Abstract
Objective
This study aimed at investigating the correlation between recurrent visual hallucinations (VHs) and regional cerebral blood flow (rCBF) in patients with dementia with Lewy bodies (DLB).
Methods
In 147 DLB patients, the correlation between noise pareidolia scores and rCBF in brain perfusion single photon emission computed tomography (SPECT) was evaluated. The 147 subjects comprised 52 probable and 95 possible DLB patients, of whom 107 did not have visual hallucinations and 40 had visual hallucinations. Brain perfusion SPECT was then performed, and memory impairment was assessed using the Mini-Mental State Examination (MMSE), while the optical illusion “pareidolia” (the tendency to see a specific image in a random visual pattern) was evaluated using noise pareidolia test. The correlations between rCBF and MMSE or noise pareidolia scores were then analyzed.
Results
The rCBF and MMSE scores were positively correlated, and rCBF was correlated with MMSE scores in a region that was consistent with a previously reported memory-related site. There was no correlation between noise pareidolia scores and occipital CBF, but there were weak correlations between noise pareidolia scores and rCBF in the bilateral frontal lobes (Brodmann area [BA]8 and BA9), left cingulate cortex (BA31), and left angular and supramarginal gyri (BA39 and BA40) in DLB patients.
Conclusion
Weak correlation was found between noise pareidolia scores and rCBF in several sites (BA8, BA9, BA31, BA39 and BA40) other than in occipital lobe. These findings suggest that DLB hallucinations may be manifested by more complex brain network disorders, rather than by primary visual cortex disorders alone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dementia with Lewy bodies (DLB) was first established in a series of studies by Kosaka et al. beginning in 1976 [1,2,3,4,5]. DLB is recognized as the second major form of dementia in the older Japanese population. The main symptoms of DLB are visual hallucinations, fluctuating cognitive impairment, and parkinsonism. Currently, DLB occurs in about 9.7–24.7% of patients with dementia [6]. Visual hallucinations are a core feature of DLB and are found in approximately 70% of all DLB patients [7,8,9]. Brain functional imaging studies using positron emission tomography (PET) and single photon emission computed tomography (SPECT) have revealed that both cerebral glucose metabolism and cerebral blood flow (CBF) is thought to cause are reduced in the posterior cerebral cortex region (visual cortex region) of DLB patients [10,11,12,13]. In the previous investigations, several regional sites of brain damage that causes visual hallucinations in DLB patients have been speculated (e.g., frontal, parietal and occipito-temporal cortex), but have not yet been clarified [14].
Faces are often present in DLB hallucinations and optical illusions. Among multiple types of pareidolia tests (which evaluate the tendency to see a specific image or pattern in a random visual pattern or stimulus), the face task is an excellent method for inducing high-frequency optical illusions, and the noise pareidolia test is commonly used for dementia patients in clinical practice [15, 16]. The prevalence of hallucinations is much higher in DLB than in other dementia subtypes, and hallucinations are thus a very important symptom for the differential diagnosis of DLB from other diseases.
In the current study, we selected DLB patients who were diagnosed based on the DLB diagnostic criteria [17] revised in 2017, and evaluated the correlation between noise pareidolia test (developed by Tohoku University) [15, 18] scores and regional CBF (rCBF) using head magnetic resonance imaging (MRI) and brain perfusion SPECT.
Subjects and methods
Subjects
We retrospectively selected subjects from the patients admitted to the infirmary at our institution for the evaluation of dementia from January 2018 to December 2019. Patient characteristics were collected, including sex, age, Mini-Mental State Examination (MMSE) score, and noise pareidolia test scores. All patients were examined by neurologists and psychiatrists and underwent standard neurological and neuropsychological examinations, laboratory tests, head MRI, and brain perfusion SPECT. The clinical data revealed fluctuations in cognitive functions, recurrent visual hallucinations, and spontaneous parkinsonism. These symptoms were used to diagnose probable DLB, possible DLB, and mild cognitive impairment (MCI) caused by DLB, based on the criteria proposed by the Consortium on DLB International Workshop [17].
