatively influence ethanol production. Acetic acid is ubiquitous in the hydrolysate, so it is desirable for the fermenting microorganism to be tolerant to this compound. Even at low concentrations (e.g., 5 g/L), these acid products affect the growth and fermentable productivity of both gram-negative and gram-positive bacteria. Acetic acid may inhibit biotechnological processes such as vinification or fermentation of biomass hydrolysate in which Saccharomyces cerevisiae plays a central role. This yeast can use acetate as a source of carbon and energy; however, in a concentrated glucose medium, the enzymes required for acetate catabolism are strongly suppressed and acetic acid becomes a stressor. In general, the toxic effects are like those in bacteria, which enters the cell cytosol inhibiting the activity of metabolic enzymes, inducing oxidative stress, and depleting ATP cells [9].
In the dilute acid pretreatment, condensation between HMF and furfural occurs when the monomer products of lignin degradation condense and precipitate on the fiber surface. HMF and furfural are results of pentose and hexose dehydration, respectively [6, 10], while the degradation of lignin forms monomers p-coumarilic alcohol, p-hydroxyphenyl predecessor (H), trans-coniferyl alcohol, guaiacyl predecessor (G), and trans-synaptic alcohol, predecessor of syringyl (S) [11]. HMF and furfural are known as key intermediates to form pseudo-lignin, which hinder enzymatic hydrolysis of the pretreated material [12]. High temperatures, low pH, and oxygen presence were found to be crucial conditions for pseudo-lignin formation [6]. Pseudo-lignin can be broadly defined as an aromatic material that is not derived from native lignin, consisting of carbonyl, carboxylic, aromatic, and aliphatic structures that, in an acidic medium, condense and precipitate on the biomass fibers. Its presence produces a positive value of Klason’s lignin and also inhibits significantly the enzymatic hydrolysis of cellulose [12].
Lignin acts as a natural glue for cellulosic fibers and is produced by enzyme-mediated radical coupling from three monolignols. The syringyl unit (S) has two methoxy groups; the guaiacyl unit (G) has a methoxy group, while the third monolignol contains a p-hydroxyphenylpropane unit (H). The syringyl to guaiacyl ratio (S/G) is a significant parameter in delignification processes and is also important in determining the amount of ethanol that can be obtained from the fermentation of hydrolyzed biomass. Studies have shown a significant decrease in lignin content with an increase in S/G ratio. The xylose yield was also high with an increase in S/G ratio in some types of biomass. Therefore, an increase in ethanol production would be expected with an increase in the S/G ratio [11].
Lignin modification or removal increases the digestibility of the plant cell wall. In fact, selection of sugarcane varieties with low lignin content is a strategy to generate a low recalcitrance biomass [13]. Recalcitrance in grasses varies according to cell type and maturation. It was observed that enzymatic digestibility can occur unevenly in different regions of the same internode. The outermost fraction and the shell are more recalcitrant, while the pith-rind interface and the pith are more digestible, with an inversely proportional correlation between the area occupied by vascular bundles and the efficiency of cellulose hydrolysis [14]. Studies conducted with 11 sugarcane hybrids revealed that the conversion of glucan to glucose by commercial cellulase was increased in samples with low lignin content, and chemical delignification increased the conversion to values above 80%. In general, experimental hybrids with low lignin content showed the highest digestibility [15, 16].
Understanding the lignin contribution on the recalcitrance of sugarcane biomasses/tissue is essential in the biomass conversion into biofuels and chemicals. Lignin and pseudo-lignin limits enzymatic hydrolysis by inhibiting cellulase enzymes [10, 17]. Due to differences in chemical composition, inhibition may have a different impact between sugarcane fractions (node, internode, leaf, external fraction, and bagasse) [2]. Therefore, this study evaluated the behavior of sugarcane fractions exposed to diluted acid pretreatment with prior partial delignification, presence of inhibitors, and pseudo-lignin formation in enzymatic hydrolysis glucose yield.
Material and Methods
Sugarcane Sample Preparation
Sugarcane biomasses (culm, leaf, and bagasse) harvested manually without burning leaves were kindly donated by Sugarcane Technology Center (CTC-Piracicaba, SP, Brazil). Sugarcane bagasse was received after juice extraction at the refinery and was successively submerged in distilled water, with constant water renewal for 3 days, in order to remove sucrose.
The biomasses (external fraction, node, and internode) were separated from the culm of the plant, as described elsewhere [2, 14]. The external fraction (containing epidermis) was separated by hand cutting using a stainless-steel knife to obtain a 2-mm-thick strips. Epidermis-free culms were subsequently manually cut with a stainless-steel knife into transverse sections by visual identification to obtain the node fractions (connection point between internode) and internode (epidermis-free culm region). Node and internode were mill-mechanically pressed for juice extraction and submerged in distilled water, with constant water renewal for 3 days, in order to remove remaining sucrose. These obtained biomasses/fractions, together with the sugarcane leaves, were oven dried at 55 °C for 48 h and then ground in a 20-mesh knife mill (825 μm), obtaining the untreated in natura biomass (IN) and stocked to assay [2].
Pretreatments
Extractive-Free Biomass
Approximately 3 g of IN biomass was subjected to constant washing in Soxhlet apparatus with ethanol (92.8%) for 8 h and subsequent water-washing for the same period. After drying (55 °C for 24 h) and weighing, 300 mg of each fraction of the biomass was used individually in the chemical characterization process, as described later, and the remaining of EF biomasses was stocked for essays [18].
Partial Delignified Biomass
The delignification process was reproduced following α-cellulose extraction [19]. Approximately 5 g of IN biomass was placed in a Schott flask containing 200 mL deionized water, 1.88 g sodium chlorite, and 0.63 mL glacial acetic acid. Three additional doses, equivalent to the first dosage of sodium chlorite and glacial acetic acid, were added hourly after 2, 3, and 4 h, totaling 7.52 g of sodium chlorite, 2.52 mL of acetic acid after 5 h. The reaction was conducted in a water bath at 70 °C. The biomass was filtered, the liquid fraction was discarded, and the mass recovery (MR) was washed with deionized water (800 mL heated water at 70 °C and 2000 mL room temperature water) until the pH of the filtrate was neutral [19]. After drying the biomass, 300 mg of each DL fraction was used for chemical characterization and remaining was stored for future analysis.
