Abstract
Herein, we combined metabolic evolution with fluorescence-activated cell sorting (FACS) of cells stained with the lipophilic dye BODIPY for isolation of SCO-overproducing strains of Yarrowia lipolytica. Metabolic evolution was implemented for enrichment of high SCO-accumulating mutant population which were then sorted by fluorescence signals using flow cytometry coupled with FACS. A mutant isolated by this approach exhibited 1.5- and 1.2-fold higher SCO titer and content, respectively, than the wild type under batch culture of sugarcane bagasse hydrolysate complex media. In addition, the mutant had whole-cell fatty acid composition different from that of the wild type with higher oleic and linoleic acids. Dual-stage fed-batch process applied to the mutant yielded high SCO titer of 49.7 g/L from hydrolysates, a fourfold improvement over batch process. This study highlights evolution-based in conjunction with fluorescence-based high-throughput screening as a powerful strategy for attaining high single-cell oil-accumulating phenotype in Y. lipolytica exploited for sustainable biodiesel and oleochemicals synthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial oil referred to as single-cell oil (SCO) has received applied interest as an attractive energy source both for the sustainable production of biodiesel and other important oleochemicals [1]. SCO processes from microbial conversion of low-cost lignocellulose feedstocks have attractive features including sustainability and renewability relative to vegetable oils. To meet economic feasibility requirements for the oil industry, it is essential to develop lignocellulose-based SCO production process that can achieve high production efficiency in titer, yield, and productivity [2].
Yarrowia lipolytica has emerged as a promising candidate for use to enhance the biorefinery of lignocellulose-based feedstock and utilization of byproducts, since the organism is capable of utilizing a wide variety of industrially relevant substrates that are of zero or negative acquisition cost, including glucose from agro-industrial wastes, glycerol from industrial biodiesel manufacturing, and other hydrophobic substrates, i.e., fatty acids from organic municipal waste and alkanes from petroleum sludge [3, 4, 8]. Y. lipolytica can accumulate over 90% of its lipids in the form of triacylglycerols (TAGs) with fatty acid profiles similar to those of vegetable oils, representing an attractive source of oil suitable for biodiesel production [5]. Hence, the primary goal of this study is to achieve high SCO titer from sugarcane bagasse hydrolysates for commercially attractive through strain and bioprocess optimization of Y. lipolytica.
Flow cytometry (FC) is a fast, accurate, and versatile bioprocess-monitoring tool used for single-cell and cell population heterogeneity studies [6, 7]. This fluorescence-based high-throughput technique can assist with the screening of cell population and process conditions thereby accelerating bioprocess development and scale-up. FC can be implemented for a real-time monitoring of cell growth, intracellular product accumulation, or cell metabolic activity during fermentation to provide useful information for dynamic process control strategies in industrial bioprocess [4, 8]. Flow cytometry in association with lipid-binding fluorescence dyes (e.g., Nile Red, BODIPY) has been used for quick and accurate estimates of oil content on a single-cell basis [9, 10]. FC can be equipped with fluorescence-activated cell sorting (FACS) which permits isolation of cells with desirable characteristics based on fluorescence intensity. FACS analysis to enrich and isolate high-lipid mutants has been demonstrated in several species of microalgae [11,12,13]. However, as far as the authors are aware, this FACS approach for the enrichment and isolation of strains with high lipid content has not yet been accomplished in oleaginous yeasts.
In this work, we present metabolic evolution-coupled FACS approach based upon buoyancy-based enrichment together with FACS high-lipid sorting strategy which enables an efficient isolation of Y. lipolytica mutant with high SCO synthesis. The evolved cell population of Y. lipolytica obtained from buoyancy screening were subjected to high-throughput FACS analysis to isolate for cell with high fluorescence intensity corresponding to high SCO content. The isolated mutant was analyzed for fatty acid composition and implemented in our previously developed dual-stage fed-batch for high-titer SCO production from sugarcane bagasse hydrolysates. The development of SCO-overproducing strain and efficient SCO production bioprocess in this study could provide a significant move towards sustainable and economically viable industrial SCO production.