The characteristics of the patients are shown in Table 1. In this study, 147 DLB patients were included; cases with comorbidities (such as cerebrovascular diseases and idiopathic normal pressure hydrocephalus), insufficient data, and indistinguishable cases were excluded.
Noise pareidolia test
Visual illusions in DLB can be evaluated by many types of tasks. In many hospitals, the evaluation of visual illusions is commonly performed using the “noise pareidolia test” (Nishio Y. Title of subordinate document. In: The noise pareidolia test. A simple neuropsychological test evoking and measuring visual hallucination-like illusions in dementia with Lewy body and other dementia disorders. https://figshare.com/articles/dataset/The_noise_pareidolia_test/3187669. Dataset posted on 21 Apr 2016.), which was developed by Tohoku University [18]. The face task in the pareidolia test is an excellent way to induce the “pareidolia” optical illusion and is known as the noise pareidolia test.
The pareidolia test (which includes both the scene pareidolia test and the noise pareidolia test) generally requires approximately 15 min to administer, but the noise pareidolia test alone requires just 5.5 ± 3.0 min for patients with dementia [16]. We prioritized convenience and selected only the noise pareidolia test to use in the outpatient department, despite its reduced sensitivity and specificity compared with the full pareidolia test for the detection of DLB visual hallucinations.
The examiner performed the noise pareidolia test without knowing the definitive diagnosis of each patient. In the face task in this test, there may or may not be a face present in a black and white pattern. If a face is seen, the subject is asked to answer "yes" and point to where it is. If no face is seen, the subject should answer "no". Subjects were instructed to answer “yes” only in tasks where a face was clearly visible. In this task, 40 image stimuli were presented one by one, and subjects were asked whether or not they could see a face in each stimulus. If a subject spent more than 10 s without a reaction, the examiner asked the subject to respond to the stimulus. If the subject answered "it looks like XX", the examiner asked the subject to respond only with what they perceived to be clearly visible.
The noise pareidolia test contains 40 image stimuli and the face is actually drawn in 8 image stimuli, and 32 images without a face. Subjects were requested to say whether a face was present and point to the place where they observed a face after a detail explanation and three training trials were done. Each picture was presented within maximum 30 s. Regardless of whether the responses were correct, no feedback was given to subjects. The noise pareidolia test has a maximum score of 40 points. Subject responses are evaluated as one of three following reactions: (1) “pareidolia”, in which subjects falsely found faces in images without a face; (2) “miss”, in which subjects did not detect the embedded faces, or (3) “correct”, in which subjects correctly responded “nothing exists” to the noise stimuli or correctly detected the embedded image in the images that contained faces (e.g., the scoring is shown as “correct” 36/40, “pareidolia” 4/40 and “miss” 0/8. The scoring method is described in detail at the previous URL).
The so-called pareidolia score is defined as the sum of both the scene pareidolia score and the noise pareidolia score [16]. However, in our outpatient department, the number of optical illusions (pareidolia) in the noise pareidolia test was counted as the noise pareidolia score only, instead of using the pareidolia score. In this study, the cut-off score for the noise pareidolia test was set as 2/3 (sensitivity 60%, specificity 92%), which was helpful from a differential diagnosis perspective (rather than using the pareidolia score) [16, 18].
Brain perfusion SPECT and MRI
Brain perfusion SPECT scans were initiated in the resting state with the eyes closed, 15 min after each patient was administered an injection of 111 MBq of N-isopropyl-p-[123I] iodoamphetamine. All SPECT scans were performed using a rotating dual-headed gamma camera (E-CAM, Siemens, Erlangen, Germany) with a low–medium energy, general purpose collimator. Brain perfusion SPECT images were obtained with a 128 × 128 matrix, 2.5 min/rotation × 12 rotations. For SPECT image reconstruction, a Butterworth filter (cutoff frequency: 0.58 cycles/cm, order: 8) was used. Attenuation correction was performed using Chang’s method (µ = 0.09 cm−1) and scatter correction was performed with a triple energy window.