Each sugarcane fraction (bagasse, leaf, external fraction, node, and internode) in its three forms (IN, EF, and DL) was pretreated (PT) with diluted sulfuric acid (20% mass acid/mass of material or 2% m/v) for 30 min at 121 °C in autoclave (1:10 solid-liquid ratio) (Fig. 1). After the reaction, the pretreated biomass sample was vacuum-filtered and the liquid fraction was collected for quantification of released sugars and inhibitors in acid medium. MR was washed with distilled water until the pH of the filtrate reached neutrality. After the MR was dried at 55 °C for 24 h, the material was stocked in plastic flask for future analyses.
Chemical Characterization
Untreated biomasses (EF and DL) and acid pretreated of each sugarcane fraction (IN-PT, EF-PT, and DL-PT) were chemically characterized to determine lignin content, cellulose, and hemicellulose. Approximately 300 mg of dry material was hydrolyzed with 3 mL of 72% sulfuric acid at 30 °C for 1 h, with periodic homogenization with glass stick. The reaction was stopped with 84 mL of distilled water and autoclaved at 121 °C for 1 h. The reaction mixture (solids in the solution) was filtered with a porous plate crucible. The liquid fraction was collected to determine soluble lignin by spectrophotometer UV-Vis at 215 and 280 nm and sugar quantification by HPLC (hexoses and pentoses). Solid residue was oven-conditioned at 105 °C to determine insoluble lignin values [18]. All treatments and analyses were performed in triplicate. Values obtained in HPLC were used to calculate the anhydrous sugars, for example, glucose released converted in glucan/cellulose present in the biomass, with hydration factor of 0.9. For xylose and arabinose, the factor was 0.88 and acetic acid of 0.72.
Pseudo-Lignin Content
One gram of MR, from the acid pretreatment, was inserted into filter paper bags that were extracted in Soxhlet with 1,4-p-dioxane/water solution (9:1) for 8 h. After solvent recovery and evaporation, the residual mass of pseudo-lignin was weighed [20]. The pseudo-lignin percentage was determined by the relation between mass solubilized per the amount of material used.
Infrared (FT-IR)-attenuated total reflectance (ATR) of the pseudo-lignin extracted samples was examined between 4000 and 400 cm−1 at 25 °C with 2-cm−1 resolution, 32 scans per spectrum. The ATR method used in a FTIR-VERTEX 70/BRUKER spectrophotometer with a source: HeNe laser (emits radiation in the mid-infrared region); Detector: DLaTGS.
Cellulose Accessibility
Sugarcane fractions (bagasse, leaf, external fraction, node, and internode) acid-untreated (EF and DL) and diluted acid pretreated (IN-PT, EF-PT and DL-PT) were evaluated for the exposed cellulose area, accessible to enzymatic action. The quantification occurred by the adsorption of the dye of Direct Blue (internal specific surface) and Direct Orange (external specific surface). For this, 0.5 mL of phosphate buffer (pH 6) was added together with 50 mg of dry biomass in six centrifuge tubes. To each tube, both blue and orange dyes were added, in six different concentrations (0.06, 0.25, 0.375, 0.5, 0.75, and 1 mL each), and the volume was completed up to 5 mL with distilled water. After constant agitation at 120 rpm at 70 °C for 6 h, the samples were centrifuged at 5600 rpm for 5 min and the supernatant was evaluated in a spectrophotometer at 624 and 244 nm. The amount of dye was assessed by the difference between the final and initial concentration [21]. Direct Orange and Direct Blue concentrations were obtained through the following equations:
A is the solution adsorption at 450 or 624 nm, ɛ is the extinction coefficient of each component in its respective wavelength, L is the cuvette’s length (1 cm). DO is direct orange concentration and DB is direct blue concentration. The extinction coefficients were determined through a standard curve of the dyes and the angular coefficient of their absorptions at 455 and 624 nm. Extinction coefficients used in this study were: EO/455 = 25.61; EB/455 = 0.86; EO/624 = 3.1; and EB/624 = 16.45 Lg−1 cm−1 [21].
Enzymatic Hydrolysis
Sugarcane biomass untreated (EF and DL) and acid pretreated (IN-PT, EF-PT, and DL-PT) were subjected to enzymatic hydrolysis in triplicate using 15 FPU/g of material (Cellic® Cetec–Novozymes, 60 FPU/mL). The reaction was performed with 0.1 g of material in 5 mL (2% solid loading) of 0.05 mol/L sodium citrate buffer, pH 4.8, 50 °C for 24 h at 120 rpm [13]. After this reaction period, the hydrolysate was water boiled for 5 min and centrifuged (2500 rpm for 15 min at 4 °C), and the liquid fraction was evaluated by high-performance liquid chromatography (HPLC). The values obtained were used to calculate the enzymatic digestibility of the material (anhydroglucose released in relation to the glucan/cellulose content) from the cellulose content present in the biomass. The equation used to obtain the glucose yields follows:
where glucose is the concentration of glucose released during enzymatic hydrolysis (g/L); biomass is the dry biomass concentration at the beginning of the enzymatic hydrolysis (g/L); f is the cellulose fraction in dry biomass (g/g); 1.11 is the conversion factor of cellulose to glucose equivalents.
High-Performance Liquid Chromatography
Sugar monomers and acid acetic were quantified using a HPLC with an Aminex® column (Bio-Rad) HPX-87 H 300 × 7.8 mm, mobile phase 0.050 mol/L H2SO4, flow of 0.4 mL/min, oven at 65 °C and RID detector, isocratic method, and dilute acid treatment. Sugar degradation products (furfural and hydroxymethylfurfural) were evaluated in a C18 column (NST) 150 mm × 4.6 mm × 0.5 μm, mobile phase water/acetonitrile (8:1) with 1% acetic acid, 0.8 mL/min flow, 35 °C and 20 mL injection volume, detector UV-Vis 274 nm—isocratic method. All treatments and analyses were performed in triplicate.