Materials and Methods
Strains, Media, and Material
Y. lipolytica (Biotec Culture Collection, BCC64401) was used as the control strain for all studies. Strains were routinely maintained on YPD agar (10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose, 25 g/L agar). Seed cultures used in batch and fed-batch process were prepared in YPD media (10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose) pH 5.5 on an incubator shaker at 200 rpm for 24 h prior to use. Media for batch and fed-batch was hydrolysates complex (HC) media, composed of sugarcane bagasse hydrolysates supplemented with peptone at specified concentration, 10% (v/v) molasses, 0.75 g/L (NH4)2SO4, 0.35 g/L KH2PO4, and 0.07 g/L MgSO4·7H2O. Sugarcane bagasse was collected from a local field in Ratchaburi, Thailand. The biomass was physically processed using a SM2000 cutting mill (Retsch, Haan, Germany) and sieved through a 0.25–1-mm mesh.
Preparation of Sugarcane Bagasse Hydrolysates
Sugarcane bagasse hydrolysates were prepared by first milling dried bagasse to a particle size of 0.25–1 cm. The processed bagasse was pretreated with diluted sulfuric acid by mixing 30% solid with 0.5% (w/v) H2SO4 and was steam pretreated at 121 °C for 30 min. The pretreated bagasse was neutralized with 4 M KOH followed by enzymatic hydrolysis at 15 FPU/g-solid CTEC2 enzyme dosage (Novozymes, Denmark), 50 °C incubation for 96 h to release sugars [14]. The hydrolyzed slurry at accumulated solid load of 22% water-insoluble solid (WIS) was harvested through centrifugation and filtration and was diluted to a concentration as specified prior to use for SCO fermentation. The pH of hydrolysates was adjusted to 5.5 using 4 M KOH prior to use for fermentation.
Metabolic Evolution with Buoyancy Screening
The wild-type strain of Y. lipolytica was enriched for its floating ability through a serial dilution experiment containing 7.5% WIS bagasse hydrolysates and 2.5 g/L peptone as depicted in Fig. 1a. For each evolution round, the yeast cell was allowed to grow for 48 h at 30 °C, initial pH 5.5, and 100 rpm agitation. The hydrolysate complex (HC) media was composed of 53.2 g/L glucose, 1.8 g/L acetate, and 0.2 g/L furfural. At the end of each evolution round, buoyancy screen was carried out by settling the culture with no shaking for 0.5–1 h to specifically isolate a subpopulation of cells with high buoyancy property which floated on the surface of the culture media. These high-buoyancy cells on top of the culture media were transferred to the next round of evolution. The evolved culture after 20 rounds of serial dilution was subjected to screening and sorting in flow cytometry analysis.
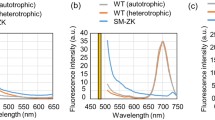
Schematic diagram describing work plan for development of SCO-overproducing strain of Y. lipolytica. a Strain optimization through buoyancy-based metabolic evolution was implemented for enrichment of floating cell population. Serial dilution under aerobic growth on sugarcane bagasse hydrolysates was performed. b The enriched population was sorted through flow cytometry equipped with fluorescence-activated cell sorter. Dot plots of forward scatter (FSC-H) and BODIPY fluorescence intensities of the 20th round evolved cell populations were analyzed to establish sorting gates used for isolation of mutants with high BODIPY fluorescence signals. c Individual clones isolated from sorted cell population were compared for their SCO accumulation. Forty-one individual clones isolated from flow cytometry/FACS were screened by fluorescence-based lipid assay after 24 h of growth in optimized hydrolysate complex medium. The average with standard deviation from duplicate experiments is shown for the control strain (wild type, red) and isolated strains (blue and green). Cell and SCO concentration were analyzed by optical density- and fluorescence-based assay, respectively. The best-performing strain Y203A (shown in light green) was chosen for further characterization
Cell Staining
Evolved cell culture was stained with BODIPY 493/503, a lipophilic fluorescent dye binding to intracellular neutral lipids. The BODIPY staining procedure was modified from the protocol reported in Friedlander et al. [10]. Briefly, evolved cell culture was spun down, washed, and resuspended in phosphate-buffered saline (PBS) solution. Forty microliters washed cell sample was mixed with 60 μL 1 mM BODIPY 493/503 solution, incubated at 4 °C for 30 min. After staining, cells were spun down and resuspended to appropriate cell concentration in water prior to flow cytometry and fluorescence-activated cell sorting analysis.