MRI scanning was performed using a 3 T Achieva or a 1.5 T Ingenia (Philips, Best, Netherlands). The scan protocol included sagittal T1-weighted three-dimensional whole-brain images (Achieva: slice thickness 1.2 mm, 140 slices, matrix size 256 × 256, field of view 25.6 × 25.6 cm, echo time 3.11 ms, repetition time 6.7 ms, flip angle 8°; Ingenia: slice thickness 1.2 mm, 140 slices, matrix size 192 × 192, field of view 24.0 × 24.0 cm, echo time 4.0 ms, repetition time 8.6 ms, flip angle 8°).
For the relative CBF image production, all voxel counts from each SPECT image were normalized by dividing them by the cerebellar counts. Because in DLB brain whole cerebral CBF is often diffusively decreased, global count normalization may underestimate the relative CBF values.
Data analysis
The statistical voxel-based analysis was performed using Statistical Parametric Mapping 12 (SPM12) software (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/), and correlations between the images and cognitive scores were then evaluated.
Each individual SPECT image was co-registered to the individual MRI image, and the MRI image was segmented into gray matter (GM), white matter, and cerebrospinal fluid using the SPM12 segmentation program. The individual GM image was then spatially normalized onto the template image using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) technique [19], and the normalized parameter was adopted to the co-registered relative SPECT image. The SPECT image was then spatially normalized to the Montreal Neurological Institute space. All images were smoothed using a 12-mm Gaussian filter. Next, SPM12 was used for voxel-wise multiple regression analysis between GM or CBF images and MMSE or noise pareidolia test scores. The significance peak was set at p < 0.001, uncorrected, and the voxel extent threshold was set at 300.
Results
Over a period of 2 years, 199 patients were clinically diagnosed with DLB in our institution. Of these, 52 patients were excluded, including 17 patients with cerebrovascular accidents, six patients with idiopathic normal pressure hydrocephalus, five patients without sufficient data, five patients who were indistinguishable, and one patient with corticobasal degeneration. Thus, 147 patients were diagnosed with DLB based on sufficient data. As shown in Table 1, the patient characteristics were as follows: 56 males and 91 females; average age 77.5 ± 6.1 years (range: 50–93 years; average age: males 78.3 years, females 77.0 years), average MMSE score 23.3 ± 4.0 points (range 13–30 points; average score: males 23.6 points, females 23.1 points), and average pareidolia score 7.1 ± 7.0 points (range 0–30 points). The 147 DLB patients consisted of 52 probable and 95 possible DLB patients. Of these, 107 patients did not have visual hallucinations and 40 patients had visual hallucinations. There were 96 patients with parkinsonism, 32 patients with rapid eye movement (REM) sleep behavioral abnormalities, 26 patients with cognitive fluctuations, 15 patients with MCI caused by DLB, and 2 patients with other dementias. Of the 40 patients with hallucinations, 27 were positive for pareidolia (noise pareidolia score ≥ 3) and 13 were negative for pareidolia (noise pareidolia score ≤ 2) in the noise pareidolia test. Of the remaining 107 patients without hallucinations, 63 were positive for pareidolia (noise pareidolia score ≥ 3) and 44 were negative for pareidolia (noise pareidolia score ≤ 2). Visual cognitive impairment or visuospatial cognitive impairment was observed in 56 patients, and Parkinson's disease (PD) was identified in 96 patients.
The results of the statistical image analysis in this study are shown in Fig. 1 and Table 2. The GM volume in the bilateral medial frontal lobes and bilateral medial temporal lobes was positively correlated with MMSE scores (Fig. 1a, Table 2). Furthermore, there was a positive correlation between rCBF and MMSE scores in the medial temporal lobes, including the hippocampus and parahippocampal gyrus, posterior and anterior cingulate gyrus, inferior temporal lobe, and basal frontal area (Fig. 1b, Table 2).