Scanning Electron Microscopy
For scanning electron microscopy (SEM) analysis, untreated and pretreated banana pseudostem samples were washed with deionized water and dried at 45 °C for 24 h. Samples were mounted onto stubs using carbon double-sided tape, coated with 5 nm platinum, and examined in a FEI Quanta FEG 450 scanning electron microscope, operating at an accelerating voltage of 1 kV [5].
Statistical Analysis
Results were reported as average of at least three replicates and standard deviation shown. Tukey test was applied to the main results (pseudo-lignin content and enzymatic hydrolysis) to identify similar statistical values (identified with the same letter in figures/tables). DX6Trial-expert software was used (ANOVA), with 95% of significance level (p < 0.05).
Results and Discussion
Chemical Characterization of Untreated and Pretreated Material
The composition of the biomass revealed cellulose contents of 37.4% for bagasse, 37.7% for leaf, and 37.5% for external fraction, while node and internode presented 36.4% and 42.6%, respectively. The content of hemicellulose in bagasse was 26.1% for bagasse, 27.4% for leaf, and 27.8% for external fraction, while node presented 31.5% and internode 29.1%. The lignin contents for bagasse were 19.4%, leaf 33.7%, external fraction 31.0%, node 23.4%, and internode 25.3% (Table 1).
The data showed that the highest content of cellulose was in the internode, while in the other fractions, its level was equivalent. On the other hand, the node presented the highest values of hemicellulose, with similar level compared with other fractions. The highest content of lignin was found in leaf and the smallest was in bagasse. The fractions showed different values of extractives in its composition. Bagasse showed 6.6%, leaf 11.4%, external fraction 12.2%, node 7.2%, and internode 10.6% of extractives (Table 1).
Pretreated biomasses were chemically characterized to determine changes in their components. Partial delignified and diluted acid pretreated (DL-PT) fractions presented higher cellulose content compared with the other treatments (highest content was observed with internode, with 61.7% and the lowest with leaf, with 53.6%). Sodium chlorite removed the lignin in the biomass while sulfuric acid mainly removed the hemicellulosic compound. The delignification process applied preserved hemicellulosic fraction (Table 1). Delignification with sodium chlorite partially removed lignin from biomass, in the condition applied. However, this procedure probably removed other compounds present in biomass such as extractives (pigments, waxes, alkaloids, terpenes, flavonoids, ashes, silica, and sugars) that may contribute to the formation of pseudo-lignin [22].
The highest lignin content was observed with bagasse (35.4%) and leaf (51.9%) EF-PT and with the external fraction (53.7%), node (45.7%), and internode (50.4%) IN-PT. However, the lowest content was observed with bagasse (5.4%) and node (15.2%) partially delignified (DL), leaf (16.8%), external fraction (11.2%), and internode (15.2%) DL-PT (Table 1).
Heterogeneity of sugarcane fractions can influence its conversion into value-added products. Studies reported that epidermis-free internode was composed of 39.58% cellulose, 27.87% hemicellulose, and 19.88% lignin, while node contained 41.17% cellulose, 25.58% total hemicellulose, and 21.32% lignin (similar lignin content compared with internode and node) [3]. Different regions of the same internode of sugarcane have differences in their chemical compositions. After dividing the internode into four parts, from the periphery to the center, it was observed that the outermost fraction showed higher levels of lignin (20–25%) while the innermost fraction (pith) showed higher levels of glucan (41–54%), compared with the other sections [14]. In the present study, similar results were found as those reported in the literature, indicating that fractions share similarities in their chemical compositions.
Seven sugarcane varieties were extractives determined and showed a range from 1.9 to 7.5% [16]. Higher values of extractives were reported, 15% and 12% for sugarcane bagasse and straw, respectively [22]. The extractives can be influenced by the presence of soluble sugars or environmental contamination. The content of extractives, as well as the chemical composition of structural sugars and lignin present in sugarcane, may vary according to their genotype, tissue, and environmental conditions during planting (location, rainfall, available nutrients, temperatures, etc.); this reflects on their development and provokes diversity in its constitution. Sugarcane bagasse presents great heterogeneity and extractive represents on average 6.61% of its composition [23]. Studies with sugarcane bagasse diluted acid pretreated (175 °C, 40 min reaction time, and acid load 1.25% m/m H2SO4) revealed 48% cellulose, 19% xylan, and 26% lignin [24]. Cellulose content was similar to that found in the present study, but differences in hemicellulose removal may have been caused by differences in pretreatment severity (acid content, temperature, and time reaction).
Sugarcane bagasse and straw pretreated with diluted acid (H2SO4 4.5% m/m for 15 min at 175 °C) showed an increase in the lignin content to 25.70% and 27.78%, respectively [18]. Probably, the lignin increase was due to the formation of pseudo-lignin. Moreover, the hemicellulose removal increases the cellulose and lignin percentage. In fact, studies with node and internode extractive-free acid pretreated (2.9% sulfuric acid m/v at 130 °C for 30 min) showed that most of the hemicellulose was removed and enriched the lignin content. The residual lignin content was enriched 6.64% for the internode (19.88% initial) and 8.33% for the node (21.32% initial) [3]. In the present study, the biomasses that were subjected to acid treatment had higher lignin values when compared with untreated biomasses (EF), while biomasses that were subjected to partial delignification and pretreated with acid (DL-PT) had higher cellulose values (Table 1).
This study demonstrated that after acid PT, bagasse showed a higher recovery yield (MR) among in natura (original) (IN-PT) with 62.94%, while external fraction presented greater recovery yield among the extractive-free and dilute acid pretreated biomasses (EF-PT) with 68.61%. Effective acid pretreatment aimed to solubilize most of the hemicellulosic fraction of the biomass, which resulted in high concentration of xylose in the liquid fraction. On the other hand, the effect of delignification had the principle of extracting large amounts of lignin present in the biomass.
