Abstract
Objective
123I metaiodobenzylguanidine (MIBG) scintigraphy is a useful tool for the diagnosis of pheochromocytomas (PHEOs), but some PHEOs are difficult to differentiate from cortical adenoma (CA) or normal adrenal uptake by visual evaluation alone. A new semi-quantitative analysis using 123I MIBG SPECT/CT is thus expected. Herein, we introduce the tumor-to-liver count ratio (T/L) and the tumor-to-muscle count ratio (T/M).
Methods
We examined the cases of 21 patients with PHEOs (10 males, 11 females; age 24–80, median 61 years) and 23 patients with CA (15 males and 8 females, age 30–78, median 58 years). The visual scoring based on 123I MIBG planar images (planar score) and SPECT images (SPECT score) was used as the conventional evaluation. Using 123I MIBG SPECT/CT findings, we calculated the semi-quantitative values of the count ratio using the maximum or mean count of the tumor and the liver or muscle as the reference organ (T/Lmax, T/Lmean, T/Mmax and T/Mmean). Each evaluation of the PHEOs and CAs was compared, and the diagnosing performance was evaluated based on an ROC analysis.
Results
The area under curve (AUC) values were as follows: the planar score, 0.833; SPECT score, 0.813; T/Lmax, 0.986; T/Lmean, 0.975; T/Mmax, 0.955; and T/Mmean, 0.933. The AUC for T/Mmax was significantly higher than those of the planar score, and SPECT score by ROC analysis (p < 0.01 each).
Conclusion
The semi-quantitative value of 123I MIBG SPECT/CT is more useful than the conventional visual evaluation for differentiating PHEOs from CAs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pheochromocytomas (PHEOs) are common adrenal tumors arising from chromaffin cells of the adrenal medullas [1]. It is important to differentiate PHEOs from other adrenal tumors for better clinical management and treatment outcomes. CT and MRI are reported to be good modalities for diagnosing PHEOs, but sometime these methods may not work well depending on the tumors' histopathological conditions [2, 3]. PHEOs may contain variable histopathological components such as lipid, necrotic lesion, cystic changes, and more which make it difficult to differentiate PHEOs from cortical adenoma (CA) [4].
Metaiodobenzylguanidine (MIBG) scintigraphy is also a good imaging tool for suspected PHEO [5]. MIBG is a guanidine analogue similar to norepinephrine that can enter chromaffin cells through both active uptake via norepinephrine transporters (NETs) [6] and passive diffusion, and MIBG is stored in the neurosecretory granules through vesicular monoamine transporters (VMATs) [7]. The diagnosis of abnormal MIBG uptake is usually done by a visual evaluation in both planar images and single photon emission computed tomography (SPECT) images [8]. In the actual clinical diagnosis, the degree of MIBG uptake of a tumor is determined by a visual assessment with a comparison of the MIBG uptake of normal tissues such as liver and muscle [9]. SPECT/CT hybrid images enable a better anatomical separation of organs compared to conventional planar images, and thus low adrenal uptake can be evaluated more accurately without overlap with the liver or other organs. This makes it possible to achieve a better differentiation of PHEOs and CAs. However, as long as a visual assessment is used, it can be difficult to differentiate PHEOs with low uptake from other adrenal accumulations [10]. New quantitative interpretation criteria are thus needed for SPECT/CT that are different from the criteria used for conventional planar imaging.
The MIBG uptake of tumors in 123I MIBG SPECT varies widely among individuals. Fendler et al. reported that the tumor-to-liver count ratio was a useful semi-quantitative parameter for assessing neuroblastomas [11]. We speculated that the tumor-to-liver count ratio would also be useful to differentiating PHEO and CA. We conducted the present study to evaluate the usefulness of a semi-quantitative analysis of 123I MIBG SPECT/CT for differentiating PHEO and CA.
Methods
Patient selection
A total of 155 patients underwent 123I MIBG SPECT/CT at our institution between January 2011 and February 2015. Among these patients, there was 21 PHEO patients (10 males and 11 females, age 24–80, median age 61 years) and 23 CA patients (15 males and 8 females, age 30–78, median age 58 years). All of the PHEOs were diagnosed histopathologically. Eleven of the CAs were diagnosed histopathologically, and the other 12 were diagnosed based on CT or MR performed more than 2 years after MIBG scintigraphy showed no change in size or characteristics. There was no patient who had two or more adrenal tumors. Serum test of the cortisol (normal range 6.2–19.4 μg/dl), adrenocorticotropic hormone (ACTH, normal range 7.2–63.3 pg/ml), epinephrine (normal range < 100 pg/ml), norepinephrine (normal range 100–450 pg/ml), and dopamine (normal range < 20 pg/ml) were examined within a month prior to the 123I MIBG examination. Table 1 summarizes the patient and tumor characteristics. The reason for 123I MIBG examination, diagnosis of MR examination and other individual parameters of all cases are shown in the supplement table (Supplement 1). This study was approved by our hospital's Institutional Review Board.