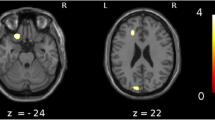
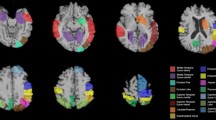
Areas of significant correlations with Mini-Mental State Examination (MMSE) scores or pareidolia scores in dementia with Lewy bodies (DLB) patients. a Areas of significant correlation between gray matter volume and MMSE scores in patients with DLB are shown (p < 0.001, uncorrected). The bilateral medial frontal lobes and bilateral medial temporal lobes are highlighted (p < 0.001, uncorrected). b Areas of significant correlation between regional cerebral blood flow (rCBF) and MMSE score in DLB patients are shown (p < 0.001, uncorrected). The bilateral medial temporal lobes, posterior and cingulate gyri, inferior temporal lobe, and basal frontal area are highlighted as significantly correlated areas. c Areas of significant correlation between rCBF and pareidolia scores in DLB patients are shown (p < 0.001, uncorrected). The bilateral frontal and cingulate gyri, left posterior cingulate gyrus, and left parietal cortex are highlighted as significantly correlated areas
There were no significant correlations between regional GM and noise pareidolia scores nor between rCBF and noise pareidolia scores in 40 patients with visual hallucinations; then we performed the analysis in the whole 147 patients. Analysis of the correlation between GM and noise pareidolia scores revealed no significant areas; however, rCBF and noise pareidolia scores were weakly correlated in the bilateral frontal and cingulate gyri (Brodmann area [BA]8 and BA9), left posterior cingulate gyrus (BA31), and left parietal cortex (BA39 and BA40). In contrast, there was no significant correlation between the occipital CBF and noise pareidolia scores (Fig. 1c, Table 3). In 40 DLB patients with hallucinations, there was no significant difference in the correlation between occipital CBF and noise pareidolia scores (positive scores or negative scores) in noise pareidolia tests.
From the above results, there was no correlation between noise pareidolia scores and occipital CBF by using this analysis method, but there was a weak correlation of rCBF in several sites (BA8, BA9, BA31, BA39 and BA40) other than in occipital lobe.
Discussion
Features of this study
In this study, we first confirmed that memory impairment correlates with rCBF around the hippocampus based on analysis of the correlation between MMSE and rCBF or GM volume. By using this analysis method, we obtained results that are consistent with previous reports. We have proved that this analysis method can be analyzed without problems.
Next step, we attempted to investigate the correlation between the optical illusion (visual hallucinations) and rCBF, based on the analysis of correlation between noise pareidolia scores and rCBF or GM volume.
Prior to the analysis, this sample size (n = 147) was larger than previously reported, and we speculated that t value would be at least 5 or higher in the correlation between the MMSE and CBF around the hippocampus. However, in fact, t value was ranged from 3.39 to 4.91, the maximum t value was 4.91 in Table 2. In the correlation between the noise pareidolia scores and the rCBF in several sites other than in the occipital lobe obtained by this analysis, t value was as low as 3.46 to 4.51 in Table 3. The maximum t value was 4.51 in Table 3; we conservatively described the correlation as “weak” between the noise pareidolia scores and the rCBF in several sites obtained by this analysis other than in the occipital lobe. In this paper, we defined t value ≤ 5 as “weak (or weakly)”.
Here, we revealed that a reduction in occipital CBF was not associated with an increase in noise pareidolia scores in DLB patients; rCBF in several sites other than in the occipital lobe showed a weak correlation with the noise pareidolia scores. There have been few functional imaging studies investigating visual hallucinations and optical illusions in DLB patients. In voxel-based analyses in previous pioneering studies [20,21,22,23], there was no significant correlation between pareidolia and occipital CBF.
The features of this study are as follows: while the total number of patients included in previous reports is small, a key feature of the present study was the relatively large number of patients: 147 patients were included based on the latest DLB diagnostic criteria [17], revised in 2017. In fact, in this study of only 40 patients with visual hallucinations analysis, no significant difference in the correlation between the occipital CBF and noise pareidolia scores (positive scores or negative scores) was found.
In the present study, the reason for the relatively few cases of movement disorders were likely because our division is a "dementia outpatient" unit consisting of neurology and psychiatry; for many cases, cognitive decline was the main reason for consultation. It is probable that many of the patients showed slight parkinsonism.
