Abstract
Sugarcane (Saccharum officinarum) residue (straw) has been identified as a promising feedstock for bioenergy production, but excessive straw removal may impair soil macrofauna and related ecosystem services. To quantify straw removal effects on abundance, richness, and diversity of soil macrofauna, four experiments were conducted in São Paulo state, Brazil, under different edaphoclimatic conditions. A secondary goal was to evaluate seasonal changes on soil macrofauna and identify linkages between those changes and soil chemical and physical attributes. Four straw removal treatments (NR, no removal, LR, low removal, HR, high removal, and TR, total removal) were evaluated. Macrofauna and other soil attributes were sampled within the 0- to 0.30-m depth increment. Soil macrofauna were impaired by TR with the magnitude of response being related to both edaphoclimatic conditions and management practices. Numerous interactions among seasons, straw removal rates, and soil macrofauna were found, especially for total abundance and diversity of organisms. Partial straw removal (HR and LR) may be a strategy to protect soil health and increase bioenergy production with minimal effects on soil macrofauna, although long-term experiments are needed to confirm our hypothesis. The NR treatment generally had better soil quality as indicated by greater soil moisture, macropore number, soil organic carbon (SOC) content, and soil fertility, which led to a higher abundance of most macrofauna organisms. Total removal resulted in greater soil compaction and decreased macrofauna abundance, especially in clay soils. Our findings confirm that an integrated approach using soil indicators as guidelines should be adopted to better predict sustainable straw management practices for sugarcane in Brazil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crop residues play a fundamental role in sustaining soil biodiversity, serving as the primary source of carbon (energy), protecting the soil from direct solar radiation, regulating soil temperature and moisture, and providing fauna shelters and habitat [1, 2]. Soil invertebrates with a body length > 2 mm are called macrofauna [3] and include Oligochaeta, Coleoptera, Hymenoptera, Hemiptera, Isopoda, Araneae, Geophilomorpha, and other taxa [4]. These macroinvertebrates act directly or indirectly on key soil processes and associated ecosystem services [5,6,7,8,9,10], such as soil aggregation [11], water regulation through the creation of biopores that enhance water infiltration and percolation [12], decomposition and incorporation of soil organic matter, nutrient recycling [13], and by providing habitat for other organisms [6]. Therefore, soil fauna act positively to achieve the United Nations Sustainable Development Goals, especially those related to food and freshwater security, land recovery, and climate change mitigation [14].
Simplified cropping systems such as those currently used in sugarcane fields have reduced the diversity of crop residues and negatively affected soil macrofauna communities [15] when compared with those with full crop rotations. Simplification decreases food web, abundance of food, lowers habitat diversity, and can result in proliferation of some groups of organisms that in high populations may become agricultural pests [16].
Brazil, the largest sugarcane producer in the world, has a long history of burning sugarcane fields before harvesting. This practice has direct and indirect detrimental effects (i.e., killing of soil organisms and removal of crop residues that serve as a food source and habitat for fauna) [17, 18]. Since the early 2000s, sugarcane producers have gradually been changing from pre-harvest burning followed by manual harvest to a mechanized harvesting system that is currently being used on 94% of the cultivated areas in south-central Brazil [19]. The gradual adoption of mechanized (green cane) harvesting has resulted in the annual deposition of a thick straw layer (10 to 20 Mg ha−1) on the soil surface [20] and therefore increased industry interest in using the straw for bioelectricity or cellulosic ethanol production [21]. This interest, however, has raised new concerns regarding how straw removal may impact soil biodiversity and sustainability of sugarcane production. Those effects, particularly on macrofauna, are still poorly investigated and thus provide the basis for this investigation.
Studies of Abraão [22] and Portillo et al. [23] have shown that leaving high amounts of sugarcane straw on soil surface increased abundance, richness, and diversity of soil macrofauna. Abreu et al. [24] also observed that changes in macrofauna are dependent on climatic conditions which can be buffered by maintaining straw in the field.
Based on those previous studies, we hypothesized that (i) total sugarcane straw removal for bioenergy production would negatively affect soil macrofauna by directly or indirectly impacting soil attributes and (ii) the magnitude of macrofauna responses would be dependent on edaphoclimatic conditions. Our primary goals were to (i) evaluate changes in abundance, richness, and diversity of the macrofauna in clayey and sandy soils during early and late harvesting seasons after 4 years of different straw removal treatments, (ii) correlate soil macrofauna changes induced by straw removal with soil physical and chemical attributes, and (iii) investigate temporal changes/alterations in macrofauna across the rainy and dry seasons in a clay soil.
Material and Method
Description of the Study Areas
The same field experimental design was replicated at four sites in São Paulo state in southeastern Brazil. Two experiments were conducted on clayey soils near Iracemápolis City (clay soil 1 and 2) and two on sandy soils surrounding Quatá City (sandy soil 1 and 2) (Table 1). The experiments were conducted in the center of large commercial sugarcane areas where sandy and clay fields had been cultivated for 30 and 50 years, respectively. All sites were also located about 1.5 to 2 km from native forest fragments.
Treatments, Experiment Design, and Timeline of Experimental Period
Four sugarcane experimental sites were planted in 2012, straw removal treatments were established in 2013, and continued following cane cycle (Fig. 1). In each region, one clay and one sandy site (no. 1) were established during early harvest (April), and another clay and sandy site (no. 2) in September (Fig. 1). Crop-water balance (mm), rainfall (mm), and mean air temperature (°C) for each site are shown in Fig. S1.
A randomized block design with four treatments and four replicates (16 plots) was replicated at each experimental site (i.e., a total of 64 plots within the whole study). Each plot was 10-m long and 12-m wide with eight sugarcane rows spaced at 1.5-m intervals. There was a 3-m space between each set of plots. After harvest, the straw remaining on soil was quantified by casting a 0.25-m2 metal frame randomly throughout the field. This procedure was repeated ten times. Straw moisture was measured directly in the field using an AL-104 Agrologic* sensor with E-831 Electrode coupling. Based on the amount of straw (dry basis), four treatments were established: no removal (NR), low removal (LR), high removal (HR), and total removal (TR) with 15, 10, 5, or 0 Mg ha−1, respectively, of straw remaining on the soil surface. The actual amount of straw removed for each treatment was carried out manually using rakes and forks. Immediately after each subsequent harvest (i.e., 1st, 2nd, 3rd, and 4th ratoons), the treatments were re-established on the same plots (Fig. 1).
Yearly fertilization to each plot consisted of 120 kg ha−1 of N (ammonium nitrate) and 120 kg ha−1 of K (potassium chloride). Following the 3rd ratoon harvest and continuing until the end of soil macrofauna sampling, no fungicides, bactericides, or insecticides were applied. Herbicides, however, were uniformly applied following the management strategies established by each sugarcane mill. Clay soil 1 and sandy soil 1 received amicarbazone (1.5 kg ha−1) and diuron (1.5 L ha−1) 50 days before macrofauna sampling. Clay soil 2 and sandy soil 2 received diuron + hexazinone (3 kg ha−1) and tebuthiuron (2.0 L ha−1) 20 days before sampling.