123 I MIBG scintigraphy and SPECT/CT acquisition
Each of the patients received a thyroid blockade with a saturated solution of potassium iodide before 123I MIBG scintigraphy (100 mg/day starting 3 days before tracer injection and continuing for 1 day). Whole-body planar images and SPECT images were obtained 24 h after an intravenous injection of 302 ± 67 MBq of 123I MIBG. All examination data in this study were acquired using SPECT/CT (Symbia T6, Siemens Medical Solutions, Knoxville, TN, USA). Whole-body anterior and posterior images were acquired at a speed of 7 cm/min with low/medium-energy general-purpose collimators, a 256 × 1024 matrix, and a 159-keV photopeak with 20% windows. Subsequently, SPECT/CT images of the patient's abdominal section including bilateral adrenal glands were obtained with a gamma camera within the same gantry. SPECT images were acquired in a step-and-shoot mode, with 50 projections (a duration of 40 s at each projection), a noncircular orbit over 360°, low/medium-energy general-purpose collimators, a 128 × 128 matrix, and a 159-keV photopeak with 20% windows.
A reconstruction technique was performed on three-dimensional ordered subset expectation maximization iterative reconstructions, with eight iterations and ten subsets. CT-based attenuation correction and no scattered correction were applied to the SPECT images. The CT scan parameters were 130 keV, ≤ 30 mAs (due to the minimization of radiation exposure), a 512 × 512 matrix, a 2 × 2.5-mm collimation, and a 5-mm section thickness.
Measurements
Whole-body planar images and SPECT images were reviewed by two nuclear medicine physicians with 5 years’ experience (Y.K.) and 20 years' experience (S.B.) in consensus. The visual evaluation of the whole-body planar image and SPECT images in each patient was performed with the following scoring: score 3 = a lesion with higher uptake than the liver. Score 1 = a lesion with lower uptake than the liver. If the lesion uptake seemed to be the same as that of the liver, the score was 2. This scoring system was based on the criteria we use in our daily clinical practice.
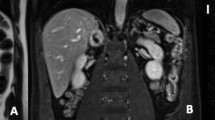
The semi-quantitative evaluation of SPECT/CT results was performed using the tumor-to-liver count ratio (T/L) and the tumor-to-muscle count ratio (T/M). For the calculation of the T/L and T/M ratios, we used the counts per pixel in the segmented tumor volume of interest (VOI), a 5-cm-dia. ball-shaped VOI set in the right liver lobe as the liver VOI, and a 1-cm-dia. sphere-shaped VOI set in the right or the left lateral dorsal muscle as the muscle VOI (Fig. 1). The locations of these VOIs were determined with reference to the SPECT/CT image. The maximum (max) and average (mean) counts of these VOIs were calculated using an IntelliSpace Portal 6.0 workstation (Koninklijke Philips N.V., Amsterdam, Netherland). The T/L and T/M ratios using the max or mean count per pixel of each VOI were calculated as follows:
Example of setting the volume of interest (VOI) in the reference lesion and tumor (a planar image, b, e CT images, c, f SPECT images, d, g SPECT/CT fusion images with VOIs). A 5-cm-dia. ball-shaped VOI was set in the right liver lobe as the liver VOI, and a 1-cm-dia. ball-shaped VOI was set in the right or the left lateral dorsal muscle as the muscle VOI
The reviewers were blinded to the prior interpretations and the results of prior imaging studies when they performed the visual evaluations and semi-quantitative evaluations.
The size and the CT attenuations of adrenal tumors were measured by CT which were performed with the same SPECT/CT examination.
Statistical analyses
The visual scores of the whole-body planar image (planar score) and the SPECT images (SPECT score) were compared between the PHEOs and CAs using the χ2 test. The semi-quantitative values were compared between the PHEOs and CAs with Mann–Whitney’s U test. The diagnostic performance of the planar score, SPECT score and semi-quantitative values were calculated with a receiver operating characteristic (ROC) analysis. Probability (p) values < 0.05 were considered significant. All statistical analyses were performed using JMP 12 software (SAS Institute, Cary, NC).