Comparisons between the present study and pioneering imaging studies of visual hallucinations in DLB and PD
In the present study, DLB patients did not show the expected predominant decrease in occipital lobe CBF. Using SPECT imaging with voxel-based analysis techniques, Colloby et al. revealed that rCBF is significantly reduced in the left inferior parietal lobes (BA40) and left frontal gyrus (BA8) compared with healthy subjects [20], which is similar to our results. Notably, in the present study, a correlation between pareidolia and the occipital lobe (primary visual cortex V1) was not identified. In addition, Nagahama et al. reported that local CBF was decreased in the bilateral angular gyrus (BA39), left fourth occipital gyrus (BA19), and right supramarginal gyrus (BA40) using SPECT imaging with voxel-based statistical analysis techniques [21]. Their findings relating to BA39 and BA40 are compatible with our results; furthermore, rCBF was not reduced in the primary visual cortex (BA17) or secondary visual cortex (BA18), but was reduced in the associative visual cortex (BA19). From this result, the reduction of rCBF in the associative visual cortex (BA19) is presumed to be caused by disorders of the brain network between the frontal and occipital lobes, rather than of the occipital lobe alone.
It has previously been reported that about 40% of patients with PD with dementia have visual hallucinations, and approximately 70% of PD patients experience optical illusions [24,25,26,27,28,29,30]. Both PD and DLB are pathologically classified as α-synucleinopathies and are so-called Lewy body diseases (LBD). In Lewy body diseases, the same physiological mechanism of visual hallucinations is inferred. Similar to DLB patients, patients with PD with visual hallucinations do not demonstrate a decrease in occipital lobe blood flow, but rCBF is reportedly reduced in the left insula (BA13), bilateral superior temporal gyrus (BA38), left supramarginal gyrus (BA40), left frontal pole (BA10), left anterior cingulate gyrus (BA24), left precuneus (BA7), right anterior cingulate (BA32), right parietal superior lobule (BA5), right frontal superior lobule (BA11), and right frontal inferior lobule (BA12) in imaging studies using MRI with voxel-based morphometry analysis techniques [22, 23].
Hypothesis of the mechanism of visual hallucinations in DLB
Visual sensory impairment is thought to be involved in the development of complex visual hallucinations in DLB patients [31]. A large number of different mechanisms have been proposed to underlie complex visual hallucinations, and the possible causes and brain disorder sites of complex visual hallucinations can be summarized as follows: (1) visual pathway (obstacle pathway, extending from the retina to V1), (2) occipital lobe (V1, V2, or V3 lesion), (3) epilepsy, (4) brain stem (e.g., midbrain peduncle hallucinations), (5) sleeping disorder (e.g., REM sleep and narcolepsy), (6) dysfunctional imbalance of neurotransmitters (e.g., acetylcholine or dopamine system failure), and (7) disorders of resting brain networks (e.g., dorsal attention system failures and default mode network abnormalities) [31,32,33,34].
In DLB patients, it has previously been reported that brain functional imaging tests, such as PET and SPECT, show decreased metabolism and decreased blood flow in the occipital lobe (visual cortex) [10,11,12,13]. The occipital lobe can cause many simple hallucinations as a whole; moreover, V1 disorders cause simple hallucinations, whereas V2 and V3 disorders lead to many complex hallucinations [33, 35, 36].
Dopamine abnormalities in the retina have been identified in PD patients, and the possibility of dopamine abnormalities cannot be ruled out in DLB patients. Acetylcholinesterase inhibitors improve visual hallucinations and attention in DLB patients, which suggests that cholinergic abnormalities may also be involved in visual hallucinations [33, 34].
Possibility of the involvement of attention disorders and brain network dysfunction
In the present study, the possible reasons underlying the lack of a correlation between CBF in the occipital lobe and pareidolia test scores were speculated to be as follows: (1) CBF reductions in other regions of the brain may have been larger than that in the occipital lobe, and the CBF reduction sites were scattered, which may have affected the whole brain; (2) many early cases of DLB were included in the present study; and (3) in the case of early DLB, the influence of the secondary visual cortex may be greater than that of the primary visual cortex.
