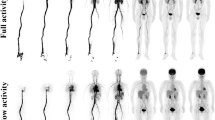
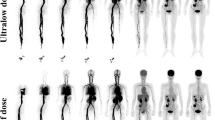
Abstract
Objective
To explore the feasibility of ultralow-activity 18F-FDG total-body dynamic PET imaging for clinical practice in patients with lung adenocarcinoma.
Methods
Eight of 18 patients were randomly injected with 18F-FDG with full activity (3.7 MBq/kg) for total-body dynamic PET imaging, while 10 received one-tenth activity (0.37 MBq/kg). The generated time-to-activity curves (TACs) according to the regions of interest (ROIs) were processed by PMOD through standard FDG two-tissue compartment model fitting. The kinetic constant rates (K1, K2, K3, and Ki), radiation dose, prompt counts, and data storage size were analysed between the full- and ultralow-activity groups. The SUVmax-Tumour/SUVmax-Liver and SUVmax-Tumour/SUVmax-Muscle on static PET images were also assessed.
Results
Each of the fitted models has a satisfactory goodness-of-fit with R2 greater than 0.9 except 3 (3/234) in ultralow-activity group, where one in pancreas (R2 = 0.851), another one in muscle (R2 = 0.868), and the third one in bone marrow (R2 = 0.895). All the fitted models in the full-activity group had a better goodness-of-fit than those in the ultralow-activity group. However, no significant differences were found in any of the kinetic metrics or image quality between the two groups except in the reduction of radiation dose and data storage size.
Conclusions
The 10 × reduction of injected 18F-FDG could achieve comparable kinetic metrics and T/N ratios by total-body dynamic PET imaging in lung adenocarcinoma patients. Ultralow-activity total-body PET imaging is feasible for clinical practice in oncological patients without obesity, especially in dynamic PET scanning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Positron emission tomography/computed tomography (PET/CT) with F-18-fluorodeoxyglucose (18F-FDG) has been widely applied to the diagnosis, staging, restaging, and treatment efficacy evaluation of tumours [1,2,3,4]. Despite the potential value of providing both anatomical and functional information for clinical decisions in oncological patients, radiation exposure remains a main concern for patients. Therefore, the demand for reducing the radiation dosimetry of PET/CT imaging without affecting the image quality has been increasing.
Recently, total-body PET/CT scanner, with a long axial field of view (AFOV) has ushered in the new era of PET/CT around the world. As we known, the Biograph Vision Quadra PET/CT LAFOV system (Siemens Healthineers, Knoxville, TN, USA) [5], the PennPET EXPLORER (built at the University of Pennsylvania) [6], and the uEXPLORER (United Imaging Healthcare, China) [7,8,9] have been successfully transitioned to clinical or research use. The uEXPLORER with a long AFOV up to 194 cm has been found to have a higher sensitivity by a factor of up to 40 × theoretically and a better signal-to-noise ratio of more than a sixfold increase [10,11,12,13]. Due to the improved sensitivity, it allows for the reduced injected dose while maintaining the image quality in PET/CT imaging [10].
Considering the above advantages, our team has demonstrated that total-body PET/CT with a half-dose (1.85 MBq/kg) 18F-FDG activity can achieve comparable image quality to conventional PET/CT and even better than that of conventional PET/CT with routine clinical full-dose 18F-FDG in lung cancer [14]. In addition to the evaluation of static PET/CT imaging at a low injected dose, we also revealed that total-body dynamic PET imaging using a 10 × reduction in injected activity could achieve relevant kinetic metrics of 18F-FDG and comparable image contrast with full-activity imaging in healthy volunteers [15].
However, the kinetic metrics of 18F-FDG in oncological patients did not exactly correspond to those in healthy volunteers due to the Warburg effect with a high ratio of anaerobic glycolysis in tumours [16]. It is even more important to evaluate whether there is a significant difference in kinetic metrics of 18F-FDG between ultralow-activity and full-activity in oncological patients, such as the 18F-FDG net uptake rate constant (Ki), which has been used in differentiating malignant from benign lung lesions [17] and metastatic lymph nodes [18]. Furthermore, Ki has also been proven to be more sensitive in detecting tumour response to cancer treatment [19, 20].
