Abstract
Objective
Technetium-99 m sestamibi (99mTc-MIBI) scintigraphy can identify non-viable left ventricular (LV) myocardium. However, the optimal cut-off value and the details of decreased 99mTc-MIBI uptake of the non-viable LV myocardium in patients with dilated cardiomyopathy (DCM) have not been well established. This study aimed to evaluate the decrease in 99mTc-MIBI uptake in each segment and in the whole LV myocardium, and to determine cut-off values for identifying non-viable LV myocardium in DCM patients.
Methods
Overall, 53 DCM patients with reduced LV ejection fraction (LVEF ≤ 40%) who underwent 99mTc-MIBI scintigraphy and any optimization of heart failure treatments were evaluated. LV myocardium was classified as viable or non-viable based on the absolute increase in LVEF of ≥ 10% unit leading to an LVEF of > 40% at follow-up, respectively. The decrease in myocardial 99mTc-MIBI uptake in each of the 17 segments was evaluated using three indices determined by different thresholds or standard references: segmental %uptake, rest score, and defect extent. Changes in the whole LV myocardium were evaluated by the minimum %uptake, and the summed rest score (SRS) and extent of LV defect were obtained using summed data of 17 segments.
Results
Segmental evaluation indicated a mild decrease in 99mTc-MIBI uptake in 18 patients with viable LV myocardium, whereas focal severe decrease in uptake was observed in patients with non-viable LV myocardium. In the receiver-operating characteristic curve analysis, the cut-off values of minimum %uptake, SRS, and LV defect extent for predicting non-viable LV were 39% (p < 0.01, area under the curve [AUC]: 0.87), 10 (p < 0.01, AUC: 0.91), and 23% (p < 0.01, AUC: 0.92), respectively.
Conclusions
In DCM patients, myocardial 99mTc-MIBI %uptake of < 40% indicated non-viable myocardium. The focal and severe decrease in uptake in approximately more than a quarter of the LV myocardium may indicate non-viable LV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The assessment of myocardial viability is important for managing patients with reduced left ventricular (LV) systolic function owing to ischemic cardiomyopathy (ICM) or non-ICM, especially dilated cardiomyopathy (DCM). Single photon computed tomography (SPECT) using technetium-99 m sestamibi (99 mTc-MIBI) can provide information to evaluate myocardial perfusion and viability. When assessing myocardial viability, quantitative perfusion SPECT (QPS) is used to evaluate defect severity by 99mTc-MIBI segment uptake. Myocardium presenting severe defect, defined as < 50% uptake to a maximum of the LV myocardium (%uptake), is classified as “non-viable” in the recent American Society of Nuclear Cardiology imaging guidelines [1]. This classification is based on reports that evaluated myocardial recovery following coronary revascularization in patients with ICM [2]. In contrast to ICM patients who have healthy myocardium, DCM patients usually have diffuse LV systolic dysfunction indicating unhealthy myocardium. It has not been established whether this threshold of %uptake for identifying non-viable myocardium can be applied in DCM patients.
Some studies using cardiac magnetic resonance (CMR) reported that the extent of non-viable myocardium detected via late gadolinium enhancement (LGE) is diverse in DCM [3]. Although the severity and spread of decreased 99mTc-MIBI uptake in LV myocardium can be evaluated using a rest score or defect extent calculated using the data of healthy subjects with different thresholds [4,5,6,7], the details of the extent of non-viable myocardium detected by 99mTc-MIBI uptake, indicating declining viable myocardium amount, and its association with myocardial viability require clarification. This study aimed to evaluate the decrease in 99mTc-MIBI uptake in each segment and in the whole LV myocardium using three indices—%uptake, rest score, and defect extent—and to determine the cut-off values for identifying non-viable LV in DCM patients.