Recently, increased attention has been paid to disorders of the dorsal attention system [37] in DLB patients, and it has been proposed that dysfunction of this system may cause incorrect visual processing and lead to complex hallucinations. In addition, dysfunction of the resting brain attention network may also be involved in the development of visual hallucinations [33, 34, 38]. Therefore, we propose that pareidolia may also be affected by attention disorders [14, 39,40,41]. Visual hallucinations may be caused not only by disorders of the primary visual cortex (i.e., the occipital lobe cortex), but also by complex interactions caused by “anterior” brain dysfunction (e.g., in the frontal lobes), such as attention disorders and network disorders with other brain regions [34, 41]. It is speculated that disorders of the resting brain network [38] that are related to the attention and default mode network [32, 38] are able to evoke pareidolia.
Future prospects
In DLB patients with visual hallucinations, it has been proposed that the distribution of Lewy bodies is not only a disorder of the primary visual region of the occipital lobe but also a disorder of a more complex brain network, thus resulting in visual hallucinations. We hypothesize that visual hallucinations can occur not only as a result of simple disorders of the primary visual cortex, such as the occipital lobe cerebral cortex, but also as a result of more complex physiological mechanisms, such as impairment of the visual pathway, the secondary visual cortex in brain networks, and the involvement of neurotransmitters [31, 32]. Through further research to clarify the physiological mechanism of complex visual hallucinations in DLB patients and the site of local brain damage in functional connectivity and brain networks, by combining many imaging modalities of brain function, the earlier diagnosis of DLB will be possible in the future. We expect that the elucidation of the visual hallucination mechanism in DLB will allow for its early diagnosis based on imaging biomarkers, and earlier therapeutic treatment interventions will improve patients’ symptoms.
Limitations
There are some limitations to this study. First, this study is a retrospective study, there was no correlation between noise pareidolia scores and occipital CBF'; however, there was a weak correlation between noise pareidolia scores and rCBF in several sites other than in occipital lobe. Regarding the sample size, since this investigation is a retrospective study of patients who visited our outpatient department, it may be necessary to secure the number of samples by long-term observation in order to perform more accurate analysis.
Second, no clear correlation was found between noise pareidolia scores and occipital CBF, but a weak correlation between noise pareidolia scores and rCBF in several sites other than in occipital lobe. We considered that the noise pareidolia scores in the noise pareidolia test may be an indirect scale rather than a direct scale of visual hallucinations.
Third, we may see pareidolia in the noise pareidolia test, ultimately, as an interaction of complex regional cerebral blood flow (activity) in the brain. As a result, we believe that we could identify a weak correlation between the noise pareidolia scores and rCBF in several sites other than in occipital lobe.
Furthermore, in order to analyze the brain network (complex interactions and functional connectivity) in detail, we have improved the evaluation method of the noise pareidolia test, developed an evaluation scale that directly reflects the visual hallucinations, and we thought that it will be necessary to develop brain functional imaging analysis in combination with the other modality.
Conclusion
In the present study, DLB hallucinations did not significantly correlate with occipital hypoperfusion, as we had expected before performing the study, but were weakly correlated with CBF in the bilateral inferior parietal lobes (BA40). Previous reports are also consistent with our findings of a weak correlation between visual hallucinations (optical illusions) and rCBF in the left precuneus (BA39) and bilateral frontal gyrus (BA8 and BA9). Visual hallucinations in DLB had a weak correlation with rCBF in several sites other than in occipital lobe, as has been previously reported. A mechanism of DLB visual hallucinations may involve more complex brain network disorders (e.g., multiple brain network disorders such as “default mode networks” and “attention networks”) rather than a primary visual cortex disorder alone.
References
Kosaka K. Lewy bodies in cerebral cortex, report of three cases. Acta Neuropathol. 1978;42(2):127–34.
Kosaka K. Diffuse Lewy body disease in Japan. J Neurol. 1990;237(3):197–204.
Kosaka K, Oyanagi S, Matsushita M, Hori A. Presenile dementia with Alzheimer-, Pick- and Lewy-body changes. Acta Neuropathol. 1976;36(3):221–33.