Flow Cytometric Cell Sorting
Y. lipolytica cells stained with BODIPY fluorescent dye were analyzed and sorted using flow cytometry and fluorescence-activated cell sorting (FACS), Becton Dickinson (BD) FACSAria II (BD Biosciences, USA). BODIPY-stained cell samples from wild type and evolved populations were analyzed using flow cytometry equipped with a 556-nm longpass splitter and 585/42-nm bandpass filters and were sorted in FACS at a sort rate of 10,000 cell count. BODIPY fluorescence was excited with a 488-nm laser, and emission was captured by 530/20-nm filters for fluorescence detection. Dot plots were used to establish gates for isolating cells with high fluorescence intensity from the evolved population which presumably would be putative SCO-overproducing mutants (see Fig. 1b), since BODIPY-based flow cytometry approach permits a direct correlation between lipid content and fluorescence intensity on a single-cell basis. Cell sorting gates were then positioned based on cells exhibiting higher fluorescence intensity than the wild-type population (result not shown). All cells that fell into these gates were collected and the sorted cells were plated onto YPD agar for isolation of individual clones. The isolated clones were designated and tested for their SCO production capacity.
Batch and Fed-Batch Process for SCO Production
All batch and fed-batch fermentation experiments were carried out in 250- and 500-mL Erlenmeyer flask, respectively, at 30 °C and initial pH 5.5 with no pH control. The batch fermentation was performed in 7.5% WIS bagasse hydrolysates supplemented with 2.5 g/L peptone, equivalent to C:N ratio of 67.4 mmol/mmol, with a working volume of 25 mL. Batch HC media composition was 53.2 g/L glucose, 1.8 g/L acetate, and 0.2 g/L furfural. For fed-batch fermentations, seed cultures were inoculated into 25-mL batch medium containing approximately 8 g/L glucose from hydrolysates and 0.37 g/L peptone supplementation. The 22% WIS hydrolysate feed media, composing of 156.2 g/L glucose, 5.3 g/L acetate, and 0.6 g/L furfural, was initiated after 1-day batch cultivation. One-stage fed-batch media (carbon-limited) was at initial C:N ratio of 67.4 mmol/mmol, while second-stage fed-batch media (nitrogen-limited) was at initial C:N ratio of 134.8 mmol/mmol based on initial concentrations of glucose and peptone available. The nitrogen concentration in peptone as determined from suppliers was 0.15 g-nitrogen/g-peptone, respectively. The feed media was applied in fed-batch culture at feeding profiles as indicated in “Results and Discussion” section. The final working volume for fed-batch process was at 100 mL. Process parameters included an inoculum volume of 10%, initial pH at 5.5, a temperature of 30 °C, and agitation at 100 for batch and 200 rpm for fed-batch. Samples (1–2 mL) for cell dry weight and SCO analyses and residual glucose concentration determination were taken every 24 h for fermentation kinetics analysis and fermentations lasted 5–10 days. The samples withdrawn were centrifuged at 11,000 rpm for 5 min, and the yeast cell and supernatant were separately collected. All experiments were performed in duplicate.
Analytical Methods
Cell Concentration
The cell dry weight was measured by washing 1–2 mL of collected cell pellet with deionized water before drying at 100 °C for 24 h and subsequently measuring weight of dry cell. The OD measurement at 595 nm of washed cell sample was converted to dried cell weight based on calibration curve.
SCO Extraction and Analysis
Single-cell oil from 1 mL culture samples of Y. lipolytica was extracted following the procedure described by Schneiter and Daum [15]. The collected cell pellets were washed with deionized water, resuspended in a 1.5 mL of chloroform/methanol (2:1) solution, and then vortexed on high with glass beads for 15 min. The organic solution was washed with 200 μl of water followed by 200 μl of water/methanol (1:1) solution before being dried overnight and weighed to quantify SCO production. For SCO quantification using fluorescence assay in 96-well plates, 40 μL washed cell samples fixed with ethanol at 4 °C for 30 min was mixed with 60 μL master solution containing 1 M potassium iodide, 1 mM BODIPY 493/503, 1 μL dimethyl sulfoxide, 3 μL of 60% PEG4000, and 20 μL water. Fluorescence was measured at excitation 484 nm and emission 510 nm. Fluorescence correlated with SCO content measured offline was used for SCO quantification: Fluorescence = 1219.4×[SCO] + 19,895.
Fatty Acid Analysis
SCO were analyzed for fatty acid fractions as previously described in Blazeck et al. [16]. Briefly, fatty acids were derived via a one-step transesterification reaction before injection into a capillary gas chromatography equipped with a flame ionization detector and helium as the carrier gas as previously described. Fatty acid standard ranging from C12 to C18 chain lengths was used to determine the fatty acid profiles of Y. lipolytica strains.