Methods
Study participants
Overall, 195 consecutive DCM patients were screened to identify individuals who were aged ≥ 20 years, had reduced LV ejection fraction (EF) ≤ 40%, and were admitted to Osaka University Hospital between December 2014 and December 2018. DCM was diagnosed according to the World Health Organization/International Society and Federation of Cardiology criteria [8]. Coronary angiography was performed to exclude significant coronary stenosis. We excluded 131 patients who had no assessable 99mTc-MIBI scintigraphy data or did not plan to optimize heart failure treatments including up-titration of cardioprotective drugs; seven patients who had no echocardiographic data for evaluating LV viability one year after 99mTc-MIBI scintigraphy, and four patients who underwent cardiac surgery before 99mTc-MIBI scintigraphy were also excluded, resulting in a final cohort of 53 patients. Baseline data, including echocardiographic data, laboratory data, and the use of cardioprotective drugs, angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker (ARB), and beta-blockers, were collected on the day of 99mTc-MIBI scintigraphy. Transthoracic echocardiographic indices were obtained using standard methods as previously described [9]. All patients either received or we tried to optimize medical therapies during the follow-up period. Of the 53 patients, 25 had available data on LGE using CMR, and these data were assessed in a subgroup analysis. The study followed the tenets of the Declaration of Helsinki and Good Clinical Practices. For this retrospective analysis of clinically acquired data, the institutional review board of our hospital waived the requirement for patients’ written informed consent (No.19210–2).
Protocol and analysis of 99m Tc-MIBI
A dose of 740 MBq of 99mTc-MIBI was administered intravenously under resting conditions. SPECT imaging was performed using a dual-head rotation camera (Brightview, Philips, Amsterdam, the Netherlands) equipped with a cardiac high-resolution collimator and resting images were obtained 30 min after injection. Projection images were obtained over 180° extending from the 45° right anterior oblique to the 45° left anterior oblique position, with an acquisition time of 40 s per image on a 64 × 64 matrix size with a 1.85 acquisition zoom and 20% symmetric window centered at 140 keV. For SPECT reconstruction, a Butterworth filter (order 8), which served as a postfilter with a cut-off frequency of 0.275 cycles/pixel, was used without correction for attenuation or scatter.
SPECT slices were assembled in polar maps for assessing regional myocardial tracer uptake. The regional tracer uptake was quantified using three indices: segment %uptake, segment rest score, and segment defect extent in 17 segments of the LV myocardium, as suggested by the QPS protocol. The ratio of each pixel count to the highest value of pixel count of the LV myocardium on the image of QPS was calculated as a segment %uptake [4], and the lowest value of segment %uptake was obtained as the minimum %uptake of the LV myocardium for whole LV assessment. The segment rest score was qualified automatically according to a five-point scoring system: 0, normal; 1, mild defect; 2, moderate defect; 3, severe defect; and 4, absent tracer, in 17 segments [5, 6]. The summed rest score (SRS) was calculated using the sum of the segment rest score of 17 segments for the whole LV assessment. The segment defect extent was defined as the proportion of segment volume with tracer uptakes of < − 2.5 standard deviations of the normal database value [10] to each segment volume, and the LV defect extent was defined as the proportion of the total segment defect extent to the whole LV volume [7].
Evaluation of LV viability
The viability of the whole LV was evaluated because the reliability of the regional assessment of corresponding LV reversibility using echocardiography and 99mTc-MIBI scintigraphy was low and LV recovery, called reverse remodeling, generally occurred in the entire LV. Viable LV myocardium was defined as achieving LV reverse remodeling, and was defined as an absolute increase in the LVEF of ≥ 10% unit leading to an LVEF of > 40% during follow-up [11] without a cardiac event (cardiac death or left ventricular assist device implantation).
Statistical analyses
Data were expressed as median and interquartile range for continuous variables and were compared using the Wilcoxon–Mann–Whitney test for continuous variables and the χ2 test or Fisher’s exact test for categorical variables. A receiver-operating characteristic (ROC) curve was generated, and the area under the curve (AUC) was assessed. The cut-off values of the minimum %uptake, SRS, and LV defect extent for detecting non-viable LV myocardium were determined. Two-tailed p values of < 0.05 were considered statistically significant. JMP Pro 14.0.0 (SAS Institute, Cary, NC, USA) software was used for all the analyses.