Thus, considering the previous paper on the evaluation of static PET/CT imaging in a low injected dose in patients with lung cancer, the aim of the present study was to investigate the feasibility of ultralow-activity 18F-FDG total-body PET/CT dynamic imaging for clinical practice in lung adenocarcinoma patients.
Materials and methods
Patients
This study was approved by the Institutional Review Board of Zhongshan Hospital, Fudan University, and conducted after signing the written informed consent from each participant.
Fifteen of 35 patients who suspected lung cancer and underwent total-body dynamic PET/CT scanning were randomly selected for the full-activity group, whereas the remaining twenty were in the ultralow-activity group. Eight patients were eventually enrolled in the full-activity group, whereas ten were in the ultralow-activity group (Fig. 1). The missing patients were consisted of 10 undesired pathological results, 6 undesired body mass index (BMI), and 1 undesired other tumour history. The inclusion criteria were as follows: (1) no history of malignancy or organ excision before dynamic PET/CT scanning; (2) all patients were confirmed to have primary lung adenocarcinoma by postoperative pathological results after dynamic PET/CT scanning. The exclusion criteria were as follows: (1) distant organ or tissue metastasis on PET/CT findings; (2) a history of chronic disease such as diabetes, liver cirrhosis, renal/heart failure, hyperthyroidism, or hyperparathyroidism; (3) BMI (kg/m2) > 28.
Total-body dynamic PET/CT imaging
All patients were required to fast for 6 h prior to dynamic PET/CT imaging. Before the 18F-FDG injection, height, weight, and blood glucose level were measured and recorded on site. During dynamic PET imaging, patients were instructed to avoid speaking and moving.
A uEXPLORER PET/CT scanner (United Imaging Healthcare) was used for dynamic PET imaging. According to the guidelines of the European Association of Nuclear Medicine for 18F-FDG PET/CT oncological examination [1], an activity of 4.1 ± 0.33 MBq/kg was injected in 18F-FDG full-activity patients, while 0.41 ± 0.03 MBq/kg was injected in the ultralow-activity group. Then, 60 min dynamic PET scanning was started simultaneously with the injection of 18F-FDG in a vein near the ankle in all groups.
Before a diagnostic CT (tube current modulation of 120 kVp, spiral pitch factor of 0.9875, and slice thickness of 1 mm) in our scanning, a low-dose CT was performed first for attenuation correction (AC).
Dynamic PET reconstruction
The PET images were reconstructed into a 239 × 239 × 679 matrix with voxels of 2.85 mm3 by the 3D list-mode OSEM algorithm, including high-resolution TOF and point-spread function modelling (OSEM–TOF–PSF) [21, 22]. For the dynamic PET image analysis, a series of PET images were divided into 55 frames with two kinds of schemes: a frame of 5 s for the initial 3 min (36 × 5 s) and a frame of 3 min thereafter until the end (19 × 180 s). Representative frames of PET images are shown in Fig. 2. Finally, the prompt events on PET images and total storage size were recorded.
Generation of tissue activity curves (TACs)
A dedicated postprocessing workstation (uWS-MIR001, United Imaging Healthcare) with dynamic analysis software was used to process the reconstructed PET images and low-dose AC CT images. ROIs were drawn within 12 main organs and tumour lesions of the lung. Two-dimensional ROIs were placed on the target tissues instead of three-dimensional ROIs to reduce the effect of unwanted areas beyond the boundary. For the tumour lesion, the freehand ROIs were placed on the solid components of the tumour to cover the largest tumour portion from the brightest slice of the PET image. For the blood pool, the ROI of the ascending aorta apart from the vessel wall was selected in this study, which was consistent with our previous studies [15, 23]. For the brain tissues, the ROIs were placed on the bilateral parietal cortex, white matter, cerebellum cortex, and medulla. For the liver, three ROIs apart from vessels were placed on the right lobe (upper, middle, and lower areas), and another one was placed on the middle of the left external lobe. For the spleen, the ROI was placed on the largest transaxial section. For the pancreas, the ROI was placed on the body area on the largest transaxial section. For the thyroid and kidney, the ROIs were placed on the middle areas of each side. For the muscle, bone marrow and bone, the ROIs were placed on the bilateral psoas major (on the same slice of PET image), bilateral medullary cavities in the upper areas of the femurs (on the same slice of PET image), and the third lumbar body, respectively. Finally, TACs were extracted from the above ROIs including lung tumour lesions.