Sugars and Inhibitors in the Diluted Acid Hydrolysate
Hydrolysate (liquid fraction) obtained after the PT of the biomasses was used to quantify released hexoses, pentoses, and degradation products (inhibitors). Untreated and diluted acid pretreated fractions (IN-PT) presented higher soluble glucose contents compared with other biomasses. Extractive-free and diluted acid pretreated (EF-PT) presented the highest glucose release with bagasse (2.57 g/L) and the lowest with the external fraction (0.63 g/L). DL-PT had the highest release of glucose with the internode and the lowest with the external fraction (2.09 and 0.50 g/L, respectively) (Table 2).
The highest concentration of xylose was observed with the external fraction (24.5 g/L) and the lowest with the leaf (18.37 g/L) (IN-PT). The pretreatment of extractive-free biomass (EF-PT) resulted in higher concentration of xylose in the node and the lower in the leaf (24.14 g/L and 17.61 g/L, respectively). Among the delignified biomasses, the largest removal of xylose occurred in the node (23.69 g/L) and the lowest occurred in the external fraction (18.27 g/L) (Table 2).
Acetic acid (6.0 g/L) was observed in the node hydrolysate (EF-PT) in highest concentration, while the lowest concentration was observed in the leaf (DL-PT) with 1.67 g/L. In the external fraction hydrolysate (IN-PT), the highest HMF concentration (2.07 g/L) was observed and the lowest HMF concentration occurred in the node (0.05 g/L). Among the EF-PT treatments, HMF concentrations ranged from 0.04 g/L (bagasse and internode) to 0.01 g/L (leaf, external fraction, and node), while DL-PT biomass hydrolysate showed 0.01 g/L (bagasse, leaf, external fraction, and node) and 0.02 g/L (internode) of HMF. Concentrations of furfural observed between treatments and fractions varied between 0.11 g/L (node DL-PT) and 0.06 g/L (leaf, external fraction, and node, IN-PT) (Table 2).
Studies with diluted acid pretreatment of sugarcane bagasse (0.5–4% H2SO4 v/v 121 °C for 1 h, solid-to-liquid ratio 1:15) revealed glucose concentrations ranging from 1.7 to 3.41 g/L (for 1.0 and 2.0 H2SO4 v/v, respectively) [25]. Sugarcane bagasse pretreated using 1% sulfuric and acetic acid (m/v) at 190 °C/10 min showed concentrations of 3.67 g/L and 3.09 g/L glucose in the hydrolysate for solid-to-liquid ratios of 1.5:10 and 1:10, respectively [26]. In the present study, the glucose values obtained for the fractions were similar to those reported in the literature (except for the external fraction). The low concentrations of glucose in the hydrolysate (DL-PT) correspond to the amorphous cellulose that was hydrolyzed, which is in accordance with acid pretreatment to remove the hemicellulosic fraction, retaining in MR a high cellulose content.
Xylose concentration of 41.54 g/L was determined in the hydrolysate of sugarcane bagasse dilute sulfuric acid pretreated (1% w/w, at 121 °C for 150 min, 20% solids) [27]. However, sugarcane bagasse pretreated with 1% sulfuric acid and 1% acetic acid (m/v) (at 190 °C/10 min, 1.5:10 and 1:10 solid-to-liquid ratios) showed 9.04 (1.5:10) and 9.33 (1:10) g/L xylose [26]. Xylose rates observed in the present study are among the values reported in the literature. The concentration of xylose released in the hydrolysate undergoes direct action of the acid concentration applied to biomass. However, severe pretreatment conditions (high acid loads, temperature, and reaction time, etc.) may favor the formation of sugar degradation products that are precursors to the formation of pseudo-lignin [10] and other undesirable toxic compounds for fermentation process [28].
In the hydrolysate of sugarcane bagasse pretreated with sulfuric acid (1% H2SO4, at 121 °C/150 min, 20% solids load), 2.95 g/L of acetic acid was detected [27]. However, pretreatment conducted with 1% sulfuric acid and 1% acetic acid (m/v) at 190 °C/10 min 1:10 solid-to-liquid ratios) showed 2.89 g/L of acetic acid [26]. As the pretreatment severity increased (time and temperature), a gradual increase in the concentration of acetic acid was observed in the hydrolysate [29]. Studies conducted with 64 different microorganisms revealed that only 5 were able to grow in the presence of 5 g/L acetic acid [30]. The acetic acid contents observed in the present study were slightly above the average reported in the literature. Thus, some acetic acid contents (leaf and node EF-PT) could be potential inhibitors of microbial growth, while other contents might be close to the considered inhibitory. The detoxification of the lignocellulosic biomass hydrolysate is an important step that can improve the efficiency of the industrial process, mitigating the effects caused by toxic compounds on the metabolism of microorganisms [28].
Temperature is an important factor to degradation product formation. At 180 °C and 200 °C, HMF concentrations increased from 0.01 to 0.11 g/L, while furfural increased from 0.26 to 2.42 g/L, respectively (hot water pretreatment/20 min, 1:20 m/v). However, increasing the reaction time to 30 and 40 min increased the furfural to 0.51 and 0.73 g/L, respectively. The increase of temperature and reaction time provoked a greater degradation of sugars [29]. In fact, higher concentrations (9.6%) of furfural were observed with high severity treatments (1.25% H2SO4 m/m, 185 °C/40 min) [24]. In the present study, the concentrations of HMF observed can be explained by the presence of sulfuric acid in the reaction medium, while the furfural concentrations were due to the shorter time and reaction temperature.
Furfural and HMF were observed in low concentration, independent of the sugarcane biomasses, compared with the literature (Table 2). Pretreatment severity can interfere in sugar release and its degradation. Higher content of HMF was observed in the external fraction (2.07 g/L) and internode (1.41 g/L) IN-PT, if compared with EF-PT and DL-PT. Furfural content was lower than some literature studies for all the pretreatments and biomasses. A large amount of glucose was observed in the hydrolysate of external fraction IN-PT (10 g/L). This higher content was probably because of sucrose present in the biomass. The external fraction was hand cut from sugarcane, and probably, it comes with certain sucrose content. Studies with external fraction reported a washing step before processing, removing sucrose content [2]. Concerned with industrial process of material, in the present study, the external fraction was not washed. These data corroborate to the low MR after dilute acid pretreatment (Table 1) and higher HMF values caused by the degradation of glucose present in sucrose (Table 2). This glucose concentration was not observed in the other external fraction pretreatment because extractive removal and chlorite delignification probably solubilized sucrose before PT.