Results
The baseline LVEF (median, 23%) and LV end-diastolic dimension (median, 68 mm) values were consistent with those characteristic of DCM (Table 1). Viable LV, which was defined as achieving LV reverse remodeling one year after 99mTc-MIBI evaluation, was identified in 18 patients (34%).
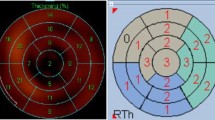
The segmental 99mTc-MIBI uptake was evaluated using three indices in patients with viable and non-viable LV myocardium (Fig. 1). Segmental %uptakes decreased mildly in patients with viable LV myocardium (Figs. 1A 2A, and focally and severely in those with non-viable LV myocardium (Figs. 1D 2D. Highly decreased %uptake, which was evaluated as a score of 3 or 4, or segment defect extent of > 50% based on the values of healthy individuals, was rare but observed mainly in the apex and inferior segments of viable LV myocardium (Figs. 1B, C, 2B, C, while they were more frequently and heterogeneously observed in non-viable LV myocardium (Figs. 1E, f, 2E, F). The minimum %uptake, SRS, and LV defect extent were distinctly different between the patients with viable and non-viable LV myocardium (median of minimum %uptake: 42% vs. 34%, p < 0.05; median of SRS: 5 vs. 17, p < 0.05; median of LV defect extent: 10% vs. 30%, p < 0.05, Fig. 3). These distributions suggested that the clinical utility of these indices was better for identifying non-viable LV myocardium rather than viable LV myocardium.
99mTc-MIBI uptake of segments in patients with viable left ventricular (LV) myocardium and non-viable LV myocardium. The upper panel shows the distributions of segmental %uptake (A), rest score (B), and defect extent (C) of quantitative perfusion SPECT (QPS) in patients with viable LV myocardium. The lower panel shows the distributions of segmental %uptake (D), rest score (E), and defect extent (F) in patients with non-viable LV myocardium. Blue box: segment score 0, green box: segment score 1, yellow box: segment score 2, red box: segment score 3, dark red box: segment score 4. A anterior; AL anterolateral; AP apical; AS anteroseptal; I inferior; IL inferolateral; IS inferoseptal; L lateral; S septal; SPECT single photon computed tomography.
The representative cases of 99mTc-MIBI uptake of segments in patients with viable and non-viable LV myocardium. The upper panel shows the segmental %uptake (A), rest score (B), and defect extent (C) of quantitative perfusion SPECT in the patient with viable LV myocardium (minimum %uptake: 41%, summed rest score: 3, LV defect extent: 4%). Segmental rest scores of 2 points in the apex and 1 point in the basal inferoseptal segment were observed, and segment defect extents (black) of 28% in basal inferoseptal, 6% in basal-inferior, 7% in mid-inferior, and 28% in the apex segments were observed. The lower panel shows the segmental %uptake (D), rest score (E), and defect extent (F) in the patient with non-viable LV myocardium (minimum %uptake: 15%, summed rest score: 31, LV defect extent: 54%). (D) Segment %uptake of ≤ 39% (blue) was observed in many segments. Scores of 3 or 4 were observed in the lateral, inferolateral, basal-inferior, and apex segments; segment defect extent of > 50% was observed in many segments. SPECT single photon computed tomography, LV left ventricular
The minimum % uptake, summed rest score (SRS), and LV defect extent in patients with viable and non-viable LV myocardium. The minimum %uptake (A), SRS (B), and LV defect extent (C) were significantly different between the patients with viable and non-viable LV myocardium (median [interquartile]: 42% [41–45] vs. 34% [27–41], 5 [3–7] vs. 17 [10–25], 10 [5–12] vs. 30 [18–47], all p < 0.05). SRS summed rest score; LV left ventricular
In the ROC curve analysis, the cut-off values of minimum %uptake, SRS, and LV defect extent for determining non-viable LV myocardium were 39% (sensitivity: 66%, specificity: 100%, AUC: 0.87, p < 0.01), 10 (sensitivity: 89%, specificity: 94%, AUC: 0.91, p < 0.01), and 23% (sensitivity: 71%, specificity: 100%, AUC: 0.92, p < 0.01), respectively (Fig. 4). The AUCs were all similar (all p > 0.05). The change in EF in patients without a cardiac event correlated well with minimum %uptake, SRS, and LV defect extent (Fig. 5, r = 0.61, − 0.67, and − 0.66, all p < 0.01, respectively).