Mathematic model fitting and parameter generation
PMOD Kinetic Modelling (Version 3.2, Zürich, Switzerland), independent of the built-in workstation, was used to process the complete TAC data through the standard FDG two-tissue compartment model fitting. In each model fitting, the data of the ascending aorta were chosen for the temporal whole-blood activity (CB(t)). In addition, CB(t) was corrected according to a previous study [24] to estimate the plasma input function (CP(t)), where the haematocrit was different by sex (0.42 for men and 0.36 for women), and the equilibration time constant was 0.2346 min−1. The kinetic rate constants (Fig. 3) including K1 (the transport of 18F-FDG forward from plasma to tissues), K2 (the transport of 18F-FDG backward from plasma to tissues), K3 (the intracellular 18F-FDG phosphorylation), K4 (the intracellular 18F-FDG dephosphorylation), and the fraction of blood volume in tissue (νB) codetermined the temporal tissue activity of 18F-FDG (CPET(t)), including CB(t) for spillover correction and CP(t) for input curve. Finally, the Ki was calculated through the following equation:
In addition, the evaluation of goodness-of-fit in each model fitting was through the recorded Akaike information criterion (AIC) [25, 26], the Schwarz criterion (SC) [27], and the determination index (R2). Representative model fittings are shown in Fig. 4.
Statistical analysis
All data are presented as the mean ± standard deviation (SD), including median and Coefficient of Variation (CoV%) if necessary. The statistical software SPSS 17 (IBM SPSS Inc., Chicago, Illinois, USA) and GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA) were used to perform statistical analyses. The chi-squared test and Fisher’s exact test were performed for comparisons of categorical variables, while Student’s t test or the Mann–Whitney U test was used for continuous variables depending on the Kolmogorov–Smirnov test. A p value less than 0.05 for the two sides was considered statistically significant.
Results
The general patient characteristics
The general patient characteristics are shown in Table 1. There was no significant difference between the full-activity and ultralow-activity groups in age, sex, height, weight, BMI, blood glucose, tumour pathological grade, tumour long diameter or SUVmax of the tumour lesion, except for the core variable of injected 18F-FDG activity (p < 0.001).
Kinetic metrics of 18F-FDG between the full-activity and ultralow-activity groups
The kinetic constant rates K1, K2, K3, and Ki of 18F-FDG in normal organs as well as lung tumours are shown in Table 2 and Table 3. No significant differences in the above kinetic constant rates in measured tissues were found between the full-activity and ultralow-activity groups.
The goodness-of-fit of model fitting
Each of the fitted models had a satisfactory goodness-of-fit with R2 greater than 0.9 except 3 (3/234) fitted models with R2 less than 0.9 (Table 4). All the above 3 fitted models were in ultra-low-activity group, where one in pancreas (R2 = 0.851), another one in muscle (R2 = 0.868), and the third one in bone marrow (R2 = 0.895). In addition, all the fitted models in the full-activity group had relatively smaller AICs and SC, as well as larger R2 values than the ultralow-activity group, with a significant difference in some of the measured organs.
Comparisons of Ki, SUVmax, and T/N ratio in lung tumour lesions
The Ki in tumour lesions was not significantly different between the full-activity and ultralow-activity groups, nor was the SUVmax of the tumour (Fig. 5). However, the CoV% of SUVmax was higher than Ki in both groups. In addition, the SUVmax-Tumour/SUVmax-Liver (Median 1.58, range 0.66–3.05 vs. Median 1.84, range 0.84–4.45) as well as SUVmax-Tumour/SUVmax-Muscle (Median 8.11, range 2.69–17.41 vs. Median 8.01, range 2.85–19.81) were also not significantly different between full-activity and ultra-low-activity group (Fig. 6).