Sugars Analysis
The collected supernatant was filtered using a 0.2-μm sterile filter and stored at −20 °C prior to analysis. Concentration of glucose and xylose in hydrolysates were measured by HPLC equipped with Aminex HPX-87P column (Bio-Rad, USA) and a refractive index detector at 80 °C with H2O as the mobile phase at a flow rate of 0.6 mL/min. The concentrations were calculated from the calibration curve of standard solution.
Yield and Rate Calculation
Yield was calculated based on total product synthesized (e.g., single-cell oil or yeast cell dry weight) per total glucose available in the hydrolysate culture. The productivity was determined as the overall rate based on product produced per total cultivation time. Single-cell oil content was determined as the percentage of gram of SCO produced per gram of cell dry weight.
Results and Discussion
Production of single-cell oil (SCO) from low-cost lignocellulosic feedstock, e.g., agricultural residues, is essential for economic viability and sustainability of biodiesel and oleochemical industry. This work described the implementation of metabolic evolution equipped with flow cytometry and fluorescence-activated cell sorting technology for rapid isolation of high SCO-accumulating strains of Y. lipolytica from sugarcane bagasse hydrolysates.
Metabolic Evolution Using Buoyancy Selection
Genetic fine-tuning of the whole metabolism using metabolic evolution scheme has been proven as an effective strategy to obtain strains with concurrent enhanced bioproducts and cell growth [17]. Oleaginous cells can be screened via buoyancy selection where cells with high lipid accumulation phenotype process high buoyancy property and can be separated based on the floating ability [18]. Hence, we sought to develop high SCO-accumulating cells by coupling metabolic evolution with growth- and buoyancy-based screening. Briefly, the wild-type strain of Y. lipolytica as a starting cell population was evolved through a serial dilution experiment of batch culture. At the end of each evolution round, the culture was settled with no shaking to specifically isolate a subpopulation of cells with high buoyancy property which floated on the surface of the culture media. These high-buoyancy cells on top of the medium were transferred to the next round of evolution. An overview of metabolic evolution of Y. lipolytica for isolation of SCO-hyperaccumulating mutants is given in Fig. 1a. In this way, the cells with high-buoyancy, floating phenotype correlating with high SCO content in the sugarcane bagasse hydrolysates would be enriched over evolution time. Analyzing SCO accumulation in the evolved cells’ population of selected round of subculturing revealed a constant increase in SCO content over evolution time (result not shown), confirming the enrichment of high SCO-accumulating cells through metabolic evolution process.
Isolation of SCO-Overproducing Mutant Through Flow Cytometry and FACS
Yeast cell population after the 20th round of subculturing in iterative metabolic evolution in sugarcane bagasse hydrolysates was stained with BODIPY dye, a fluorescence-based straining lipid bodies [10] and subjected to screening in flow cytometry equipped with FACS. Flow cytometry analysis reveals two-dimensional dot plot of forward scatter, FSC-H, used as a readout of cell size, and BODIPY fluorescence intensity, used as a readout of SCO content from a pool of approximately 20,000 mutants (Fig. 1b). The plot shows BODIPY fluorescence distribution with the mean of fluorescence intensity values, as represented in arbitrary fluorescence units, of 6278 a.u. and that there was a uniform increase in BODIPY staining in the evolved population by 4.8-fold compared with the wild type (result not shown). A higher mean fluorescence signal distribution in the pool of mutants compared to wild type indicates the presence of mutants’ population that accumulate higher SCO than a wild-type population. BODIPY-stained, evolved cells were then sorted using FACS for high SCO mutants’ isolation. The dot plot was used for establishing sorting gates used for screening. Sorting gates from the dot plot shown in Fig. 1b were selected on the evolved cell population to maximize BODIPY fluorescence intensity signal correlating to SCO content at minimized forward scatter. Evolved cells exhibiting high-fluorescence-intensity phenotype that fell in the high-lipid gates were sorted and collected on plates. The isolated cells were plated on YPD agar and incubated for 2 days to recover isolated colonies.