Receiver operating characteristics curves (ROC) of the minimum % uptake, SRS, and LV defect extent for predicting non-viable LV myocardium. The optimal cut-off values for the minimum %uptake, SRS, and LV defect extent for predicting non-viable LV myocardium were 39% (p < 0.01, sensitivity: 66%, specificity: 100%, area under the curve [AUC]: 0.87), 10 (p < 0.01, sensitivity: 89%, specificity: 94%, AUC: 0.91), and 23% (p < 0.01, sensitivity: 71%, specificity: 100%, AUC: 0.92), respectively. SRS summed rest score; LV left ventricular
The relationships between the change in LV ejection fraction and the indices of 99m Tc-MIBI uptake. The change in LV ejection fraction (LVEF) from baseline to follow-up (ΔLVEF) correlated with minimum %uptake (A), SRS (B), and LV defect extent (C) (r = 0.61, − 0.67, and − 0.66, all p < 0.01, respectively). The black lines indicate the regression line. ΔLVEF, the change of left ventricular ejection fraction from baseline to follow-up; SRS summed rest score, LV left ventricular
The three indices were well correlated with each other, even calculated by a different threshold or standard indicating different implications to the severity or extent of damaged myocardium (Fig. 6). Minimum %uptake, SRS, and LV defect extent also correlated with LV diastolic dimension (r = − 0.37, 0.47, and 0.48, all p < 0.01, respectively) and the duration from the first heart failure hospitalization (r = − 0.38, 0.44, and 0.49, all p < 0.01, respectively), but not with the brain natriuretic peptide level. These indices were associated with non-viable LV myocardium after adjusting for LV diastolic dimension or the duration from the first heart failure hospitalization.
The relationships among SRS, minimum %uptake, and LV defect extent. A significant correlation between SRS and minimum %uptake (A, r = − 0.86, p < 0.01), LV defect extent and minimum %uptake (B, r = − 0.85, p < 0.01), or SRS and LV defect extent (C, r = 0.97, p < 0.01) was observed. Patients with viable LV myocardium are indicated by block circles and those with non-viable LV myocardium by red circles, and black lines indicate the regression line. SRS, summed rest score; LV, left ventricular
In the subgroup analysis of 25 DCM patients with 99mTc-MIBI scintigraphy and CMR data, seven patients had LGE on CMR, indicating less myocardial viability. When patients had signs indicating non-viable LV using LV defect extent—high AUC in the three indices, no patient had LV reverse remodeling regardless of CMR findings (Fig. 7). In contrast, among the patients who did not have signs indicating non-viable LV myocardium using 99mTc-MIBI scintigraphy, no patient had LGE.