Comparisons of prompt events and storage size
The prompt counts from PET data, including the true, scatter, and random counts, were larger in the full-activity group than in the ultralow-activity group. Furthermore, the effective counts in each frame were also larger in the full-activity group. (Table 5).
Discussion
To our knowledge, the present study was the first to explore the difference in 18F-FDG kinetic constant rates between full-activity and ultralow-activity lung adenocarcinoma patients through total-body dynamic PET imaging. Despite one tenth of the injected dose, the kinetic constant rates in ultralow activity were not significantly different from those in full activity.
In our previous research [15], we demonstrated that ultralow-activity total-body dynamic PET imaging allows equal performance to full-activity PET imaging for investigating kinetic metrics of 18F-FDG in healthy volunteers. Oncological patients, however, were the biggest beneficiary group when performing PET/CT imaging rather than healthy volunteers. In addition, the presence of tumours may affect the 18F-FDG metabolism of other organs. Therefore, it is meaningful to further evaluate the 18F-FDG kinetic constant rates in oncological patients between different injected dose groups by the total-body PET scanner.
Due to the high sensitivity of the total-body PET/CT scanner, we successfully decreased the injected 18F-FDG dose up to one tenth of the common dose again for dynamic PET imaging. Two-dimensional ROIs were preferred in our study instead of three-dimensional ROIs to avoid unwanted areas beyond the target organ. Despite the ultralow injected dose, no significant differences in the Ki or SUVmax in tumour lesions were found when compared to the full-activity group. Moreover, the Ki of lung adenocarcinoma in our study was similar to Yang’s study [18] despite the Patlak graphic analysis in their study. However, the Ki of lung adenocarcinoma was obviously lower than that of lung squamous cell carcinoma (SCC) in Yang’s paper. This was in line with the fact that SCC displayed more FDG uptake than lung adenocarcinoma [28].
In our study, both the Ki and the SUVmax of tumour in the ultralow-activity group were higher than the full-activity group despite of no statistical difference, due to the higher proportion of the patients with poorly differentiated cancer in ultralow-activity group. However, the CoV% of SUVmax was higher than Ki in both groups, which indicated that the SUVmax was more susceptible to interference. Furthermore, the SUVmax-Tumour/SUVmax-Liver and SUVmax-Tumour/SUVmax-Muscle were also not significantly different between the two groups, which indicated that the static image quality in the ultralow-activity group was equal to that in the full-activity group. In addition to the tumour lesion, the Ki in other measured tissues was also not significantly different between the full-activity and ultralow-activity groups. Therefore, considering another study [14] from our team about half-dose 18F-FDG compared with conventional PET/CT using full-dose FDG in lung cancer, we have strongly demonstrated the feasibility of low-activity both in static and dynamic PET/CT scanning by total-body PET/CT in lung cancer patients.
The tenfold reduction in injected 18F-FDG dose is a huge attractive advantage of decreasing radiation exposure. However, research on low-activity PET/CT scanning is rare due to the intrinsically noisy nature of PET modalities, especially dynamic PET scanning. Furthermore, the low signal-to-noise ratio (SNR) of dynamic PET data is a major obstacle which can cause the severe deviation in the evaluation of dynamic parameter [29]. To achieve equivalent image quality and lesion detectability when compared with full activity, the lowest PET counts need to reach 5 million in low-activity scanning [30]. In our study, the median effective count in each frame was 42.54 million in the ultralow-activity group, which exceeded the minimum requirement described above. Despite no denoising method in our dynamic study while the nonlocal means (NLM) method in Wu’s study [31], the random counts and noise equal counts (NEC) were also decreased in ultralow-activity compared with full activity, which may improve the image quality despite the lower total counts.
In addition to the advantage of decreasing radiation exposure, the lower storage of PET/CT scanning data in ultralow activity, especially in dynamic scanning, is another attractive point that relieves the burden of storage in clinical workstations. The median size of the data in the full-activity group was more than 631 GB, while it was 63.6 GB in ultra-low-activity group. The nearly tenfold reduction in data size offered the huge possibility to store more research data in the limited storage capacity of clinical workstations.