Soil Macrofauna Sampling and Taxonomic Identification
Soil macrofauna sampling was based on two objectives. First, the evaluation of straw removal effects over a period of 4 years under different edaphoclimatic conditions. For this purpose, samples were collected from all four sites 80 days after the 4th ratoon harvest (i.e., at the beginning of the 5th ratoon when crop canopy was still open) (Fig. 1). Macrofauna (Fig. 1) were evaluated in clay and sandy soil no. 1 in July 2017 which was the winter (dry season), while clay and sandy soil no. 2 were evaluated in December 2017 which was the summer (rainy season). The second objective was to quantify seasonal changes in soil macrofauna communities at the same site between the rainy and dry seasons. Therefore, an additional evaluation was performed only for clayey soil 1 during rainy season in November 2016, when sugarcane canopy was completely closed (Fig. 1).
Soil macrofauna samplings were conducted by collecting monoliths (0.25 × 0.25 m wide and 0.30 m deep) and dividing them into 0.0 to 0.10 -, 0.10 to 0.20-, and 0.20 to 0.30-m increments, using a method adapted from Tropical Soil Biology and Fertility [29] and illustrated in Fig. S2. A total of 192 monoliths were collected to evaluate straw removal effects (i.e., 4 sites × 4 treatments × 4 repetitions ×3 soil depths). An additional 48 monoliths were collected to investigate seasonal macrofauna changes (i.e., 1 sampling × 4 treatments × 4 replications ×3 depth increments × 1 site). The monoliths were excavated from the center of each plot to minimize border effects. Macroinvertebrates found in sugarcane straw were included in samples from the 0- to 0.10-m soil depth. Soil moisture was measured at the time of sampling using the GS3 moisture sensor plugged into a Decagon® ProCheck hand-held device.
Macroinvertebrates were carefully extracted from the monoliths using tweezers and sorted by hand in a large tray. Collected organisms were stored in a 70% ethanol solution for subsequent laboratory identification and counting. Taxa were identified in the laboratory on the order level, [30], except for the Formicidae family, which was counted separately from the Hymenoptera (ants) due to the high abundance and family behavior. The identified groups were Araneae (spiders), Coleoptera (beetles), Dermaptera (earwings), Diplura (bristletails), Diptera (flies), Geophilomorpha (centipedes), Hemiptera (true bugs), Hymenoptera (wasps, bees, sawflies), Isoptera (termites), and Oligochaeta (earthworms).
Soil Chemical and Physical Attributes
While performing soil macrofauna evaluation in all four sites (except for the additional evaluation performed on clayey soil 1-rainy season), soil samples were collected for analyses of soil chemical and physical attributes. All samples were collected at 0.0 to 0.10-, 0.10 to 0.20-, and 0.20 to 0.30-m soil layers. Soil organic carbon (SOC), pH (CaCl2 0.01 mol L−1), available phosphorus (P), and bases sum (BS—Ca++, Mg++, and K+) were determined using disturbed samples according to the methodology proposed by Raij et al. [27]. Total soil organic C concentration (TOC) was measured by dry combustion method using a LECO® Carbon Analyzer model CN-628 [31]. For soil physical evaluations, undisturbed soil samples were collected using a metal ring (0.05 m in diameter and 0.05 m in height). In the laboratory, soil samples were saturated and carried over to a tension table (− 6 kPa) to determine macroporosity (MaP, m−3 m−3) by the difference between microporosity and total porosity as described by Teixeira et al. [28]. Bulk density (BD, Mg m−3) was calculated by dividing soil dry mass after drying at 105 °C for 48 h by the cylinder volume, as described by Teixeira et al. [28].
Data Analyses
Soil macrofauna data were statistically analyzed for each site (clay soil 1 and 2, sandy soil 1 and 2) for the full 0 to 0.30-m depth increment by summing organisms for the three sampled depths. Soil macrofauna were analyzed for total abundance (number of individuals per square meter) of each taxonomic group, richness (number of taxa), and diversity (Shannon index). Normality of the data was confirmed by the Shapiro-Wilk test (p > 0.05). Data transformations were not required to meet assumptions for analysis of variance (ANOVA). The macrofauna data for each site (clay soil 1-dry, clay soil 2-rainy, sandy soil 1-dry, sandy soil 2-rainy) were analyzed with a one-way ANOVA to test straw removal and season interactions. Clayey soil 1 was also evaluated with a two-way ANOVA to test temporal effects of straw management and season (dry vs rainy). For all analyses, when main or interactive treatments were significant (p < 0.05), mean values were compared using Tukey’s test. By recognizing that the Formicidae family has a colony behavior and high abundance in comparison with other taxa, the same analyses were done excluding this family (Table S2).
In addition to these indices, the magnitude of response for each macrofauna taxon to partial (LR and HR) or total straw removal (TR), were compared with NR by calculating a V index (Eq. 1) as proposed by Wardle [32] and, more recently, by Jiang et al. [33]. The V index ranged from − 1 to 1, with negative values indicating that straw removal (i.e., LR, HR, and TR) inhibited a given taxon, whereas positive values indicate that the straw removal stimulates the presence of a given taxon. V index = 0 indicate equal abundance with or without straw removal.
where aM, abundance of individuals in managed systems (i.e., straw removal—LR or HR or TR); aNM, abundance of individuals in non-managed systems (i.e., no-straw removal—NR). In cases where values of aM = NM = 0 the equation was not applied and V index was considered equal to 0 (i.e., no treatment effect).
A principal component analysis (PCA) was also performed to investigate relationships among macrofauna taxa abundances, soil chemical, and physical attributes. Soil macrofauna community and soil attributes were subjected to PCA using Bray-Curtis distance, after a Hellinger transformation to eliminate unit effects and distribution range differences. For this analysis, only the measurements taken during the dry season (July 2017) for clayey soil 1 were considered. All statistical analyses were conducted using R software [34], version 3.2.2, with the vegan community ecology package [35].
Results
Soil Macrofauna in Sugarcane Fields Under Straw Removal
Eleven taxonomic groups ((1 subclass (Oligochaeta), 8 orders, 1 suborder (Isoptera) and 1 family (Formicidae)) were found distributed across five evaluations and four straw removal treatments (Fig. 2; Table S1). The Formicidae family was the most abundant group, representing 90.6% of the total collected macrofauna, followed by the Hymenoptera (3.1%), Oligochaeta (1.8%), Coleoptera (1.6%), Geophilomorpha (1.0%), Hemiptera (0.7%), Dermaptera (0.5%), Araneae (0.2%), Diplura (0.2%), Isoptera (0.1%), and Diptera (0.1%). However, the abundance and representativeness of each taxon vary according to each site and evaluation.
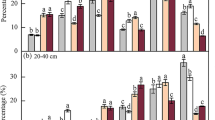
Abundance of soil macrofauna (ind. m−2) per taxon, total abundance (ind. m−2), richness, and Shannon index (H′) in the 0–0.30 m soil layer as a function of straw removal treatments (NR, no removal; LR, low removal; HR, high removal; TR, total removal). In the charts, means followed by the same lowercase, in each site and evaluation, do not differ macrofauna indexes among straw removal treatments according to Tukey’s test (p < 0.05). Absolute values of abundance and standard error per taxon are presented in Table.S1. *except Formicidae
Soil macrofauna were more abundant in clay soils with 8380 ind. m−2 in clayey soil 1 (rainy season), 9744 ind. m−2 in clayey soil 1 (dry season), and 3208 in clayey soil 2. This high abundance in clay soils was due to the Formicidae family that represented 91%, 98%, and 94% of the total organisms identified in those evaluations. In sandy soils, a total abundance of 316 ind. m−2 was found in soil 1 and 676 ind. m−2 in soil 2 (Fig. 2; Table S1).