Pseudo-Lignin Content
The pseudo-lignin formation was observed in the highest content for all fraction IN-PT, followed by the EF-PT and in smaller amount DL-PT (Fig. 2). Pseudo-lignin content in IN-PT was 10.82% for bagasse, 6.92% for leaf, 6.55% for external fraction, 10.25% for node, and 11.70% for internode. However, pseudo-lignin content decreased with EF-PT, with 6.91% for bagasse, 4.85% for leaf, 4.17% for external fraction, 7.76% for node, and 7.04% for internode. The DL-PT showed the lower pseudo-lignin contents of 1.14%, 2%, 2.55%, 2.38% and 0.07%, respectively for bagasse, leaf, external fraction, node, and internode (Fig. 2). The results suggested that to remove extractives before the acid pretreatment may reduce pseudo-lignin formation. The previous delignification with sodium chlorite was more effective in suppressing pseudo-lignin formation (Fig. 2).
Partial delignification prior to dilute acid pretreatment may reduce the concentration of secondary compounds forming pseudo-lignin by removing an amount of lignin (Table 1). Biomass extractives and silica were suggested to contribute to the formation of pseudo-lignin during pretreatment with sulfuric acid [22]. According to pretreatment applied to biomass, the increase in severity (temperature, reagent loading, time, etc.) results in an impact on the amount of xylan removed, increasing also the possibility of generating furfural. A study showed that the increase in acid loading in sugarcane bagasse pretreatment (above 2% v/v) decreases the glucose and xylose in the hydrolysate, while furfural and acetic acid increase [25]. Degradation of xylose may contribute to the formation of pseudo-lignin, which may impair the enzymatic hydrolysis process. In acid medium, the pseudo-lignin formed contributed with a significant increase of the lignin in bagasse and sugarcane straw [22]. Due to the low lignin content in biomass, which condenses with sugar degradation products (largely responsible for the formation of pseudo-lignin) during acid pretreatment, there was also a smaller amount of pseudo-lignin formation in the biomasses. Possibly, the IN-PT biomass had higher pseudo-lignin content due to the presence of extractives in the material, which may undergo reactions with furfural and HMF and lignin during dilute acid pretreatment. The contribution of extractives in pseudo-lignin formation can be evidenced by comparing the same biomass between IN-PT and EF-PT.
FT-IR
The extracted pseudo-lignin was evaluated using FT-IR. FTIR analyses of the pseudo-lignin of the fractions evaluated in this study were consistent with the those reported in the literature. Bands between 1750 and 1700 cm−1 represent the C=O stretching in carboxylic acids, ketones, ester groups of carbohydrates, conjugated aldehydes; region between 1600 and 1510 cm−1 represents aromatic ring skeletal vibration (C=O stretching); region between 1460 cm−1 represents the C–H deformation, and 1410 cm−1 represents aromatic skeletal vibrations combined with deformation C–H in plane. The band at 1327 cm−1 corresponds to the syringyl ring condensed with guaiacyl ring (G ring replaced at position 5), C–C, C–O, C=O stretch, secondary OH aromatic, C–H in plane deformation (typical for G units), and primary OH; 1221 cm−1 represents G ring (C=O stretch), 1200 cm−1 and 1050 cm−1 represent C–O stretching in alcohols, ethers, or carboxylic acids. Band at 800 cm−1 represents C–H out-of-plane bending at positions 2, 5, and 6 (G units), while 870 cm−1 represents C–H out-of-plane at positions 2 and 6 (S units) (Fig. 3) [12, 31].
Figures 3 a and b correspond to in natura (IN-PT) and extractive-free (EF-PT) pseudo-lignin bands, respectively. Fractions represented in these spectra showed similar band patterns, assuming similarity between the structures. However, pseudo-lignin extracted from partially delignified with sodium chlorite (Fig. 3c) showed changes in the bands between 1750 and 1700 cm−1 indicating an increase in the number of C=O in conjugated and unconjugated, respectively. Strong bands in 1700 cm−1 region represent carbonyl and carboxylic groups present in the pseudo-lignin (stretching in ketones). Band at 1600 cm−1 is attributed to the stretching of the aromatic ring; however, this band was not clearly identified in pseudo-lignin, probably due to the delignifying effect (Fig. 3c). The reduction in the band intensity corresponding to 1600 cm−1 and 1510 cm−1 indicates a reduction in the stretching of the C=O bonds (aromatic ring vibrations, the latter mainly in G units), probably due to the effect of the delignification. The absence of the band at 1270–1260 cm−1 indicates a lower proportion of unit G in the delignified fractions (Fig. 3c) compared with the pseudo-lignin from IN-PT and EF-PT (Fig. 3 a and b). The band in the region of 1120 cm−1 represents higher levels of S units in the delignified material (Fig. 3c).
Cellulose Accessibility
The exposed cellulose surface on sugarcane biomasses was evaluated by adsorption of Direct Orange and Direct Blue dyes (Table 3). Untreated fractions showed low dye adsorption. External fraction showed total adsorption of 11 mg/g, node of 24 mg/g, while internode and leaf showed 106 and 145 mg/g, respectively. After the acid pretreatment (IN-PT), all the materials increased the dye adsorption from 1.5 to 22 times. Pretreatment carried out with 20% m/m of sulfuric acid reported total adsorption of dye of 86 mg/g by the external fraction. Node showed 69 mg/g of adsorption, while internode and leaf presented total adsorption of dye of 628 mg/g and 666 mg/g, respectively [21].
Fractions that had a greater adsorption of dyes suggest greater accessibility to cellulose, probably due to the structure modification [19]. The removal of hemicellulose and lignin generates pores and allows the adsorption of Direct Orange dye on the exposed cellulose. The partial delignification process provoked an increase in the cellulose accessibility by removal of lignin. The DL provoked an increase in the dye adsorption on to bagasse sample of 27 times compared with untreated (EF). The acid pretreatment of the de DL bagasse resulted in a decrease of the dye adsorption, however adsorbed 13 times more dye than untreated bagasse. The decrease of dye adsorption after the pretreatment of partial delignified bagasse could be related to a reorganization of cellulose component and effect of dry process [32]. Direct Blue dye has less affinity for cellulose than Direct Orange dye, penetrating areas where cellulases cannot reach. Therefore, the accessibility to cellulose can be measured not only by the total amount of adsorbed dye, but also by the greater amount of Direct Orange adsorbed in relation to Direct Blue (higher concentration of adsorbed Direct Orange means greater cellulose surface exposed to cellulases) [21].