Forty-one individual clones were picked for further analysis and the selected mutants, as well as the wild type, were cultured in batch containing HC media for 48 h before quantification of total SCO content via fluorescence-based lipid assay. All of isolated mutants by flow cytometry and FACS show higher SCO content than the wild type (Fig. 1c). The result is a confirmation that buoyancy-based metabolic evolution equipped with high-throughput screening by flow cytometry and FACS can be effectively used to isolate strains with enhanced SCO production. This result also emphasizes that a large number of mutants in a pool can be quickly screened and sorted for the SCO production phenotypes. We selected, out of the 41 isolated clones, the best-performing strain Y203A for further batch and fed-batch characterization as Y203A culture exhibited the highest improvement in total SCO content based on fluorescence-based lipid assay relative to the wild-type culture.
Strain Characterization
Isolated mutant Y203A was compared with wild type for growth and SCO production in batch cultivation of HC media (Fig. 2). The Y203A mutant grew at a specific rate of 0.10 h−1, while the wild type grew slightly at slower rate of 0.07 h−1. After 3 days, the sorted mutant Y203A produced SCO at titer of 10.01 ± 0.32 g/L, 52% higher titer compared to the wild type. The mutant achieved SCO yield of 0.20 g/g based on total glucose, SCO content of 67.3%, and had an overall productivity of 0.14 g/L h. Improved SCO production in the mutant was in part due to faster glucose uptake and growth rates of the mutant than the wild type. At present, it is not yet known whether the high SCO and high-biomass phenotypes are linked or due to separate mutations. Comparative omics analysis will be required as part of future study to identify mutations responsible for high-SCO phenotype observed. Connected with higher SCO titer, Y203A exhibited 67% of cell dry weight SCO content which represented a 24% increase over the wild-type strain. A 12% decrease in the lipid-free biomass titer was also observed in Y203A at the expense of higher SCO synthesis compared to wild type. Performance summary of isolated mutant Y203A in comparison with wild type in batch fermentation is given in Table 1. The results clearly prove flow cytometry coupled with FACS, in combination with buoyancy-based metabolic evolution, as a powerful technique with less labor and time-consuming approach for the enhancement of the SCO content of Y. lipolytica. The results also show flow cytometry/FACS as a rapid and reliable method for screening a large number of strains and isolating mutant strains with increased SCO accumulation from the cell population saving time and money in the investigation of strains for industrial applications. This approach presented here should be broadly applicable to other lipid-related high-throughput screens in oleaginous yeasts for enhanced SCO production. Furthermore, this SCO-overproducing mutant Y203A could serve as a genetic resource to elucidate genes and molecular processes contributing to lipid hyperaccumulation as well as serving as a recipient of future metabolic engineering for further enhancement of SCO synthesis.
Strain performance of wild type and isolated mutant Y203A. Kinetics of cell growth (circle), SCO production (diamond), and glucose consumption (square) by the wild type (a) compared to the mutant Y203A (b) in batch culture of hydrolysate complex media. Comparative performance between the wild type and isolated strain Y203A for SCO titer and content (c) and SCO yield and productivity (d) in batch culture. The isolated strain outperformed wild type for SCO production efficiency
Overall, Y203A revealed high SCO production capacity in titer, content, and production rate; thus, the mutant clearly operated at different metabolic processes in contrast to the parental, wild-type strain. Y203A could benefit for even higher SCO titer when being used in fed-batch fermentation process.
Comparative Lipid Profiling
In addition to measurements of total SCO production, we evaluated lipid content of the isolated mutant Y203A in comparison with the wild type by gas chromatography. The fatty acid profile of wild type and sorted mutant Y203A cultures was compared in Fig. 3, showing differences exist between the fatty acid profiles of wild type and mutant cultures. The major fatty acids in both strains were oleic acid, linoleic acid, palmitoleic acid, palmitic acid, stearic acid, myristoleic acid, and myristic acid, which is consistent with a lipid profile commonly found in Y. lipolytica grown on glucose [19]. Y203A strain appeared to accumulate more oleic and linoleic acids than the wild type. In contrast, palmitic, palmitoleic, myristoleic, and myristic acids were present in sorted mutant cultures at lower levels to that of the wild type. Nevertheless, the content of SCO has composition that is compatible with the production of biodiesel or other oleochemicals, thereby can be used to replace high-priced vegetable oil feedstock [20].