The highest richness was also observed in clay soil 1 (rainy season) with results ranging from 2.0 to 5.0 taxa among straw removal treatments, followed by dry season measurements from in the same site—1.3 to 3.5 taxa (Fig. 2). In clayey soil 2, the richness varied from 1.5 to 3.3 taxa, and in sandy soils it varied from 0.5 to 2.0 taxa (Fig. 2). The diversity (Shannon index) varied from 0.10 to 1.11 in clayey soil 1 for both evaluations; from 0.21 to 0.56 in clayey soil 2, from 0.16 to 0.58 in sandy soil 1, and from 0.02 and 0.41 in sandy soil 2.
Our findings showed site-specific soil macrofauna responses to sugarcane straw removal. Significant changes (p < 0.05) induced by straw removal on total abundance and diversity index were observed in clayey soil 1 in both seasons. In rainy season (i.e., rainy summer–closed canopy), TR reduced macrofauna abundance as compared with HR, but not from LR and NR (Fig. 2). By disregarding Formicidae data, the abundance became substantially reduced in TR (36 ind. m−2) as compared with NR (340 ind. m−2) (Table S2). The diversity index was higher in HR and NR than in LR, with no differences as far as TR (Fig. 2). However, no significant differences were identified when Formicidae data were not considered in the evaluation (Table S2).
In dry season (i.e., dry winter with open canopy), macrofauna indexes were more sensitive to straw removal effects, with higher abundance in LR than in other treatments (Fig. 2). However, when Formicidae data were not included, HR and TR were lower under NR and LR. Straw removal also induced the reduction of macrofauna richness and diversity in this site and season. (Fig. 2; Table S2).
In clayey soil 2 (i.e., later harvesting–rainy summer), differences in abundance occurred when Formicidae data were not included, wherein TR showed lower abundance than HR with no differences as far as LR and NR (Table S2). Higher straw removal rates (HR and TR) reduced macrofauna richness in comparison with LR and NR (Fig. 2). However, no changes were identified when Formicidae data were not included (Table S2). Similarly, to clayey soil 1 during rainy season, no differences in richness were found in clayey soil 2.
As for sandy soils, the impacts of straw removal were not significant (Fig. 2). Treatments differed significantly only with the exclusion of Formicidae data from sandy soil no. 1 (Table S2). At this site, abundance and diversity indexes were lower in HR and TR as compared with LR and NR, while richness decreased as a result of increases in straw removal (Table S2). In sandy soil no. 2 (i.e., later harvesting–rainy summer), no significant differences were identified among straw removal treatments (Fig. 2; Table S2).
Behavior of Taxonomic Groups Under Straw Removal
Overall, soil macrofauna was sensitive to sugarcane straw removal, but V index revealed that each taxonomic group (taxon) responds differently to this management, providing responses reflecting site-specific conditions (Fig. 3). Analyses of each taxon showed that Araneae was stimulated under TR, except for clayey soil 1-rainy season, where those organisms were inhibited. Coleoptera was stimulated under LR and inhibited under HR and TR treatments (V index < 0.0). In clayey soil 1 during rainy season, Coleoptera was also inhibited under TR but mildly affected under HR and LR. Oligochaeta appeared only in clayey soil 1 and sandy soil 1, where abundance decreased as straw removal increased, being extremely inhibited under TR. The Formicidae family was also inhibited under TR clayey soils, where high abundance of these organisms is found. In sandy soils, where abundance is low, Formicidae was stimulated by straw removal, occurring only under TR and HR. Geophilomorpha occurred mostly in clayey soil 1, being inhibited under straw removal, as seen in both evaluations. Values of V index from Diplura, Dermaptera Diptera, Hemiptera, Hymenoptera, and Isoptera oscillated between positive, null, and negative values across areas, indicating that sensitivity responses from those taxa to straw removal vary according to soil types, harvesting seasons, and time of evaluation.
V Index for the soil macrofauna taxa in the 0–0.30 m layer as a function of straw removal rates (NR, no removal; LR, low removal; HR, high removal; TR, total removal) in clayey soil 1 (rainy and dry season), clayey soil 2, sandy soil 1, and sandy soil 2. *In clayey soil 1, symbols with solid contour line represent data from dry season, whereas symbols with dashed contour line represent data from rainy season.
Soil Macrofauna Community Linked to Soil Chemical and Physical Attributes
The ordination of soil macrofauna and its relationship with soil chemical and physical attributes were examined by PCA in all four sites (Fig. 4). In clayey soil 1, only the dry season was considered in this analysis. Absolute values of soil chemical and physical attributes are presented in the supplementary material (Table S3). In clayey soil 1, the first two axes explained 53.7% of the variance (Fig. 4a, b). At this site, PCA ordination showed that plots under HR and TR were mainly associated with high soil compaction (higher BD), indicating negative correlation with the abundance of all macrofauna organisms, except for Araneae (Fig. 4a, b; Table S3). On the other hand, LR and NR were associated with greater soil quality and higher soil macrofauna abundance (Fig. 4b). Oligochaeta, Geophilomorpha, Diptera, Hemiptera, Coleoptera, and Formicidae were positively correlated with NR and LR, especially when associated with higher soil moisture, MaP and available P (Fig. 4a, b; Table S3). In clayey soil 2, the two first axes explained 53.6% of the variance. TR was also associated with high BD and Araneae abundance, while other organisms were more associated with HR, LR, and NR, and positively correlated with soil moisture, SOC, P, SB, and especially with higher MaP (Fig. 4c, d; Table S3).
Results of principal component analysis (PCA) of soil macrofauna and soil attributes across straw removal managements (NR, no removal; LR, low removal; HR, high removal; TR, total removal) at clayey soil 1 (a, b), clayey soil 2 (c, d), sandy soil 1 (e, f), and sandy soil 2 (g, h). MaP, soil macroporosity; SOC, soil organic carbon; P, available phosphorus; BS, bases sum (K+, Ca+2, Mg+2)
In sandy soils (1 and 2), the two first axes explained 42.6% and 48.2% of the variance, respectively (Fig. 4e, f; g, h). In sandy soil 1, data showed that Formicidae was positively correlated with TR and BD, associated with low values for P, while the abundance of other taxa were positively correlated with NR and LR, especially when associated with moisture, SOC, P and BS (Fig. 4e, f; Table S3). In sandy soil 2, greater values of soil chemical and physical indicators were positively correlated with NR and LR. Coleoptera and Hymenoptera, the most abundant groups in this site, were correlated with NR and LR, whereas other groups were mainly associated with HR and TR treatments and higher BD.
Seasonal Macrofauna Changes in Clayey Soil 1
Analysis of variance revealed interactions between straw removal and season evaluations of clayey soil 1 (Table 2). Regarding abundance, the interaction occurred in LR (F = 27.83, p < 0.0001) and HR (F = 6.60, p < 0.0178), indicating possible foraging behavior and search for soil moisture by some macrofauna organisms among treatments, depending on climatic conditions. Total abundance interactions can be explained mainly by Formicidae behavior (Table S4), which showed significant interactions among LR (F = 74.65, p < 0.0001), HR (F = 16.70, p < 0.0005), and seasons. Similar pattern was observed for Geophilomorpha (Table S4).