Despite the advantages in the ultralow-activity group described above, we found that the goodness-of-fit of model fitting in the full-activity group was superior to that in the ultralow-activity group, which indicated that a sufficient injected dose of 18F-FDG was required for dynamic PET imaging to guarantee stable model fitting. This reason also applied to the diverse goodness-of-fit in different organs, which explained that organs with high 18F-FDG metabolism, such as brain tissues, showed better model fitting, whereas those with low 18F-FDG metabolism, such as pancreas, muscle, and bone marrow, showed relatively poor model fitting with R2 less than 0.9. Furthermore, this may offer the reasonable explanation of the p value is more than but near the 0.05 in K1 value in muscle as well as K3 value. Due to the high 18F-FDG metabolism in tumours, the tumour lesion also displayed better model fitting than other organs except brain tissues, even in the ultralow-activity group.
However, there are still several unavoidable limitations in this study. First, the interclass variances of kinetic constants in some organs, especially for organs with low 18F-FDG metabolism, were still substantial in both the ultralow-activity and full-activity groups. Second, the patients in each group were not large enough and lacked a portion of advanced metastatic lung adenocarcinoma. Hence, a follow-up study on dynamic PET scanning may recruit more patients to avoid similar limitations. Third, the dynamic PET imaging with 60 min is too long for the clinical practice and too difficult for the oncological patients to avoid slight movement. For this reason, the short time dynamic PET imaging may be more attractive in the future.
Conclusion
We demonstrated that there were no significant differences in kinetic metrics or image quality after one-tenth injected dose of 18F-FDG using a high-sensitivity total-body PET scanner in lung adenocarcinoma patients compared to full-activity dynamic imaging, except in the reduction of radiation dose and data storage size. Thus, combined with our previous work related to the decreased injected dose of 18F-FDG by a total-body PET scanner, it is feasible to use one-tenth injected dose of 18F-FDG for clinical practice in oncological patients without obesity in dynamic PET scanning.
References
Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54.
Fletcher JW, Djulbegovic B, Soares HP, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49(3):480–508.
Bosch KD, Chicklore S, Cook GJ, et al. Staging FDG PET-CT changes management in patients with gastric adenocarcinoma who are eligible for radical treatment. Eur J Nucl Med Mol Imaging. 2020;47(4):759–67.
Broccoli A, Nanni C, Cappelli A, et al. Diagnostic accuracy of positron emission tomography/computed tomography-driven biopsy for the diagnosis of lymphoma. Eur J Nucl Med Mol Imaging. 2020;47(13):3058–65.
Alberts I, Hunermund JN, Prenosil G, et al. Clinical performance of long axial field of view PET/CT: a head-to-head intra-individual comparison of the biograph vision quadra with the biograph vision PET/CT. Eur J Nucl Med Mol Imaging. 2021;48(8):2395–404.
Viswanath V, Daube Witherspoon ME, Karp JS, et al. Numerical observer study of lesion detectability for a long axial field-of-view whole-body PET imager using the PennPET explorer. Phys Med Biol. 2020;65(3): 035002.
Xiao J, Yu H, Sui X, et al. Can the BMI-based dose regimen be used to reduce injection activity and to obtain a constant image quality in oncological patients by (18)F-FDG total-body PET/CT imaging? Eur J Nucl Med Mol Imaging. 2021;49(1):269–78.
Hu P, Zhang Y, Yu H, et al. Total-body (18)F-FDG PET/CT scan in oncology patients: how fast could it be? Eur J Nucl Med Mol Imaging. 2021;48(8):2384–94.
Zhang Y, Hu P, Wu R, et al. The image quality, lesion detectability, and acquisition time of (18)F-FDG total-body PET/CT in oncological patients. Eur J Nucl Med Mol Imaging. 2020;47(11):2507–15.
Cherry SR, Jones T, Karp JS, et al. Total-body PET: maximizing sensitivity to create new opportunities for clinical research and patient care. J Nucl Med. 2018;59(1):3–12.
Zhang X, Badawi RD, Cherry SR, et al. Theoretical study of the benefit of long axial field-of-view PET on region of interest quantification. Phys Med Biol. 2018;63(13):135010.