High-Titer SCO Production by Isolated Mutant in Fed-Batch Process
Isolated mutant Y203A was first implemented in one-stage fed-batch with pulse feeding as previously described in [21] to provide balanced nutrients for continued SCO production for high product titer. In one-stage fed-batch shake flask, the culture was initiated as batch for 1 day followed by pulse addition of hydrolysate feed media at C:N ratio of 67.4 mmol/mmol for 4 days. Figure 4a, b compares fermentation performance by Y203A and wild type in the single-stage fed-batch culture under the same feeding scheme. High SCO titer of 34.70 ± 0.77 g/L was reached by the mutant in one-stage fed-batch, 68.5% higher than those achieved in the wild type under the same condition (Table 1). This was also 3.4-fold improvement in titer compared to batch process, proving fed-batch as effective strategy for high SCO titer operation. Total biomass titer was also increased from 29.01 g/L by the wild type to 50.67 g/L by the mutant, partly as a result of enhanced SCO accumulation, in one-stage fed-batch cultivation. Higher biomass production by the mutant indicates that the mutant is capable of channeling carbon flux towards lipid and biosynthesis more efficiently than the wild type, and that genetic mutations occurred in the mutant may be responsible for increasing precursors and cofactors of lipid and biosynthesis. The SCO yield, content, and productivity achieved by Y203A mutant in fed-batch was 0.25 ± 0.01 g/g based on total glucose available, 68.4% content, and 0.24 g/L h, respectively (Table 1). It should be noted that SCO production performance is highly dependent on strain and cultivation conditions. Nevertheless, similar SCO yields in Y. lipolytica have been achieved by other previous works, using synthetic media and carbon sources [22, 23]. The SCO titer and yield achieved in this study also outperformed previous values reported in Jin et al. [1] for lignocellulose conversion to SCO. However, SCO titer in this work is still below the values reported by other previous works in synthetic media [10, 24].
Fermentation kinetics of one-stage fed-batch by wild type (a) and isolated mutant Y203A (b). Shown are biomass (circle), SCO production (diamond), SCO content (triangle), residual glucose (square), and cumulative volume due to pulse feeding (dashed line). Production process was conducted as batch for 1 day before switching to pulse feeding fed-batch using media containing sugarcane bagasse hydrolysates supplemented with peptone at C:N ratio of 67.4 mmol/mmol. Comparative performance between the wild type and isolated strain Y203A for SCO titer and content (c) and SCO yield and productivity (d) in one-stage fed-batch culture. The isolated mutant showed 1.6-fold improvement in SCO titer, compared to wild type
To further enhance SCO tier, Y203A mutant was also implemented in dual-stage fed-batch shake flask as depicted in Fig. 5a. Two-stage fed-batch process was initially carried out similar to one-stage fed-batch; then after 5 days, the yeast cells were harvested and then resuspended in the second fed-batch under nitrogen-limiting condition at high C:N ratio of 134.8 mmol/mmol, the goal being to maintain the culture at low C:N ratio for growth in the first phase and at high C:N ratio in the second phase to prolong SCO accumulation at higher titer. Figure 5b shows the culture time courses of batch followed by the first fed-batch under growth and the second fed-batch under limited-growth conditions through controlled C:N ratio feed media. The mutant Y203A in dual-stage fed-batch culture led to an accumulation of SCO up to 49.77 ± 1.71 g/L within 10 days of two-stage cultivation. Comparing different fed-batch modes, two-stage process was a better performing process scheme with continued increase in SCO accumulation after addition of nitrogen-limited, high C:N level hydrolysates in the late stage of fed-batch. SCO titer achieved by Y203A in dual-stage process was approximately 43.4% higher than that achieved in one-stage fed-batch. The SCO yield and productivity in dual-stage fed-batch were 0.25 ± 0.01 g/g and 0.21 g/L h, respectively. It should also be noted that the fed-batch shake flask cultivation developed in this work could yield up to 50–60 g/l biomass titer, partly as a result of enhanced SCO accumulation, which has not been achieved elsewhere under the same cultivation condition.