High abundance of Oligochaeta (earthworms), one of the most relevant indicators of soil quality, occurred mostly during rainy season. In the sugarcane crop, earthworms were positively related to the straw amount left on the soil surface (Fig. 5). Interactions among straw removal and season were also detected (Table S4). Results clearly showed that the straw removal induced a drastic reduction of earthworms, particularly in rainy season (F = 17.02, p < 0.0001), when abundance was higher under NR (236 ind. m−2), followed by HR, LR, and TR (40, 48, and 4 ind. m−2, respectively). In the dry season, however, Oligochaeta was not found under HR and TR treatments
Even though richness was significantly affected by straw removal and season, no interactions were detected (Table 2). However, significant differences were observed between seasons, with high richness occurring during rainy season in comparison with the dry season (Table 2), even disregarding Formicidae data (data not showed). Similar to total abundance, interactions between straw removal and season were found within the diversity index (Table 2). Most importantly, soil diversity responses to straw removal presented substantial variation during rainy season, but no straw removal rate produced significant decrease in soil macrofauna diversity in dry season (Fig. 2).
Discussion
Soil Macrofauna Responses to Sugarcane Straw Removal
The maintenance of crop residues covering soil surface is a key factor for the suitable functioning of soil [36] since the removal of crop residues disturbs the equilibrium among soil organisms, leading to the reduction of individuals and taxa until a new equilibrium can be reestablished [37]. The negative impacts induced by the absence of crop residues on soil macrofauna have been reported in several cropping systems (e.g., [37,38,39,40]). However, to the best of our knowledge, the effects of sugarcane straw removal rates on soil macrofauna have not been widely investigated, being restricted to a few preliminary studies [22,23,24]. Therefore, this study provides a more comprehensive approach, being the first study to encompass field experiments under different edaphoclimatic conditions in south-central Brazil.
Our results clearly revealed that TR sharply impairs soil macrofauna, causing the reduction of abundance, richness, and diversity of soil macrofauna under this conditions. Those results are in line with findings reported by Portillo et al. [23], who showed that TR treatments sustained lower richness, diversity, and abundance of soil organisms than NR treatments (maintaining 16.9 Mg ha−1 of straw) in a clayey soil experiment conducted in Mato Grosso do Sul state, Brazil. Similar results were reported by Abrão [22] who found that the macrofauna were strongly influenced by straw removal, presenting greater density, richness, and diversity when at least 50% (i.e., 7.6 Mg ha−1) of straw was left on the soil surface. Nonetheless, although TR produces detrimental effects on soil, maintaining as much sugarcane straw as possible in the field, such as under NR, may not always guarantee macrofauna abundance and richness. Therefore, intermediate straw removal rates may be a strategy to increase bioenergy production with minimum impacts on soil macrofauna, although long-term experiments are still needed to confirm those results.
It is worth noting, as demonstrated in this study, that the magnitude of soil macrofauna responses is highly influenced by edaphoclimatic conditions and management practices adopted in each sugarcane field. Under this scenario, it is very likely that in 4 years the straw removal would be even more harmful to sandy soil macrofauna since this type of soil (e.g., low soil organic matter and water holding capacity) presents more adverse conditions to sustain high biodiversity than clayey soils. Nevertheless, the highest response was observed in clayey soil 1, in both evaluations (rainy and dry seasons), showing lower soil macrofauna indexes under TR as compared with NR or partial straw removal (HR and LR). Higher responses to straw removal in clayey soils were also observed in studies by Castro et al. [41] and Carvalho et al. [42] when investigating changes on pest infestation and sugarcane yield. Likewise, those responses were associated with greater water holding capacity [43] and soil C stocks [19], influencing soil macrofauna.
Macrofauna responses to straw removal were less intense in sandy soils. This was also identified by Abreu et al. [24] who observed that macrofauna was not affected by the amount of straw left on the soil surface (sandy soil) during a rainy season in Piauí state, northeastern Brazil. When straw deposition occurs in late season (rainy summer), sugarcane tillering is fast and the effect of straw on soil macrofauna can be reduced as a result of the microclimate created by closed sugarcane canopy, as observed in sandy soil 2. Despite that, according to Abreu et al. [24], when at least part of the straw is maintained on the soil, the macrofauna can be favored in the long term. An indication of this response was observed in sandy soil 1, where abundance, richness, and diversity of macrofauna (Table S2), excluding ants, were lower under TR and HR than under LR and NR.
Moreover, site-specific management practices might impair soil macrofauna, which was identified in late harvesting sites (clayey soil 2 and sandy soil 2). Surprisingly, low abundance of soil macrofauna was found in those sites as well as a complete inhibition of some important organisms such as Oligochaeta and Geophilomorpha, even in NR treatment. In the experiments, the sugarcane was harvested shortly before macrofauna assessments (about 80 days) and studies indicated that the areas harvested in rainy season, such as clayey soil 2 and sandy soil 2, are more susceptible to soil compaction and can compromise macrofauna habitats [44, 45]. Soil compaction affects the habitat and can hinder the reestablishment of communities, especially those organisms such as Oligochaeta and Geophilomorpha.
Another potential reason for lower abundance and total inhibition of Oligochaeta and Geophilomorpha in later harvesting sites may be associated with the higher volume of herbicides applied during the period prior to the evaluation of macrofauna. The use of herbicides may momentarily eliminate individuals from some levels of the trophic web [46], compromising the development of individuals at early stages of life, such as larvae and eggs. Due to their excavating habit, those organisms come into direct contact with applied substances by ingesting contaminated soil during feeding or by absorbing them through their cuticles, thus reducing the population [47]. Previous studies also demonstrated that Oligochaeta and other invertebrates, even soil microbiota, can present an avoidance behavior, keeping away from soil treated with herbicides, depending on their concentration and amount [48,49,50,51,52,53,54,55,56]. Moreover, Silva et al. [53] observed that in a sugarcane field the fauna population can recover after a period of herbicide applications, with changes in soil fauna abundance occurring between the day of herbicide application up to 40 or 80 days thereafter.
This study included an analysis of one of the same active ingredients applied at clay soil 2 and sandy soil 2 (i.e., tebuthiuron), but in our study only a single assessment right after herbicide application (20 days) was carried out. This could explain the absence of some organisms anticipated to be responsive to straw removal. Therefore, we strongly recommend that further studies investigate the interactions between herbicides and sugarcane straw removal on soil macrofaunal communities.
In summary, these results show that TR negatively affects the abundance, richness, and diversity of soil macrofauna. However, our results were highly site-specific and based on only a single sampling event for all sites, except clayey soil 1. While these limited results are insightful, our data are still preliminary and should not be seen as conclusive as far as suitable straw removal rates for sustainable macrofauna and extra feedstock for bioenergy production are concerned.
Straw Removal and Important Organisms in Sugarcane Production System
In addition to the specific conditions from each site, results showed that each taxon may respond differently to straw removal. Some groups of macroinvertebrates are more responsive and sensitive to changes occurring in the agricultural system, being considered bioindicators of soil quality [4, 57]. Bioindicators are species, groups, or communities whose presence or absence, as well as behavior, reveal specific soil conditions [57]. Our results showed that some organisms might be more sensitive to straw removal, giving some insights that could be considered bioindicators in this system.
In spite of the avoidance behavior in later harvesting sites, earthworms were sensitive to straw removal in sites where they were found (Fig. 3). This order is among the most important bioindicators of soil quality and ecosystem functioning [58] due to their sensitivity to low soil moisture [6], soil compaction [59], pesticide [60], low organic matter, and low quantity of crop residue on soil surface [6, 61]. It suggests that indiscriminate straw removal might be harmful to the soil quality and, consequently, to sugarcane production.