Cherry SR, Badawi RD, Karp JS, et al. Total-body imaging: transforming the role of positron emission tomography. Sci Transl Med. 2017;9(381):eaaf6169.
Surti S, Karp JS. Impact of detector design on imaging performance of a long axial field-of-view, whole-body PET scanner. Phys Med Biol. 2015;60(13):5343–58.
Tan H, Sui X, Yin H, et al. Total-body PET/CT using half-dose FDG and compared with conventional PET/CT using full-dose FDG in lung cancer. Eur J Nucl Med Mol Imaging. 2021;48(6):1966–75.
Liu G, Hu P, Yu H, et al. Ultra-low-activity total-body dynamic PET imaging allows equal performance to full-activity PET imaging for investigating kinetic metrics of (18)F-FDG in healthy volunteers. Eur J Nucl Med Mol Imaging. 2021;48(8):2373–83.
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–30.
Gupta N, Gill H, Graeber G, et al. Dynamic positron emission tomography with F-18 fluorodeoxyglucose imaging in differentiation of benign from malignant lung/mediastinal lesions. Chest. 1998;114(4):1105–11.
Yang M, Lin Z, Xu Z, et al. Influx rate constant of (18)F-FDG increases in metastatic lymph nodes of non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging. 2020;47(5):1198–208.
Romer W, Hanauske AR, Ziegler S, et al. Positron emission tomography in non-Hodgkin’s lymphoma: assessment of chemotherapy with fluorodeoxyglucose. Blood. 1998;91(12):4464–71.
Wahl RL, Zasadny K, Helvie M, et al. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol. 1993;11(11):2101–11.
Zhang X, Xie Z, Berg E, et al. Total-body dynamic reconstruction and parametric imaging on the uEXPLORER. J Nucl Med. 2020;61(2):285–91.
Zhang X, Zhou J, Cherry SR, et al. Quantitative image reconstruction for total-body PET imaging using the 2-meter long EXPLORER scanner. Phys Med Biol. 2017;62(6):2465–85.
Liu G, Xu H, Hu P, et al. Kinetic metrics of (18)F-FDG in normal human organs identified by systematic dynamic total-body positron emission tomography. Eur J Nucl Med Mol Imaging. 2021;48(8):2363–72.
Wahl LM, Asselin MC, Nahmias C. Regions of interest in the venous sinuses as input functions for quantitative PET. J Nucl Med. 1999;40(10):1666–75.
Akaihe H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–23.
Akaihe H. An information criterion (AIC). Math Sci. 1976;14:5–9.
Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–4.
de Geus-Oei LF, van Krieken JH, Aliredjo RP, et al. Biological correlates of FDG uptake in non-small cell lung cancer. Lung Cancer. 2007;55(1):79–87.
Kimura Y, Naganawa M, Shidahara M, et al. PET kinetic analysis –pitfalls and a solution for the Logan plot. Ann Nucl Med. 2007;21(1):1–8.
Schaefferkoetter JD, Yan J, Townsend DW, et al. Initial assessment of image quality for low-dose PET: evaluation of lesion detectability. Phys Med Biol. 2015;60(14):5543–56.
Wu Y, Feng T, Shen Y, et al. Total-body parametric imaging using the Patlak model: feasibility of reduced scan time. Med Phys. 2022;49(7):4529–39.
Funding
This study is supported by Shanghai Municipal Key Clinical Specialty Project (shslczdzk03401), Clinical Research Plan of SHDC (SHDC2020CR3079B), Science and Technology Committee of Shanghai Municipality(20DZ2201800), Special Fund for Clinical Research of Zhongshan Hospital, Fudan University(2020ZSLC63), The Youth Medical Talents-Medical Imaging Practitioner Program of Shanghai “Rising Stars of Medical Talent” Youth Development Program (SHWRS [2020]087), The National Natural Science Foundation of China (82001863), and the Shanghai Sailing Program (19YF1408200).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lv, J., Yin, H., Yu, H. et al. The feasibility of ultralow-activity 18F-FDG dynamic PET imaging in lung adenocarcinoma patients through total-body PET/CT scanner. Ann Nucl Med 36, 887–896 (2022). https://doi.org/10.1007/s12149-022-01772-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-022-01772-2