Studies carried out with the sugarcane fractions (external fraction, node, internode, and leaves) submitted to oxidative pretreatment (1.5 g of NaClO2 and 0.5 mL of anhydrous acetic acid added in 50 mL of distilled water with 5 g of biomass, reaction temperature 70 °C and reaction time 30 min, 1, 2, and 3 h, with new loads/doses of sodium chlorite and acetic acid added at 1, 2, and 3 h) revealed that the highest rates of total dye adsorption were observed in more severe treatments. After 3 h of reaction, the external fraction showed total dye adsorption of 2076 mg/g, while the node showed 609 mg/g. Internode and leaf showed total adsorption of 1327 mg/g and 567 mg/g, respectively [21].
The porosity/accessibility of lignocellulosic biomass is a fundamental characteristic for enzymatic hydrolysis of polysaccharides, directly affected by the lignin content [13]. Higher levels of lignin in the biomass represent low porosity and worse enzymatic conversion of the polysaccharides. Consequently, the removal of lignin improves enzymatic saccharification [32]. A more severe pretreatment removing lignin could increase the cellulose accessibility. In fact, a chlorite pretreatment with harsh condition (two more loads of chemicals) increased the total dye adsorption to 2076 mg/g [17].
Enzymatic Hydrolysis
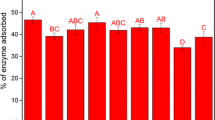
The untreated sugarcane biomasses (EF) showed lower glucose yields (Fig. 4). The pretreatment is necessary to breakdown the biomass structure and increase the cellulose accessibility, improving enzymatic action [13, 19]. The lowest conversion of the cellulose into glucose occurred in the external fraction (5%) and the highest in the internode (12%). The partial delignified samples (DL) improved the cellulose conversion, resulting in an increase of the yield of 4.4 and 3.3 times, respectively, to external fraction and internode. A partial lignin removal process increased glucose conversion yield, indicating that there was an increase in the cellulose surface area to which the cellulase enzymes had access (Fig. 4). DL-PT of biomasses had glucose yields in the range of 90% and showed similar statistical value (Fig. 4). Partial delignification followed by diluted acid pretreatment was fundamental in the biomass conversion process. The combination of these procedures resulted in the high surface area (due to the removal of hemicellulose and lignin) to the cellulase enzyme action.
Glucose yield from enzymatic hydrolysis of untreated and acid pretreated materials. EF: extractive-free biomass; DL: partial delignified biomass; IN-PT: in natura (original) acid pretreated; EF-PT: extractive-free acid pretreated; DL-PT: partial delignified acid pretreated. Equal letters indicate statistical similar values
The DL-PT biomass reached the highest glucose yields, suggesting that the removal of the lignin (DL) and the hemicellulose (PT) together is the best strategy to improve cellulose conversation. Bagasse and internode IN-PT had similar statistical value, with 54% glucose yield after enzymatic hydrolysis, and the highest conversion applying IN-PT.
The EF-PT treatment of bagasse and internode showed similar statistical value, reaching the highest levels of glucose conversion (69% to 66%, respectively). Leaf (50%) and node (51%) were also similar statistical value, and the lowest glucose conversion rate was observed again in the external fraction (31%) (Fig. 4). The difference in the increase in cellulose conversion between IN-PT and EF-PT biomasses can be explained by the absence of extractives in the biomass, which contributes to the formation of pseudo-lignin which is detrimental in enzymatic hydrolysis.
Sugarcane bagasse of 11 different varieties that were submitted to dilute acid pretreatment (0.5% m/m H2SO4 at 180 °C/15 min) and subsequent enzymatic hydrolysis showed glucose yield between 39 and 78% after 24 h of hydrolysis. After 72 h of reaction, the glucose yield reached between 61 and 94% with low recalcitrance varieties identified [19]. The present study reached similar conversion yield for bagasse with pretreatments of DL, IN-PT, EF-PT, and DL-PT (Fig. 4).
A different approach could be an acid pretreatment followed by a partial delignification. Sugarcane bagasse pretreated with dilute acid (1% m/v H2SO4 at 121 °C/10 min) and further delignification with NaOH (1–1.5% m/v at 100 °C/1 h) revealed glucose yield 56% for diluted acid pretreated material and 79% after delignified step [33].
The cellulose conversion into glucose results obtained in the present study is close or higher than that reported in the literature. However, the severity of the pretreatment influences the final hydrolysis process by the amount of hemicellulose removed and the exposed surface area of the cellulose. Factors such as the presence of extractives, lignin content (Table 2), and pseudo-lignin formation (Fig. 2) interfered with the results of glucose enzymatic hydrolysis yield (Fig. 4). The biomasses that underwent the extractive removal/pretreated (EF-PT) and delignification/pretreated (DL-PT) showed higher values of glucose yield compared with IN-PT biomass (Fig. 4). The partial delignification alone was enough to modify the biomass structure, improving the glucose yield slightly close to the IN-PT for some biomasses, such as leaf and node. Therefore, the removal of extractives and partial delignification of biomass may be a key factor in the conversion of biomass to fermentable sugars via acid pretreatment. The results suggest that the presence of these compounds during dilute acid pretreatment could generate higher levels of pseudo-lignin.
Scanning Electron Microscopy
Sugarcane biomasses were investigated for morphological changes after acid pretreatment and delignification. SEM images of untreated biomasses (IN) showed an integral rigid structure pattern among the fractions. The surfaces were characterized by the absence of degradation or fragmentation. Damage on the structure of the untreated material could be result of the milling process but revealing a tissue integrity (Fig. 5).