Beetles (Coleoptera) are an important order in sugarcane fields. Our results showed that they were stimulated by LR and inhibited by HR and TR, when compared with NR across all areas (Fig. 3). Some species of this order are beneficial to sugarcane, because they participate in the straw decomposition and contribute to nutrient cycling and translocation of organic matter in the soil, in addition to creating galleries that enhance soil aeration [58]. On the other hand, some of these species are considered important pests in sugarcane fields, such as Sphenophorus levis, known for causing losses in sugarcane production of up to $500 million per year [16]. In line with our results that showed inhibition of beetles in TR compared with NR, Castro et al. [41] recently showed that the levels of ratoon damage from Sphenophorus levis increased in NR treatment. However, these authors showed that straw removal is not fully effective to control this pest, and thus, other control methods (chemical and/or biological) are still needed. In this present study, macrofauna were not identified to species, but we encourage this level of detail for future studies.
Ants (Formicidae family) were the most abundant organisms found in our study, which is in agreement with previous studies [15, 23, 24]. As pointed out by Jiang et al. [33], ants produce local changes that tend to affect the equilibrium and the structure of communities in a number of ways. Similarly to beetles, ants can be beneficial or harmful to sugarcane fields. These organisms are known to be the “soil engineers,” improving soil structure, nutrient recycling, and food transport to other individuals, besides being microarthropod controllers in the soil, enabling them to act as regulators of population and biological control [59, 60]. On the other hand, some ant species, mainly the “cutters,” affect negatively sugarcane growth and yield. Therefore, changes in their abundance are decisive in increasing or decreasing insecticide applications in sugarcane plantations. It is worth highlighting the high inhibition of ants by TR in clayey soils where they are widely abundant. However, Formicidae family presented V index values oscillating between positive, null, and negative across the evaluated sites and treatments (Fig. 3). The absence of a specific response of ants to straw removal management may have been influenced by the fact that these organisms can easily move around and have high adaptive capacity to climatic conditions, regardless of straw removal treatments. Ants can adapt to local conditions, being one of the few taxa able to colonize areas to the detriment of others [60]. They can be found in either well-managed crop systems [61] or in poorly managed and low-complexity environments which had undergone soil degradation processes [62,63,64].
Based on this discussion, we highlight the importance of understanding the behavior of the main representatives of the soil macrofauna. This will be substantially helpful to establish appropriate bioindicators for straw removal and associated crop management.
Soil Macrofauna Community Linked to the Soil Quality Under Straw Removal Treatments
Soil macrofauna are closely linked to other soil attributes and ecosystem functions [6, 7, 65]. Our results suggested that the negative effects of straw removal on soil quality can be directly and indirectly associated with soil macrofauna changes. Therefore, as straw removal produces detrimental effects on soil, it is hard to define the cause-effect relation between changes in soil processes and functions, and changes in soil macrofauna communities.
High straw removal rates (HR and TR) favor water losses to the atmosphere, reducing soil moisture (Table S3). Previous studies showed that straw removal can intensify the effect of direct solar radiation on soil, increasing thermal amplitude in soil [43, 66] and soil erosion [67], thus compromising organism habitats. All these factors have a direct effect on soil macrofauna, especially on most sensitive communities. Our results show that higher presence macrofauna in clayey soils was positively correlated with higher soil moisture under NR and LR than in HR and TR (Fig. 4; Table S3).
Straw is also the main C input in sugarcane soils [68]. Since indiscriminate removal causes the reduction in the source for soil biota, the soil organic matter cycling are also reduced, leading to gradual depletion of soil C stocks [19]. Furthermore, as straw decomposition rates are greatly reduced in the absence of soil fauna, especially under intensive straw removal [69], nutrient recycling also decreases. The multivariate analysis revealed that the macrofauna abundance was positively correlated with higher levels of P and BS after 4 years, especially in clayey soils. This corroborates the data from Cherubin et al. [70], who reported that labile and biological P soil pools were closely associated with richness and diversity of macrofauna in the soil. Thus, the impacts of straw removal on soil macrofauna can be harmful to soil fertility and, potentially, to sugarcane production over time.
Lastly, some macrofauna groups, such as earthworms (Oligochaeta), termites (Isoptera), beetles (Coleoptera), and ants (Formicidae), play important roles in controlling soil structural dynamics [59]. In general, our results showed that the soil macrofauna abundance was positively correlated with better soil physical attributes such as MaP. In this context, macrofauna influences soil structure by either incorporating fresh organic matter in soil profile or by coating their galleries [59], since C is one of the main factors accounting for soil structure and pore stability [71]. Thereby, macrofauna act directly and indirectly on soil aggregation [11]. Some earthworm species and social insects build galleries and translocate soil aggregates into the soil profile without changing their internal organization. Most termites are able to modify the internal organization of soil aggregates, forming new biogenic aggregates [6, 11, 59]. The role of these soil engineers in soil structure was investigated by several authors [72,73,74,75]. In sugarcane areas, Franco et al. [76] verified a positive relationship between the abundance of macrofauna, particularly Isoptera and Coleoptera, and higher structural quality of soil. In a parallel study, Castioni et al. [77] also verified that reduced abundance of Oligochaeta correlates with higher BD and soil resistance to penetration under straw removal. Total removal directly affects soil physical quality and increases soil compaction [77, 78], contributing to lower soil macrofauna abundance and diversity.
Therefore, our results suggest that sugarcane straw removal affects soil quality, inducing direct changes in soil macrofauna and/or through synergistic effects that interconnect the presence and the activities of macrofauna communities with the status of soil chemical and physical attributes.
Seasonal Macrofauna Changes in Clay Soil 1
Soil organisms are sensitive to changes in the soil and the environment. Seasonal changes in macrofauna are associated with soil moisture and temperature, food source, and reproductive cycle of organisms [24, 37, 79]. Greater richness, diversity, and abundance of some soil macrofauna organisms were observed in rainy season as compared with dry season (Table 2; Table S4), which is in line with studies on different soil types, crop species, and regions [80]. Our study site is located in a humid subtropical climate zone where seasonal variations are considerable. In November (rainy season), the climate is characterized by a warm and humid summer, whose conditions are favorable to greater richness and abundance of soil macrofauna. On the other hand, the climate in July is characterized by a dry winter with lower temperatures and higher water restriction. This drought period limits the proliferation of soil organisms. According to Lavelle and Spain [4], temperature and moisture are the main factors necessary to activate metabolic regulation in macrofauna individuals and determine the spatial distribution in the habitat and the periods of higher organism activity. The stage of sugarcane development should also be taken into account, since in the rainy season the canopy was completely closed, providing greater protection and shading to the soil. This microclimate, modulated by plant canopy, was the opposite to those conditions observed in the dry period, where sugarcane was in the early growth stage at the time of macrofauna sampling. Similarly, Abreu et al. [24] reported greater macrofauna abundance in the sugarcane field in rainy season as compared with the dry season soon after harvesting.
The abundance of some macrofauna groups, such as Formicidae, was affected by the interaction of straw removal and season effects. Under water restriction, some organisms move to environments under better survival conditions [24]. Likewise, our study showed changes in the activity areas of some macrofauna organisms in intermediate straw removal rates (LR and HR) throughout seasonal evaluations. In searching for favorable conditions, some invertebrates, especially Formicidae, started being more abundant under LR treatment during the dry season. In rainy season, the same organisms were more abundant in HR treatment. Nevertheless, TR was an inhospitable environment to macrofauna, even in the most favorable climate season.