Kosaka K, Yoshimura M, Ikeda K, Budka H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree—a new disease? Clin Neuropathol. 1984;3(5):185–92.
Kosaka K, Mehraein P. Dementia-Parkinsonism syndrome with numerous Lewy bodies and senile plaques in cerebral cortex. Arch Psychiatr Nervenkr. 1979;226(4):241–50.
Vann Jones SA, O’Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med. 2014;44(4):673–83.
Ballard C, Holmes C, McKeith I, Neill D, O’Brien J, Cairns N, et al. Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer’s disease. Am J Psychiatry. 1999;156(7):1039–45.
Nagahama Y, Okina T, Suzuki N, Matsuda M, Fukao K, Murai T. Classification of psychotic symptoms in dementia with Lewy bodies. Am J Geriatr Psychiatry. 2007;15(11):961–7.
Aarsland D, Ballard C, Larsen JP, McKeith I. A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson’s disease with and without dementia. Int J Geriatr Psychiatry. 2001;16(5):528–36.
Lobotesis K, Fenwick JD, Phipps A, Ryman A, Swann A, Ballard C, et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology. 2001;56(5):643–9.
Shimizu S, Hanyu H, Hirao K, Sato T, Iwamoto T, Koizumi K. Value of analyzing deep gray matter and occipital lobe perfusion to differentiate dementia with Lewy bodies from Alzheimer’s disease. Ann Nucl Med. 2008;22(10):911–6.
Ishii K, Imamura T, Sasaki M, Yamaji S, Sakamoto S, Kitagaki H, et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer’s disease. Neurology. 1998;51(1):125–30.
Imamura T, Ishii K, Sasaki M, Kitagaki H, Yamaji S, Hirono N, et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer’s disease: a comparative study using positron emission tomography. Neurosci Lett. 1997;235(1–2):49–52.
Pezzoli S, Cagnin A, Bandmann O, Venneri A. Structural and functional neuroimaging of visual hallucinations in Lewy body disease: a systematic literature review. Brain Sci. 2017;7(7):84.
Uchiyama M, Nishio Y, Yokoi K, Hirayama K, Imamura T, Shimomura T, et al. Pareidolias: complex visual illusions in dementia with Lewy bodies. Brain. 2012;135(Pt 8):2458–69.
Mamiya Y, Nishio Y, Watanabe H, Yokoi K, Uchiyama M, Baba T, et al. The Pareidolia test: a simple neuropsychological test measuring visual hallucination-like illusions. PLoS ONE. 2016;11(5): e0154713.
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100.
Yokoi K, Nishio Y, Uchiyama M, Shimomura T, Iizuka O, Mori E. Hallucinators find meaning in noises: pareidolic illusions in dementia with Lewy bodies. Neuropsychologia. 2014;56:245–54.
Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113.
Colloby SJ, Fenwick JD, Williams ED, Paling SM, Lobotesis K, Ballard C, et al. A comparison of 99mTc-HMPAO SPET changes in dementia with Lewy bodies and Alzheimer’s disease using statistical parametric mapping. Eur J Nucl Med Mol Imaging. 2002;29(5):615–22.
Nagahama Y, Okina T, Suzuki N, Matsuda M. Neural correlates of psychotic symptoms in dementia with Lewy bodies. Brain. 2010;133(Pt 2):557–67.
Ibarretxe-Bilbao N, Ramirez-Ruiz B, Junque C, Marti MJ, Valldeoriola F, Bargallo N, et al. Differential progression of brain atrophy in Parkinson’s disease with and without visual hallucinations. J Neurol Neurosurg Psychiatry. 2010;81(6):650–7.
Meppelink AM, de Jong BM, Teune LK, van Laar T. Regional cortical grey matter loss in Parkinson’s disease without dementia is independent from visual hallucinations. Mov Disord. 2011;26(1):142–7.
Barrett MJ, Smolkin ME, Flanigan JL, Shah BB, Harrison MB, Sperling SA. Characteristics, correlates, and assessment of psychosis in Parkinson disease without dementia. Parkinsonism Relat Disord. 2017;43:56–60.