High SCO titer production through dual-stage fed-batch process by Y203A. a The dual-stage fed-batch was composed of two steps. First, a traditional fed-batch was performed in which the feed media at low C:N ratio of 67.4 mmol/mmol was pulse-fed after the batch culture. Second, after one-stage fed-batch, cell separation was conducted to collect the yeast cell which was then suspended into the second culture with high C:N ratio of 134.8 mmol/mmol. The pulse feed profiles and optimal C/N ratios were designed according to dynamic flux balance modeling described previously (Unrean et al., submitted). b Time course profiles of yeast cell (circle), single-cell oil production (diamond), SCO content (triangle), residual glucose (square) in dual-stage fed-batch under growth (low C:N ratio), and limited-growth (high C:N ratio) fermentation of bagasse hydrolysates, respectively. Shown are batch (b) followed by one-stage fed-batch (FB*) then cell separation and suspension in second-stage fed-batch (FB+) of bagasse hydrolysates
Higher SCO production efficiency in two-stage fed-batch likely resulted from a better control of C:N ratio, which limited biosynthesis and strongly triggered lipogenesis, than in one-stage process. In addition, dilution effect caused by feeding and an increase in culture volume observed in the one-stage fed-batch were minimized in the dual-stage fed-batch compared to one-stage process, due to additional step of cell separation and suspension. The result confirmed the two-stage fed-batch as an effective process to maximize titer of SCO accumulation. The high SCO titer reached in this study should permit the SCO-based lignocellulosic bioprocess to meet economic feasibility for industrialization [1]. In the integrated biorefinery process, biomass feedstock (e.g., sugarcane bagasse, rice straw) can be pretreated via fractionation to separate cellulose and hemicellulose. The cellulose stream can be directed for hydrolysis yielding mainly glucose and then fermented to SCO, while hemicellulose containing mixed glucose and xylose can be used for fermentation of other bioproducts, e.g., lactic acid, succinic acid, or ethanol, to create multi-product biorefinery. Alternatively, genetic engineering of Y. lipolytica for the utilization of xylose could also be implemented for the completed utilization of all sugars available in the biomass feedstock.
Conclusion
We isolated a high SCO-accumulating strain of Y. lipolytica using buoyancy-based metabolic evolution coupled with flow cytometry and FACS. Utilizing FACS sorting of BODIPY fluorescence-stained cell population permitted a rapid isolation of Y203A mutant strain which revealed enhanced SCO titer and content compared to the wild-type strain in batch culture. Furthermore, fatty acid profiles in the isolated strain differed significantly from the wild-type strain with higher oleic and linoleic acids’ composition. The increased fatty acid content and the altered fatty acid composition indicate that metabolic evolution enrichment equipped with high-throughput fluorescence-activated cell sorting approach yielded high SCO-accumulating mutant strain with perturbed lipid metabolism. Y203A mutant may enable the understanding of molecular and genetic mechanisms contributing to high SCO accumulation through comparative multi-omics analysis which is left for future research.
Implementing the isolated mutant in two-stage fed-batch strategy, previously developed for attaining high SCO titer production, successfully yielded up to 49.7 g/L SCO titer. Hence, the work presented here demonstrates the effectiveness of metabolic evolution equipped with FACS represents a generalized and useful tool for high-throughput isolation of oleaginous yeasts with enhanced SCO content for biodiesel or oleochemical production. This combinatorial high-throughput approach should facilitate rapid strain isolation of SCO-overproducing mutants and optimized production process to attain high SCO titer from renewable lignocellulosic biomass for economic feasibility and sustainability.
References
Jin M, Slininger PJ, Dien BS, Waghmode S, Moser BR, Orjuela A, Sousa Lda C, Balan V (2015) Microbial lipid-based lignocellulosic biorefinery: feasibility and challenges. Trends Biotechnol 33(1):43–54
Koutinas AA, Chatzifragkou A, Kopsahelis N (2014) Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleo-chemical production. Fuel 116:566–577
Fontanille P, Kumar V, Christophe G, Nouaille R, Larroche C (2012) Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica. Bioresour Technol 114:443–449