Sulfuric acid acts on plant cell walls, causing the fibrous mesh to loosen. Acid pretreatment removed on average 45% of biomass components (IN-PT), while delignification removed on average 33% (DL) and 41% more followed by acid pretreatment (DL-PT). The materials pretreated with sulfuric acid presented deformed and fractured structure after pretreatment application (IN-PT) (Fig. 5). The damage of the cell wall structure of biomass by removing hemicellulose (IN-PT) is not as aggressive as pretreatment of partial delignification followed by acid pretreatment (DL-PT). For some biomasses, it was possible to observe a progressive damage between the IN-PT and DL-PT biomasses (leaf, external fraction, and node). The biomasses suffered major changes in their structure due to the removal of hemicellulose and lignin by the action of chemical agents, resulting in loss of initial mass (Table 1) and making the cellulose fibers exposed to enzymatic action (Table 3). In fact, delignified and acid-pretreated biomass (DL-PT) presented more open and fragmented fibers compared with in natura acid pretreated (IN-PT) and untreated (IN) biomasses. Removal of xylan caused structural changes in the biomass surface. However, with the additional removal of lignin, the structural damage was more evident (Fig. 5).
Higher magnification of SEM images was possible to visualize pseudo-lignin droplets that were formed during dilute acid pretreatment and condensed onto the material fibers (Fig. 6, arrows). Pseudo-lignin was observed even on the DL-PT material, which was previously partially delignified (Fig. 6, arrows). These droplets are formed from the condensation of sugar degradation products and lignin fragments [22], with interference of extractives that can increase the content. Pseudo-lignin is detrimental to enzymatic hydrolysis because it covalently and irreversibly binds to cellulase enzymes during enzymatic hydrolysis, reducing its sugar conversion capacity. Therefore, inhibition of its formation is desired in the lignocellulosic ethanol production process.
Conclusions
This study evaluated different biomasses/fractions of sugarcane response to delignification and acid pretreatment, in regard to pseudo-lignin formation, cellulose accessibility, and enzymatic hydrolysis glucose yield. The heterogeneity of the biomasses was evidenced by the difference in the chemical composition and response to the pretreatment. Hemicellulose was removed in higher amount from all the biomasses by acid pretreatment. The partial delignification distinguished biomass response to lignin removal. Both the hemicellulose and lignin removal provoked modification in the material structure increasing the cellulose accessibility revealed by dye adsorption. The pseudo-lignin showed to be influenced by extractives, increasing its amount. Pseudo-lignin formed by the acid pretreatment showed structure similarity, except for those from delignified material that showed modification in the aromatic ring spectra profile. As a result, removing part of the hemicellulose and lignin decreased the pseudo-lignin formation, improving the cellulose accessibility improving enzymatic hydrolysis glucose yield.
Data Availability
Not applicable.
References
Bezerra TL, Ragauskas AJ (2016) Review a review of sugarcane bagasse for second-generation bioethanol and biopower production. Biofuels Bioprod Biorefin 10:634–647. https://doi.org/10.1002/bbb
Brienzo M, Ferreira S, Vicentim MP, de Souza W, Sant’Anna C (2014) Comparison study on the biomass recalcitrance of different tissue fractions of sugarcane culm. Bioenergy Res 7:1454–1465. https://doi.org/10.1007/s12155-014-9487-8
Brienzo M, Abud Y, Ferreira S, Corrales RCNR, Ferreira-Leitão VS, Souza W, Sant’Anna C (2016) Characterization of anatomy, lignin distribution, and response to pretreatments of sugarcane culm node and internode. Ind Crop Prod 84:305–313
Food and Agriculture Organization of the United Nations - FAO (2018) Faostat. http://www.fao.org/faostat/en/#rankings/countries_by_commodity
Shimizu FL, Monteiro PQ, Ghiraldi PHC, Melati RB, Pagnocca FC, Souza W, Sant’Anna C, Brienzo M (2018) Acid, alkali and peroxide pretreatments increase the cellulose accessibility and glucose yield of banana pseudostem. Ind Crop Prod 115:62–68. https://doi.org/10.1016/j.indcrop.2018.02.024
Zhuang J, Wang X, Xu J, Wang Z, Qin M (2017) Formation and deposition of pseudo-lignin on liquid-hot-water-treated wood during cooling process. Wood Sci Technol 51:165–174. https://doi.org/10.1007/s00226-016-0872-7
Ma X, Yang X, Zheng X, Chen L, Huang L, Cao S, Akinosho H (2015) Toward a further understanding of hydrothermally pretreated holocellulose and isolated pseudo lignin. Cellulose 22:1687–1696. https://doi.org/10.1007/s10570-015-0607-1
Rasmussen H, Sørensen HR, Meyer AS (2014) Formation of degradation compounds from lignocellulosic biomass in the biorefinery: sugar reaction mechanisms. Carbohydr Res 385:45–57. https://doi.org/10.1016/j.carres.2013.08.029
Trček J, Mira NP, Jarboe LR (2015) Adaptation and tolerance of bacteria against acetic acid. Appl Microbiol Biotechnol 99:6215–6229. https://doi.org/10.1007/s00253-015-6762-3
Schmatz AA, Tyhoda L, Brienzo M (2020) Sugarcane biomass conversion influenced by lignin. Biofuels Bioprod Biorefin 14:469–480. https://doi.org/10.1002/bbb.2070
Govender M, Bush T, Spark A, Bose SK, Francis RC (2009) An accurate and non-labor intensive method for the determination of syringyl to guaiacyl ratio in lignin. Bioresour Technol 100:5834–5839. https://doi.org/10.1016/j.biortech.2009.06.009
Hu F, Jung S, Ragauskas A (2012) Pseudo-lignin formation and its impact on enzymatic hydrolysis. Bioresour Technol 117:7–12. https://doi.org/10.1016/j.biortech.2012.04.037