Earthworms were also responsive to interactions among straw removal and seasons (Fig. 5). Presley et al. [66] pointed out that below a critical soil moisture level, earthworms decrease their metabolism and reproductive activity and may even desiccate or go into a period of estivation [81]. In the dry season, no individuals were found under HR and TR, while in the rainy season, when soil moisture is less restrictive, some individuals were found in those treatments. Despite the similar abundance of earthworm observed in HR and LR in rainy season, the individuals were mostly found at the 0.10–0.20 m soil layer under HR, whereas all individuals were found at the 0–0.10 m soil depth under LR (results not shown). This is an indication that under intensive straw removal these organisms gradually become more active in deeper soil layers, where they are less affected by soil warming and drying cycles.
Conclusion
This pioneering study investigating soil macrofauna responses to sugarcane straw removal under different edaphoclimatic conditions in Brazil brought promising results and offers opportunities and insights for future studies. Our findings revealed that total straw removal impaired soil macrofauna, and the magnitude of responses are closely related to soil and climatic (seasons) conditions and management practices adopted in sugarcane fields. On the other hand, partial straw removal may be a sustainable strategy to increase the bioenergy production with minimum impacts on soil macrofauna.
Formicidae, Oligochaeta, and Coleoptera were substantially affected by straw removal. While negative impacts from intensive straw removal on the abundance of earthworms are critical to high soil quality sustainability and plant growth, inhibition of some species of ants and Coleoptera (sugarcane pests) can be beneficial for plants. Therefore, the results provided by this study certainly encourage further studies on species and behavior of most representative taxa of soil macrofauna so that more appropriate bioindicators can be established for sugarcane straw removal management.
Moreover, close linkage between soil macrofauna, soil chemical, and physical processes, especially in clayey soils, were identified by this study. No removal was positively related to higher soil moisture, macropores, SOC, and soil fertility, which are normally associated with higher abundance of most macrofauna organisms. In contrast, total removal of straw was associated with higher soil compaction and lower abundance. Therefore, indiscriminate straw removal jeopardizes soil macrofauna and may substantially affect the soil quality and its capacity to provide multiple ecosystem services. Thus, an integrated approach associated with biological, physical, and chemical soil indicators should be designed and adopted to predict sustainable management strategies for sugarcane straw removal in Brazil.
References
Wanner M, Dunger W (2002) Primary immigration and succession of soil organisms on reclaimed opencast coal mining areas in eastern Germany. Eur J Soil Biol 38:137–143. https://doi.org/10.1016/S1164-5563(02)01135-4
Batáry P, Báldi A, Samu F et al (2008) Are spiders reacting to local or landscape scale effects in Hungarian pastures? Biol Conserv 141:2062–2070. https://doi.org/10.1016/j.biocon.2008.06.002
Swift MJ, Heal OW, Anderson JM, Anderson JM (1979) Decomposition in terrestrial ecosystems. Berkeley, Univ of California Press
Lavelle P, Spain AV (2001) Soil ecology. Springer Science & Business Media, Netherlands
Brussaard L (1997) Biodiversity and ecosystem functioning in soil. Ambio 26:563–570 doi: https://www.jstor.org/stable/4314670. Accessed 26 January 2019
Lavelle P, Decaëns T, Aubert M et al (2006) Soil invertebrates and ecosystem services. Eur J Soil Biol 42:S3–S15. https://doi.org/10.1016/j.ejsobi.2006.10.002
Bardgett RD, van der Putten WH (2014) Belowground biodiversity and ecosystem functioning. Nature 515:505–511. https://doi.org/10.1038/nature13855
Soliveres S, van der Plas F, Manning P et al (2016) Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 536:456–459. https://doi.org/10.1038/nature19092
Nielsen UN (2019) Soil fauna assemblages. Ecol Biodivers Conserv. https://doi.org/10.1017/9781108123518
Wall DH, Adams G, Parsons AN (2001) Soil biodiversity. In: Chapin FS, Sala OE, Huber-Sannwald E (eds) Glob. Biodivers. a Chang. Environ. Springer Verlag, New York, pp 47–82
Lehmann A, Zheng W, Rillig MC (2017) Soil biota contributions to soil aggregation. Nat Ecol Evol 1:1828–1835. https://doi.org/10.1038/s41559-017-0344-y
Léonard J, Rajot JL (2001) Influence of termites on runoff and infiltration: quantification and analysis. Geoderma 104:17–40. https://doi.org/10.1016/S0016-7061(01)00054-4
Decaëns T, Jiménez JJ, Gioia C et al (2006) The values of soil animals for conservation biology. Eur J Soil Biol 42:S23–S38. https://doi.org/10.1016/j.ejsobi.2006.07.001
Keesstra S, Pereira P, Novara A et al (2016) Effects of soil management techniques on soil water erosion in apricot orchards. Sci Total Environ 551–552:357–366. https://doi.org/10.1016/j.scitotenv.2016.01.182
Franco ALC, Bartz MLC, Cherubin MR et al (2016) Loss of soil (macro)fauna due to the expansion of Brazilian sugarcane acreage. Sci Total Environ 563–564:160–168. https://doi.org/10.1016/j.scitotenv.2016.04.116
Dinardo-Miranda LL, Fracasso JV (2013) Sugarcane straw and the populations of pests and nematodes. Sci Agric 70:305–310. https://doi.org/10.1590/S0103-90162013000500012
Pasqualin LA, Dionisio JA, Zawadneak MAC, Marcal CT (2012) Macrofauna edáfica em lavouras de cana-de-açúcar e mata no noroeste do Paraná - Brasil. Semin Ciências Agrárias 33:7–18. https://doi.org/10.5433/1679-0359.2012v33n1p7
Benazzi EDS, Bianchi MDO, Correia MEF et al (2013) Impactos dos métodos de colheita da cana-de-açúcar sobre a macrofauna do solo em área de produção no Espírito Santo – Brasil. Semin Ciências Agrárias 34:3425–3442. https://doi.org/10.5433/1679-0359.2013v34n6Supl1p3425
Bordonal R d O, Menandro LMS, Barbosa LC et al (2018) Sugarcane yield and soil carbon response to straw removal in south-central Brazil. Geoderma 328:79–90. https://doi.org/10.1016/j.geoderma.2018.05.003
Carvalho JLN, Nogueirol RC, Menandro LMS et al (2017) Agronomic and environmental implications of sugarcane straw removal: a major review. GCB Bioenergy 9:1181–1195. https://doi.org/10.1111/gcbb.12410
Menandro LMS, Cantarella H, Franco HCJ et al (2017) Comprehensive assessment of sugarcane straw: implications for biomass and bioenergy production. Biofuels, Bioprod Biorefining 11:488–504. https://doi.org/10.1002/bbb.1760
Abrao JS (2012) Niveis de Palhadas e Preparos do Solo em Cultivos de Cana-de-Acucar: Impacto Sobre a Fauna Edáfica e Epigeica. THESIS, UEMS