Back A, Rossignol T, Krier F, Nicaud JM, Dhulster P (2016a) High-throughput fermentation screening for the yeast Yarrowia lipolytica with real-time monitoring of biomass and lipid production. Microb Cell Factories 23(15):147
Ledesma-Amaro R, Nicaud JM (2016) Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog Lipid Res 61:40–50
Freitas C, Nobre B, Gouveia L, Roseiro J, Reis A, da Silva TL (2014) New at-line flow cytometric protocols for determining carotenoidcontent and cell viability during Rhodosporidium toruloides NCYC 921 batch growth. Process Biochem 49:554–562
Besmer MD, Weissbrodt DG, Kratochvil BE, Sigrist JA, Weyland MS, Hammes F (2014) The feasibility of automated online flow cytometry for in-situ monitoring of microbial dynamics in aquatic ecosystems. Front Microbiol 5:265. doi:10.3389/fmicb. 2014.00265
Back A, Rossignol T, Krier F, Nicaud JM, Dhulster P (2016b) High-throughput fermentation screening for the yeast Yarrowia lipolytica with real-time monitoring of biomass and lipid production. Microb Cell Factories 23(15):147
Hyka P, Lickova S, Pribyl P, Melzoch K, Kovar K (2013) Flow cytometry for the development of biotechnological processes with microalgae. Biotechnol Adv 31:2–16
Friedlander J, Tsakraklides V, Kamineni A, Greenhagen EH, Consiglio AL, MacEwen K, Crabtree DV, Afshar J, Nugent RL, Hamilton MA, Joe SA, South CR, Stephanopoulos G, Brevnova EE (2016) Engineering of a high lipid producing Yarrowia lipolytica strain. Biotechnol Biofuels 9:77
Velmurugan N, Sung M, Yim SS, Park MS, Yang JW, Jeong KJ (2013) Evaluation of intracellular lipid bodies in Chlamydomonas reinhardtii strains by flow cytometry. Bioresour Technol 138:30–37
Xie B, Stessman D, Hart JH, Dong H, Wang Y, Wright DA, Nikolau BJ, Spalding MH, Halverson LJ (2014) High-throughput fluorescence-activated cell sorting for lipid hyperaccumulating Chlamydomonas reinhardtii mutants. Plant Biotechnol J 12:872–882
Terashima M, Freeman ES, Jinkerson RE, Jonikas MC (2015) A fluorescence-activated cell sorting-based strategy for rapid isolation of high-lipid Chlamydomonas mutants. Plant J 81(1):147–159
Unrean P, Khajeeram S (2016) Optimization and techno-economic assessment of high-solid fed-batch saccharification and ethanol fermentation by Scheffersomyces stipitis and Saccharomyces cerevisiae consortium. Renew Energy 99:1062–1072
Schneiter R, Daum G (2006) Extraction of yeast lipids. Methods Mol Biol 313:41–45
Blazeck J, Hill A, Liu L, Knight R, Miller J, Pan A, Otoupal P, Alper HS (2014) Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat Commun 5:3131
Unrean P, Srienc F (2011) Metabolic networks evolve towards states of maximum entropy production. Metab Eng 13(6):666–673
Sitepu IR, Ignatia L, Franz AK, Wong DM, Faulina SA, Tsui M, Kanti A, Boundy-Mills K (2012) An improved high-throughput Nile red fluorescence assay for estimating intracellular lipids in a variety of yeast species. J Microbiol Methods 91(2):321–328
Silverman AM, Qiao K, Xu P, Stephanopoulos G (2016) Functional overexpression and characterization of lipogenesis-related genes in the oleaginous yeast Yarrowia lipolytica. Appl Microbiol Biotechnol 100(8):3781–3798
Wahlen BD, Morgan MR, McCurdy AT, Willis RM, Morgan MD, Dye DJ, Bugbee B, Wood BD, Seefeldt LC (2013) Biodiesel from microalgae, yeast, and bacteria: engine performance and exhaust emissions. Energy Fuel 27:220–228
Unrean P, Khajeeram S, Laoteng K (2016) Systematic optimization of fed-batch simultaneous saccharification and fermentation at high-solid loading based on enzymatic hydrolysis and dynamic metabolic modeling of Saccharomyces cerevisiae. Appl Microbiol Biotechnol 100(5):2459–2470
Qiao K, Imam Abidi SH, Liu H, Zhang H, Chakraborty S, Watson N, Kumaran Ajikumar P, Stephanopoulos G (2015) Engineering lipid overproduction in the oleaginous yeast Yarrowia lipolytica. Metab Eng 29:56–65
Liu L, Pan A, Spofford C, Zhou N, Alper HS (2015) An evolutionary metabolic engineering approach for enhancing lipogenesis in Yarrowia lipolytica. Metab Eng 29:36–45
Xu P, Qiao K, Stephanopoulos G (2017) Engineering oxidative stress defense pathways to build a robust lipid production platform in Yarrowia lipolytica. Biotechnol Bioeng 114(7):1521–1530
Acknowledgements
The authors would like to thank the National Center for Genetic Engineering and Biotechnology, Thailand, for the financial support of this project (Grant No. P-16-50341).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Unrean, P., Champreda, V. High-Throughput Screening and Dual Feeding Fed-Batch Strategy for Enhanced Single-Cell Oil Accumulation in Yarrowia lipolytica . Bioenerg. Res. 10, 1057–1065 (2017). https://doi.org/10.1007/s12155-017-9865-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-017-9865-0