Results
Visual evaluation
The average planar scores of the PHEOs and CAs were 2.52 ± 0.68 and 1.57 ± 0.59 and the average SPECT scores of the PHEOs and CAs were 2.95 ± 0.22 and 1.91 ± 0.90, respectively (Fig. 2). Both the planar score and the SPECT score of the PHEOs were significantly higher than those of the CAs (p < 0.01). With the cutoff value of score 3, the planar score's sensitivity = 0.61, specificity = 0.96, positive predict value (PPV) = 0.93, negative predict value (NPV) = 0.73, and accuracy = 0.80; the corresponding values for the SPECT score were sensitivity = 0.95, specificity = 0.65, PPV = 0.71, NPV = 0.94, and accuracy = 0.80 (Tables 2, 3). The area under the curve (AUC) values of the planar score and SPECT score were 0.833 and 0.813, respectively in ROC analysis (Fig. 3, Table 3).
Comparison of the PHEOs and CAs by each evaluation. a Planar score, b SPECT score, c T/Lmax, d T/Lmean, e T/Mmax, f T/Mmean. For the PHEOs and CAs, the average planar scores were 2.52 ± 0.68 and 1.57 ± 0.59 and the average SPECT scores were 2.95 ± 0.22 and 1.91 ± 0.90, respectively. Both the planar score and the SPECT score of the PHEOs were significantly higher than those of the CAs (p < 0.01 each). The average T/Lmax values of the PHEOs and CAs were 11.9 ± 11.9 and 0.78 ± 0.62, respectively. For the PHEOs and CAs, the average T/Lmean values were 13.8 ± 14.6 and 0.92 ± 0.82, the average T/Mmax values were 50.1 ± 64.5 and 4.16 ± 4.38, and the average T/Mmean values were 53.4 ± 76.4 and 5.25 ± 5.95, respectively. All of the semi-quantitative evaluations of the PHEOs were significantly higher than those of the CAs (p < 0.01 each)
Semi-quantitative evaluation
The average T/Lmax values of the PHEOs and CAs were 11.9 ± 11.9 and 0.78 ± 0.62, respectively. The average T/Lmean values were 13.8 ± 14.6 and 0.92 ± 0.82, the average T/Mmax values were 50.1 ± 64.5 and 4.16 ± 4.38, and the average T/Mmean values were 53.4 ± 76.4 and 5.25 ± 5.95 for the PHEOs and CAs, respectively (Fig. 2). All semi-quantitative evaluations of PHEOs were significantly higher than those of the CAs (p < 0.01 each). With the cutoff value of 2.25 for T/Lmax, sensitivity = 0.95, specificity = 0.96, PPV = 0.95, NPV = 0.96, and accuracy = 0.95. The corresponding values for T/Lmean using the cutoff value of 2.00 were 0.95, 0.91, 0.91, 0.95 and 0.93. The corresponding values for T/Mmax with the cutoff value of 5.30 were 0.95, 0.83, 0.83, 0.95 and 0.89. The corresponding values for T/Mmean with cutoff value of 12.0 were 0.86, 0.91, 0.90, 0.88 and 0.89 (Tables 2, 3). The AUCs from the ROC analysis were as follows: T/Lmax, 0.986; T/Lmean, 0.975; T/Mmax, 0.954; and T/Mmean, 0.934 (Fig. 3, Table 3).
CT attenuation and tumor size
Average CT attenuation of PHEOs and CAs was 34.4 ± 7.0 and 14.9 ± 21.3, respectively, and average tumor size of PHEOs and CAs was 38.2 ± 15.4 and 24.3 ± 11.4, respectively (Table 1). Both CT attenuation and tumor size of PHEOs were statistically higher than CAs (p < 0.01). Sensitivity, specificity, PPV, NPV and accuracy of CT attenuation were 0.95, 0.65, 0.71, 0.94 and 0.80 with cutoff value of 25.1 and of tumor size were 0.95, 0.52, 0.65, 0.92 and 0.73 with cutoff value of 20.0. AUC of tumor size and CT attenuation were 0.768 and 0.807, respectively, in ROC analysis (Tables 2, 3).