Portilho IIR, Borges CD, Costa AR et al (2011) Resíduos da cultura da cana-de-açúcar e seus efeitos sobre a fauna invertebrada epigéica. Semin Ciências Agrárias 32:959–970. https://doi.org/10.5433/1679-0359.2011v32n3p959
de Abreu RRL, de Lima SS, de Oliveira NCR, Leite LFC (2014) Fauna edáfica sob diferentes níveis de palhada em cultivo de cana-de-açúcar. Pesqui Agropecuária Trop 44:409–416. https://doi.org/10.1590/S1983-40632014000400002
Alvares CA, Stape JL, Sentelhas PC et al (2013) Köppen’s climate classification map for Brazil. Meteorol Zeitschrift 22:711–728. https://doi.org/10.1127/0941-2948/2013/0507
USDA, United States Department of Agriculture, Natural Resources Conservation Service (1998) Keys to Soil Taxonomy, Soil Survey Staff, Washington, D.C
Raij B, de Andrade JC, Cantarella H, Quaggio JA (2001) Análise química para avaliação da fertilidade de solos tropicais. Instituto Agronômico, Campinas
Teixeira PC, Donagemma GK, Fontana A, Teixeira WG (2017) Manual de métodos de análise de solo. EMBRAPA Solos, Brasília
Anderson JM, Ingram JSI (1993) Tropical soil biology and fertility: a handbook of methods. CAB Internacional, Wallingford
Triplehorn CA, Johnson NF (2011) Estudo dos insetos. Cengage Learning, São Paulo
Nelson DW, Sommers L (1982) Total carbon, organic carbon, and organic matter 1. In: Methods soil Anal. Part 2. Chem. Microbiol. Prop. American Society of Agronomy, Soil Science Society of America (SSSA. Agronomy Monograph, 9), Madison, pp 539–579
Wardle DA (1995) Impacts of disturbance on detritus food webs in agro-ecosystems of contrasting tillage and weed management practices Adv. Ecol. Res. 26(1995):105–185. https://doi.org/10.1016/S0065-2504(08)60065-3
Jiang Y, Ma N, Chen Z, Xie H (2018) Soil macrofauna assemblage composition and functional groups in no-tillage with corn stover mulch agroecosystems in a mollisol area of northeastern China. Appl Soil Ecol 128:61–70. https://doi.org/10.1016/j.apsoil.2018.04.006
Team RDC (2018) R: a language and environment for statistical computing. R Found. Stat. Comput, Vienna, Austria
Oksanen J, Kindt R, Simpson GL (2018) Package ‘vegan3d’ Static and Dynamic 3D Plots for the 'vegan' Package. http://CRAN.Rproject.org/package=vegan3d. Accessed 10 January 2019
Cherubin MR, Oliveira DM d S, Feigl BJ et al (2018) Crop residue harvest for bioenergy production and its implications on soil functioning and plant growth: a review. Sci Agric 75:255–272. https://doi.org/10.1590/1678-992x-2016-0459
Baretta D, Mafra ÁL, Santos JCP et al (2006) Análise multivariada da fauna edáfica em diferentes sistemas de preparo e cultivo do solo. Pesqui Agropecuária Bras 41:1675–1679. https://doi.org/10.1590/S0100-204X2006001100014
Gatiboni LC, Coimbra JLM, Wildner LDP, Denardin RBN (2009) Modificações na fauna edáfica durante a decomposição da palhada de centeio e aveia preta, em sistema plantio direto. Biotemas 22:45–53. https://doi.org/10.5007/2175-7925.2009v22n2p45
Errouissi F, Ben Moussa-Machraoui S, Ben-Hammouda M, Nouira S (2011) Soil invertebrates in durum wheat (Triticum durum L.) cropping system under Mediterranean semi arid conditions: a comparison between conventional and no-tillage management. Soil Tillage Res 112:122–132. https://doi.org/10.1016/j.still.2010.12.004
Bedano JC, Domínguez A, Arolfo R, Wall LG (2016) Effect of Good Agricultural practices under no-till on litter and soil invertebrates in areas with different soil types. Soil Tillage Res 158:100–109. https://doi.org/10.1016/j.still.2015.12.005
de Castro SGQ, Dinardo-Miranda LL, Fracasso JV et al (2019) Changes in soil pest populations caused by sugarcane straw removal in Brazil. BioEnergy Res. https://doi.org/10.1007/s12155-019-10019-4
Carvalho JLN, Menandro LMS, de Castro SGQ et al (2019) Multilocation straw removal effects on sugarcane yield in South-Central Brazil. BioEnergy Res. https://doi.org/10.1007/s12155-019-10007-8
Ruiz Corrêa ST, Barbosa LC, Menandro LMS et al (2019) Straw removal effects on soil water dynamics, soil temperature, and sugarcane yield in South-Central Brazil. BioEnergy Res. https://doi.org/10.1007/s12155-019-09981-w
Robertson LN, Kettle BA, Simpson GB (1994) The influence of tillage practices on soil macrofauna in a semi-arid agroecosystem in northeastern Australia. Agric Ecosyst Environ 48:149–156. https://doi.org/10.1016/0167-8809(94)90085-X
Brévault T, Bikay S, Maldès JM, Naudin K (2007) Impact of a no-till with mulch soil management strategy on soil macrofauna communities in a cotton cropping system. Soil Tillage Res 97:140–149. https://doi.org/10.1016/j.still.2007.09.006
Bartz MLC, Brown GG, da Rosa MG et al (2014) Earthworm richness in land-use systems in Santa Catarina, Brazil. Appl Soil Ecol 83:59–70. https://doi.org/10.1016/j.apsoil.2014.03.003
Papini S, Andréa MM (2004) Ação de minhocas Eisenia foetida sobre a dissipação dos herbicidas simazina e paraquat aplicados no solo. Rev Bras Ciência do Solo 28:67–73. https://doi.org/10.1590/S0100-06832004000100007
de Sousa APA, de Andréa MM (2011) Earthworm (Eisenia andrei) avoidance of soils treated with cypermethrin. Sensors 11:11056–11063. https://doi.org/10.3390/s111211056
Andréa MM, Peres TB, Luchini LC, Jr AP (2000) Impact of long-term pesticide applications on some soil biological parameters. J Environ Sci Heal Part B 35:297–307. https://doi.org/10.1080/03601230009373271
Fernandes WD, Cruz MCA, Faccenda O, Valente TO (2000) Impacto de herbicidas em uma guilda de formigas predadoras. Rev Bras Herbic 1:225. https://doi.org/10.7824/rbh.v1i3.339
de Andréa MM, Peres TB, Luchini LC et al (2003) Influence of repeated applications of glyphosate on its persistence and soil bioactivity. Pesqui Agropecuária Bras 38:1329–1335. https://doi.org/10.1590/S0100-204X2003001100012
Guerra RT, Bueno CR, Schubart HO (1982) Avaliação preliminar sobre os efeitos da aplicação do herbicida Paraquat e aração convencional na mesofauna do solo na região de Manaus-Am. Acta Amaz 12:7–13. https://doi.org/10.1590/1809-43921982121007
Da Silva RF, Scheid DL, Corassa GM et al (2012) Influência da aplicação de herbicidas pré-emergentes na fauna do solo em sistema convencional de plantio de cana-de-açúcar. Biotemas 25:227–238. https://doi.org/10.5007/2175-7925.2012v25n3p227
Prado AG, Airoldi C (2001) The effect of the herbicide diuron on soil microbial activity. Pest Manag Sci 57:640–645. https://doi.org/10.1002/ps.321
de Azevedo AR, Coronas MV (2019) Uso de testes de fuga com minhocas Eisenia andrei e Eisenia fetida para identificação da toxidade de agrotóxicos no Brasil: uma breve revisão da literatura. Ciência e Nat 40:18–26. https://doi.org/10.5902/2179460X35495