Graham JM, Grünewald RA, Sagar HJ. Hallucinosis in idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997;63(4):434–40.
Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(Pt 4):733–45.
Fénelon G, Soulas T, Zenasni F, Cleret de Langavant L. The changing face of Parkinson’s disease-associated psychosis: a cross-sectional study based on the new NINDS-NIMH criteria. Mov Disord. 2010;25(6):763–6.
Inzelberg R, Kipervasser S, Korczyn AD. Auditory hallucinations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;64(4):533–5.
Pagonabarraga J, Martinez-Horta S, Fernández de Bobadilla R, Pérez J, Ribosa-Nogué R, Marín J, et al. Minor hallucinations occur in drug-naive Parkinson’s disease patients, even from the premotor phase. Mov Disord. 2016;31(1):45–52.
Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol. 2008;115(4):409–15.
Armstrong RA. Visual signs and symptoms of dementia with Lewy bodies. Clin Exp Optom. 2012;95(6):621–30.
Oishi N, Udaka F, Kameyama M, Sawamoto N, Hashikawa K, Fukuyama H. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology. 2005;65(11):1708–15.
Onofrj M, Taylor JP, Monaco D, Franciotti R, Anzellotti F, Bonanni L, et al. Visual hallucinations in PD and Lewy body dementias: old and new hypotheses. Behav Neurol. 2013;27(4):479–93.
Muller AJ, Shine JM, Halliday GM, Lewis SJ. Visual hallucinations in Parkinson’s disease: theoretical models. Mov Disord. 2014;29(13):1591–8.
Ffytche DH, Howard RJ, Brammer MJ, David A, Woodruff P, Williams S. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci. 1998;1(8):738–42.
Ffytche DH, Howard RJ. The perceptual consequences of visual loss: “positive” pathologies of vision. Brain. 1999;122(Pt 7):1247–60.
Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20(2):150–9.
Raichle ME. The restless brain: how intrinsic activity organizes brain function. Philos Trans R Soc Lond B Biol Sci. 2015;370(1668):20140172.
Heitz C, Noblet V, Cretin B, Philippi N, Kremer L, Stackfleth M, et al. Neural correlates of visual hallucinations in dementia with Lewy bodies. Alzheimers Res Ther. 2015;7(1):6.
Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, Campdelacreu J, Gascon J, Falcon C, et al. Frontal and associative visual areas related to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease with dementia. Mov Disord. 2010;25(5):615–22.
Perneczky R, Drzezga A, Boecker H, Förstl H, Kurz A, Häussermann P. Cerebral metabolic dysfunction in patients with dementia with Lewy bodies and visual hallucinations. Dement Geriatr Cogn Disord. 2008;25(6):531–8.
Acknowledgements
We would like to express our gratitude to all hospital staff at the Hyogo Brain and Heart Center for providing us with the chance to perform this research. Special thanks to all staff in the Department of Aging Brain and Cognitive Disorders and Geriatric Delirium Rounds Team at the Hyogo Brain and Heart Center.
Funding
This manuscript received no funding.
Author information
Authors and Affiliations
Contributions
Conceptual idea for the article was by TN and KI. TN performed the literature search. TN, KS, AI, HO, and AT were involved in the clinical neurological and neuropsychological data acquisition. YK, RK, and KI were involved in the imaging data acquisition. TN, TY, and KI performed the data analysis. The first draft of the manuscript was written by TN and was reviewed by KI. All authors provided critical revisions on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Informed consent
All investigations were carried out according to the Declaration of Helsinki. We confirmed that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. All co-authors have read and approved the submission. This study was examined by the ethics committee of the Hyogo Brain and Heart Center, and informed consent was made by opt-out information disclosure.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakata, T., Shimada, K., Iba, A. et al. Correlation between noise pareidolia test scores for visual hallucinations and regional cerebral blood flow in dementia with Lewy bodies. Ann Nucl Med 36, 384–392 (2022). https://doi.org/10.1007/s12149-022-01717-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-022-01717-9