Serum tests
Average ACTH, epinephrine and norepinephrine of PHEOs were 31.5 ± 21.5, 574.4 ± 1221.6 and 1399.9 ± 1377.4 and those of CAs were 22.1 ± 18.5, 27.4 ± 22.7 and 370.4 ± 271.7, respectively. These tests showed significant difference between PHEOs and CAs (ACTH: p < 0.05, epinephrine: p < 0.01, norepinephrine: p < 0.01). Average of cortisol and dopamine showed no statistical difference between PHEOs and CAs. Sensitivity, specificity, PPV, NPV and accuracy of ACTH were 0.95, 0.43, 0.63, 0.90 and 0.69 with cutoff value of 13.3 pg/ml, those of epinephrine were 0.76, 0.83, 0.80, 0.79 and 0.80 with cutoff value of 43 pg/ml and those of norepinephrine were 0.86, 0.70, 0.72, 0.84 and 0.77 with cutoff value of 387 pg/ml. AUC of ACTH, epinephrine and norepinephrine were 0.678, 0.876 and 0.816, respectively, in ROC analysis (Tables 2, 3).
ROC analysis of the semi-quantitative evaluation and other evaluations
Our comparison of the two AUCs of the four semi-quantitative evaluations revealed that the AUC of T/Lmax was relatively larger than the others, but there was no significant difference in AUCs (Fig. 3). The comparison of the two AUCs of the T/Lmax, planar score and SPECT score revealed that the AUC of the T/Lmax was larger than those of the other evaluations (Table 4).
Discussion
The four 123I MIBG SPECT/CT semi-quantitative values examined in this study, i.e., T/Lmax, T/Lmean, T/Mmax, and T/Mmean, were all significantly higher in the PHEOs than in the CAs. There was no significant difference in the differentiation ability of these four semi-quantitative values, and all four indices were shown to have high differentiation ability. It is difficult to differentiate PHEOs from CAs by a visual evaluation when the CA is small in size and the uptake of surrounding normal adrenal glands is taken into account by the partial volume effect, or when the expressions of the MIBG transporters NET and VMAT are relatively low and the MIBG uptake of the PHEO is only slightly higher than normal. In such cases, semi-quantitative values using liver or muscle as a reference organ (e.g., with the T/Lmax, with a threshold of 2) can be used to accurately differentiate a PHEO from a CA.
Our results demonstrated no significant difference in the differentiation ability between liver and muscle as the reference organ. In actual diagnoses, the liver may be preferred because it is easier to create large ROIs for the liver. We also observed no significant difference in the differentiation ability between the analysis using the maximum value of the VOI and that using the average value of the VOI. This may be because both PHEO and CA are tumors with relatively uniform MIBG uptake.
Compared to the visual evaluation using planar images, the semi-quantitative evaluation had similar specificity but higher sensitivity. With planar images, a lesion can be considered positive when the uptake is more prominent than that of the liver, but the uptake of adrenal lesions cannot be identified when their uptake is close to or weaker than that of the liver. PHEOs, which show such mild MIBG uptake, may be misjudged as CA. In contrast, a semi-quantitative evaluation using SPECT/CT allows better identification and a more precise measurement of the adrenal tumor uptake, and it resulted in better sensitivity in this study.
Compared to the visual evaluation using SPECT images, the sensitivity of the semi-quantitative evaluation was similar, but the specificity was higher. With a visual assessment using SPECT, it can be difficult to differentiate between a PHEO and a CA. The reason for this is that even if the liver is used as the reference site, scoring will still be incomplete as long as visual evaluation is used. Therefore, PHEO with relatively weak accumulation or adenoma that includes accumulation of surrounding normal adrenal glands due to partial volume effect is evaluated as same level of accumulation as liver, which decrease the diagnostic performance. A semi-quantitative evaluation based on the MIBG uptake in the liver and muscle can set a stricter threshold and thus has higher specificity than a visual evaluation of SPECT images.
In this study, we compare size, CT attenuation, serum tests of cortisol, ACTH, epinephrine, norepinephrine and dopamine between PHEOs and CAs. In these values, size, CT attenuation, ACTH, epinephrine and norepinephrine in PHEOs were significantly larger than those of CAs. These diagnostic performances were also high. The main purpose of this study was to compare the diagnostic performance of the semi-quantitative value of 123I MIBG with that of the conventional visual evaluation. The diagnostic performance of the CT features and the blood tests were not compared with the semi-quantitative value because the conditions were not unified in this study. Also MR is a very powerful method for differentiating PHEO from CA, but it is difficult to compare with 123I MIBG semi-quantitative evaluation because MR sequence was not unified. We believe that this method is useful in cases where it is difficult to distinguish between adenoma and PHEO, such as when signal changes are caused by intra-tumoral hemorrhage, or in cases where MR imaging is difficult (e.g., presence of metal in the body such as a pacemaker, severe claustrophobia).