Brienzo M, Fikizolo S, Benjamin Y, Tyhoda L, Görgens J (2017) Influence of pretreatment severity on structural changes, lignin content and enzymatic hydrolysis of sugarcane bagasse samples. Renew Energy 104:271–280. https://doi.org/10.1016/j.renene.2016.12.037
Costa THF, Masarin F, Bonifácio TO et al (2013) The enzymatic recalcitrance of internodes of sugar cane hybrids with contrasting lignin contents. Ind Crop Prod 51:202–211. https://doi.org/10.1016/j.indcrop.2013.08.078
Masarin F, Neves AS, Milagres AMF, Ferraz A (2013) Evaluation of a simple alkaline pretreatment for screening of sugarcane hybrids according to their in vitro digestibility. Ind Crop Prod 51:390–395. https://doi.org/10.1016/j.indcrop.2013.09.033
Masarin F, Gurpilhares DB, Baffa DCF, Barbosa MHP, Carvalho W, Ferraz A, Milagres AMF (2011) Chemical composition and enzymatic digestibility of sugarcane clones selected for varied lignin content. Biotechnol Biofuels 4:1–10. https://doi.org/10.1186/1754-6834-4-55
Li M, Pu Y, Ragauskas AJ (2016) Current understanding of the correlation of lignin structure with biomass recalcitrance. Front Chem 4:1–8. https://doi.org/10.3389/fchem.2016.00045
ABNT NBR 16550:2018 Sugarcane bagasse - chemical characterization. Brazilian National Standards Organization (2018) ABNT
Brienzo M, Tyhoda L, Benjamin Y, Görgens J (2015) Relationship between physicochemical properties and enzymatic hydrolysis of sugarcane bagasse varieties for bioethanol production. New Biotechnol 32:253–262. https://doi.org/10.1016/j.nbt.2014.12.007
Hu F, Jung S, Ragauskas A (2013) Impact of pseudolignin versus dilute acid-pretreated lignin on enzymatic hydrolysis of cellulose. ACS Sustain Chem Eng 1:62–65. https://doi.org/10.1021/sc300032j
Shimizu FL, Azevedo GO, Coelho LF et al (2020) Minimum lignin and xlan removal to improve cellulose accessibility. BioEnergy Res 13:775–785. https://doi.org/10.1007/s12155-020-10120-z
de Carvalho DM, Sevastyanova O, Penna LS et al (2015) Assessment of chemical transformations in eucalyptus, sugarcane bagasse and straw during hydrothermal, dilute acid, and alkaline pretreatments. Ind Crop Prod 73:118–126. https://doi.org/10.1016/j.indcrop.2015.04.021
Melati RB, Schmatz AA, Pagnocca FC et al (2017) Sugarcane bagasse: production, composition, properties, and feedstock potential. In: Murphy R (ed) Sugarcane: production systems, uses and economic importance. Nova Science Publishers, New York, pp 1–38
Mesa L, Morales M, González E, Cara C, Romero I, Castro E, Mussatto SI (2014) Restructuring the processes for furfural and xylose production from sugarcane bagasse in a biorefinery concept for ethanol production. Chem Eng Process 85:196–202. https://doi.org/10.1016/j.cep.2014.07.012
Rai PK, Singh SP, Asthana RK, Singh S (2014) Biohydrogen production from sugarcane bagasse by integrating dark- and photo-fermentation. Bioresour Technol 152:140–146. https://doi.org/10.1016/j.biortech.2013.10.117
Rocha GJ d M, Carlos M, Isaias Barbosa S et al (2011) Dilute mixed-acid pretreatment of sugarcane bagasse for ethanol production. Biomass Bioenergy 35:663–670. https://doi.org/10.1016/j.biombioe.2010.10.018
Martins LHS, Rabelo SC, da Costa AC (2015) Effects of the pretreatment method on high solids enzymatic hydrolysis and ethanol fermentation of the cellulosic fraction of sugarcane bagasse. Bioresour Technol 191:312–321. https://doi.org/10.1016/j.biortech.2015.05.024
Candido JP, Claro EMT, de Paula CBC, Shimizu FL, de Oliveria Leite DAN, Brienzo M, de Angelis DF (2020) Detoxification of sugarcane bagasse hydrolysate with different adsorbents to improve the fermentative process. World J Microbiol Biotechnol 36:1–12. https://doi.org/10.1007/s11274-020-02820-7
Hongdan Z, Shaohua X, Shubin W (2013) Enhancement of enzymatic saccharification of sugarcane bagasse by liquid hot water pretreatment. Bioresour Technol 143:391–396. https://doi.org/10.1016/j.biortech.2013.05.103
Soares LCSR, Chandel AK, Pagnocca FC, Gaikwad SC, Rai M, da Silva SS (2016) Screening of yeasts for selection of potential strains and their utilization for in situ microbial detoxification (ISMD) of sugarcane bagasse hemicellulosic hydrolysate. Indian J Microbiol 56:172–181. https://doi.org/10.1007/s12088-016-0573-9
Singh R, Singh S, Trimukhe KD, Pandare KV, Bastawade KB, Gokhale DV, Varma AJ (2005) Lignin–carbohydrate complexes from sugarcane bagasse: preparation, purification, and characterization. Carbohydr Polym 62:57–66. https://doi.org/10.1016/j.carbpol.2005.07.011
Junior CS, Milagres AMF, Ferraz A, Carvalho W (2013) The effects of lignin removal and drying on the porosity and enzymatic hydrolysis of sugarcane bagasse. Cellulose 20:3165–3177. https://doi.org/10.1007/s10570-013-0032-2
Rocha GJM, Gonçalves AR, Nakanishi SC, Nascimento VM, Silva VFN (2015) Pilot scale steam explosion and diluted sulfuric acid pretreatments: comparative study aiming the sugarcane bagasse saccharification. Ind Crop Prod 74:810–816. https://doi.org/10.1016/j.indcrop.2015.05.074
Acknowledgements
This study was supported by Brazilian Council for Research and Development – CNPq. (process number: 401900/2016-9).
Funding
This study was supported by Brazilian Council for Research and Development – CNPq (process number: 401900/2016-9).
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schmatz, A.A., Salazar-Bryam, A.M., Contiero, J. et al. Pseudo-Lignin Content Decreased with Hemicellulose and Lignin Removal, Improving Cellulose Accessibility, and Enzymatic Digestibility. Bioenerg. Res. 14, 106–121 (2021). https://doi.org/10.1007/s12155-020-10187-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10187-8