Monquero PA, Dos Reis FC, Munhoz WS et al (2012) Solo cultivado com cana-de-açúcar: persistência e impacto de herbicidas na microbiota no solo. Rev Bras Ciencias Agrar 7:380–387. https://doi.org/10.5039/agraria.v7i3a1169
Bünemann EK, Bongiorno G, Bai Z et al (2018) Soil quality – a critical review. Soil Biol Biochem 120:105–125. https://doi.org/10.1016/j.soilbio.2018.01.030
da SP de Almeida S, Louzada JNC (2009) Estrutura da comunidade de Scarabaeinae (Scarabaeidae: Coleoptera) em fitofisionomias do cerrado e sua importância para a conservação. Neotrop Entomol 38:32–43. https://doi.org/10.1590/S1519-566X2009000100003
Bottinelli N, Jouquet P, Capowiez Y et al (2015) Why is the influence of soil macrofauna on soil structure only considered by soil ecologists? Soil Tillage Res 146:118–124. https://doi.org/10.1016/j.still.2014.01.007
Offenberg J (2015) REVIEW: Ants as tools in sustainable agriculture. J Appl Ecol 52:1197–1205. https://doi.org/10.1111/1365-2664.12496
Pereira MP d S, Queiroz JM, Valcarcel R, Mayhé-Nunes AJ (2007) Fauna de formigas como ferramenta para monitoramento de área de mineração reabilitada na Ilha da Madeira, Itaguaí, RJ. Ciência Florest 17:197–204. https://doi.org/10.5902/198050981951
Lourente ERP, da Silva RF, da Silva DA et al (2007) Macrofauna edáfica e sua interação com atributos químicos e físicos do solo sob diferentes sistemas de manejo. Acta Sci Agron 29:17–22. https://doi.org/10.4025/actasciagron.v29i1.60
Wink C, Guedes JVC, Fagundes CK, Rovedder AP (2005) Insetos edáficos como indicadores da qualidade ambiental soil. Rev Ciências Agroveterinárias 4:60–71 doi: http://200.19.105.203/index.php/agroveterinaria/article/view/5405
Andersen AN, Hoffmann BD, Muller WJ, Griffiths AD (2002) Using ants as bioindicators in land management: simplifying assessment of ant community responses. J Appl Ecol 39:8–17. https://doi.org/10.1046/j.1365-2664.2002.00704.x
Commision E, Orgiazzi A, Bardgett R, et al. (2016) Global soil biodiversity atlas. European Commission. Publications Office of the European Union, Luxembourg
Presley MLL, McElroy TC, Diehl WJ (1996) Soil mosture and temperature interact to affect growth, survivorship, fecundity, and fitness in the earthworm Eisenia fetida. Comp Biochem Physiol - A Physiol 114:319–326. https://doi.org/10.1016/0300-9629(96)00017-5
de Souza ZM, Prado R d M, Paixão ACS, Cesarin LG (2005) Sistemas de colheita e manejo da palhada de cana-de-açúcar. Pesqui Agropecuária Bras 40:271–278. https://doi.org/10.1590/S0100-204X2005000300011
Carvalho JLN, Hudiburg TW, Franco HCJ, DeLucia EH (2017) Contribution of above- and belowground bioenergy crop residues to soil carbon. GCB Bioenergy 9:1333–1343. https://doi.org/10.1111/gcbb.12411
Pimentel LG, Cherubin MR, Oliveira DMS et al (2019) Decomposition of sugarcane straw: basis for management decisions for bioenergy production. Biomass and Bioenergy 122:133–144. https://doi.org/10.1016/j.biombioe.2019.01.027
Cherubin MR, Franco ALC, Cerri CEP et al (2016) Phosphorus pools responses to land-use change for sugarcane expansion in weathered Brazilian soils. Geoderma 265:27–38. https://doi.org/10.1016/j.geoderma.2015.11.017
Tisdall JM, Oades JM (1982) Organic matter and water-stable aggregates in soils. J Soil Sci 33:141–163. https://doi.org/10.1111/j.1365-2389.1982.tb01755.x
Brown GG, da Silva E, Thomazini MJ, et al. (2018) The role of soil fauna in soil health and delivery of ecosystem services. In: Reicosky D (ed) Managing soil health for sustainable agriculture Volume 1: Fundamentals. Burleigh Dodds Science Publishing, Cambridge, pp 197–241
Hallaire V, Curmi P, Duboisset A et al (2000) Soil structure changes induced by the tropical earthworm Pontoscolex corethrurus and organic inputs in a Peruvian ultisol. Eur J Soil Biol 36:35–44. https://doi.org/10.1016/S1164-5563(00)01048-7
Pérès G, Cluzeau D, Curmi P, Hallaire V (1998) Earthworm activity and soil structure changes due to organic enrichments in vineyard systems. Biol Fertil Soils 27:417–424. https://doi.org/10.1007/s003740050452
Portilho IIR, Crepaldi RA, Borges CD et al (2011) Fauna invertebrada e atributos físicos e quimicos do solo em sistemas de integração lavoura-pecuária. Pesqui Agropecu Bras 46:1310–1320. https://doi.org/10.1590/S0100-204X2011001000027
Franco ALC, Cherubin MR, Cerri CEP et al (2017) Relating the visual soil structure status and the abundance of soil engineering invertebrates across land use change. Soil Tillage Res 173:49–52. https://doi.org/10.1016/j.still.2016.08.016
Castioni GA, Cherubin MR, Menandro LMS et al (2018) Soil physical quality response to sugarcane straw removal in Brazil: a multi-approach assessment. Soil Tillage Res 184:301–309. https://doi.org/10.1016/j.still.2018.08.007
Castioni GAF, Cherubin MR, Bordonal R d O et al (2019) Straw removal affects soil physical quality and sugarcane yield in Brazil. BioEnergy Res. https://doi.org/10.1007/s12155-019-10000-1
Silva J, Jucksch I, Iezid Maia Almeida Feres C, de Castro Tavares R (2012) Fauna do solo em sistemas de manejo com café Soil faunal in management systems with coffee. J Biotec Biodivers 3:59–71
de Aquino AM, Ferreira da Silva R, Mercante FM et al (2008) Invertebrate soil macrofauna under different ground cover plants in the no-till system in the Cerrado. Eur J Soil Biol 44:191–197. https://doi.org/10.1016/j.ejsobi.2007.05.001
Diehl WJ, Williams DL (1992) Interactive effects of soil moisture and food on growth and aerobic metabolism in Eisenia fetida (oligochaeta). Comp Biochem Physiol Part A Physiol 102:179–184. https://doi.org/10.1016/0300-9629(92)90031-K
Acknowledgments
We would like to thank LNBR/CNPEM technicians’ group for all the support in the field and laboratory activities, and the Sugarcane mills for providing the experimental sites and the logistical support during the fieldwork. We also thank the three anonymous reviewers of this manuscript for the helpful comments and suggestions that improved earlier versions.
Funding
This research was funded by LNBR/PNUD through the Project BRA/10/G31—Sugarcane Renewable Electricity (SUCRE/PNUD) and by the National Council for Scientific and Technological Development (CNPq) through the Project PROC 476718/2013-9. MRC is grateful to the São Paulo Research Foundation—FAPESP for providing the research grant (Proc # 2018/09845-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 568 kb)
Rights and permissions
About this article
Cite this article
Menandro, L.M.S., de Moraes, L.O., Borges, C.D. et al. Soil Macrofauna Responses to Sugarcane Straw Removal for Bioenergy Production. Bioenerg. Res. 12, 944–957 (2019). https://doi.org/10.1007/s12155-019-10053-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-019-10053-2