In addition, tumors other than PHEO and CA were not examined in this study. Although it has been reported that the prognosis of PHEO varies depending on the gene expression profile, we did not investigate the gene expression of the tumors in this study.
A detailed evaluation of MIBG uptake using a semi-quantitative value may enable the differentiation of tumor activity and histological types, and it may also help determine the indications for 131I MIBG treatment and the therapeutic effect. Semi-quantitative values can also be expected to be useful for MIBG-accumulating lesions in organs other than the adrenal gland, such as paragangliomas and neuroblastomas, but further investigation is needed in this regard as well.
In conclusion, our findings demonstrated that an evaluation using a semi-quantitative value is more effective than the conventional visual evaluation in both planar and SPECT images for differentiating between PHEOs and CAs, which are high-incidence adrenal tumors. A semi-quantitative value is more useful than a visual evaluation in diagnoses using 123I MIBG SPECT/CT.
References
Amar L, Servais A, Roqueplo APG, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005;90(4):2110–6.
Bessell-Brownel R, O’Malley EO. CT of pheochromocytoma and paraganglioma: risk of adverse events with i.v. administration of nonionic contrast material. Am J Roentgenol. 2007;188:970–4.
Varghese JC, Hahn PF, Papanicolaou N, Mayo-Smith WW, Gaa JA, Lee MJ. MR differentiation of phaeochromocytoma from other adrenal lesions based on qualitative analysis of T2 relaxation times. Clin Radiol. 1997;52:603–6.
Blake M, Kalra M, Maher M, Shani D, Sweeney A, Mueller P, et al. Pheochromocytoma: an imaging chameleon. Radiographics. 2004;24(Suppl. 1):S87–99.
Tenenbaum F, Lumbroso J, Schlumberger M, Mure A, Plouin P, Caillou B, et al. Comparison of radiolabeled octreotide and meta-iodobenzylguanidine (MIBG) scintigraphy in malignant pheochromocytoma. J Nucl Med. 1995;36:1–6.
Bomani J, Levison DA, Flagman WD, Home T, Bollox PM, Ross G, et al. Uptake of iodine-123 MIBG by pheochromocytomas, paragangliomas, and neuroblastomas: a histological comparison. J Nucl Med. 1987;28:973–8.
Havekes B, Lai EW, Corssmit EP, Romijn JA, Timmers HJ, Pacak K. Detection and treatment of pheochromocytomas and paragangliomas: current standing of MIBG scintigraphy and future role of PET imaging. Q J Nucl Med Mol Imaging. 2008;52:419–29.
Lumachi F, Tregnaghi A, Zucchetta P, Cristina MM, Cecchin D, Grassetto G, et al. Sensitivity and positive predictive value of CT, MRI and 123I-MIBG scintigraphy in localizing pheochromocytomas: a prospective study. Nucl Med Commun. 2006;27:583–7.
Cecchin D, Lumachi F, Marzola MC, Opocher G, Scaroni C, Zucchetta P, et al. A meta-iodobenzylguanidine scintigraphic scoring system increases accuracy in the diagnostic management of pheochromocytoma. Endocr Relat Cancer. 2006;13:525–33.
Yung B, Loke T, Tse T, Tsang M, Chang J. Sporadic MIBG bilateral adrenal medullary hyperplasia: apparent false positive MIBG scan and expected MRI findings. Eur J Radiol. 2000;36:28–31.
Fendler W, Melzer H, Walz C, Schweinitz D, Coppenrath E, Schmid I, Bartenstein P, et al. High 123I-MIBG uptake in neuroblastic tumours indicates unfavourable histopathology. Eur J Nucl Med Mol Imaging. 2013;40:1701–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kitamura, Y., Baba, S., Isoda, T. et al. Usefulness of semi-quantitative analysis in 123I metaiodobenzylguanidine SPECT/CT for the differentiation of pheochromocytoma and cortical adenoma. Ann Nucl Med 36, 95–102 (2022). https://doi.org/10.1007/s12149-021-01690-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01690-9