The incidences of LV reverse remodeling in patients stratified based on the findings of 99m Tc-MIBI and cardiac magnetic resonance. Patients were stratified according to the findings indicating non-viability on 99mTc-MIBI scintigraphy (LV defect extent of ≥ 23%) and cardiac magnetic resonance (presence of late geranium enhancement). Reverse remodeling occurred only in patients who had no signs indicating non-viable LV myocardium on both examinations. RI, 99mTc-MIBI scintigraphy; CMR cardiac magnetic resonance, SRS summed rest score, LV left ventricular
Discussion
This study has three major findings. First, the decrease in myocardial 99mTc-MIBI uptake in DCM patients with viable LV myocardium, defined as the recovery of systolic function by optimized medical therapy, was mild, and a focal and severe decrease was present in those with non-viable LV myocardium. Second, the three indices—minimum %uptake, SRS, and LV defect extent—calculated using different thresholds or standards, and reflect the degree of decreasing 99mTc-MIBI uptake of the LV myocardium—were well correlated with each other and were associated with the irreversibility of the LV myocardium. Each optimal cut-off value showed similar high predictability for the irreversibility of the LV myocardium. The LV defect extent with approximately more than a quarter of the LV myocardium in DCM may indicate a non-viable LV. Third, no patient with LV defect extent of ≥ 23% achieved LV reverse remodeling regardless of the presence or absence of LGE on CMR. These findings suggested that evaluating myocardial 99 mTc-MIBI uptake indexed by the minimum perfusion or the spread of decreased perfusion, indicating declining viable myocardium amount, will help identify LV myocardium viability, especially irreversibility in DCM patients.
Decreased uptakes and the irreversibility of LV
Highly decreased myocardial 99mTc-MIBI uptake on the rest of the image of QPS represents reduced blood flow to the myocardial scar tissue [12, 13]. In ICM patients, segments with a %uptake of ≥ 75% are considered viable, and those with a %uptake between 50 and 75% are considered to contain scar tissue, while those with a %uptake of ≤ 50% are considered to have extensive scar tissue [14]. In contrast, no study has reported an optimal cut-off value of 99 mTc-MIBI uptake to indicate irreversible myocardium in DCM patients, although it has been reported that patients with an extensively decreased myocardial 99 mTc-MIBI uptake responded poorly to heart failure therapies [15, 16]. In our study, the optimal cut-off value of the minimum %uptake was 39% for predicting non-viable LV myocardium, which did not achieve LV reverse remodeling. The minimum %uptakes between 40 and 50% were observed in 28 DCM patients, of which 17 patients (61%) had viable LV myocardium. The contractility of the myocardium with 99 mTc-MIBI uptake between 40 and 50% might improve by optimizing medical therapies. In patients with minimal %uptake > 40%, the washout rate of 99mTc-MIBI was significantly higher in the non-viable LV myocardium than in viable LV myocardium (data not shown). The washout rate may facilitate the differentiation of myocardial viability in patients with preserved %uptake. However, further study is needed due to a lack in verification of the washout rate in DCM patients accompanied by a severe perfusion defect. Our results suggest that the cut-off value of 99mTc-MIBI %uptake for indicating irreversible myocardium may be approximately 40% in DCM patients, which is likely to be low when compared to that in patients with ICM. The different levels of cut-off value may be associated with the difference in myocardial quality, which is used as a reference standard (maximum uptake), between DCM and ICM. The other possibility may be associated with the difference in the therapy, not revascularization but optimizing medication, for myocardial recovery. Different therapy may have a different impact on the recovery of myocardial function.
The minimum %uptake, which represents the most decreased segment uptake in the LV myocardium, correlated moderately with SRS or LV defect extent, which is a semi-quantitative or quantitative index of severity of the decreased uptake in the entire LV myocardium using a different calculation based on the healthy subject values. This indicates that myocardial injury may develop as both entirely spreading and locally worsening in the LV myocardium. However, its development may be diverse, especially in patients with severely impaired LV myocardium. Scores 3 and 4 in the QPS segments, indicating a high decrease in the uptake, were found more often in the apex, inferior, or inferolateral parts of the LV myocardium in our DCM cohort. However, in the healthy controls, a mild decrease in 99mTc-MIBI uptake in the inferior segments was also observed [10], suggesting that the decrease in 99mTc-MIBI uptake in the apex, inferior, or inferolateral segments of the LV myocardium might be composed by both non-diseased properties including methodological effects and myocardial injury. This was supported by the analysis with the data excluding apex, basal-inferior, mid-inferior, inferior, basal-inferolateral, and mid-inferolateral segments. The predictability of LV defect extent and SRS were consistently high, but that of minimum %uptake became worse (data not shown). On considering this, our results including the indices based on healthy database comparison suggest that the appearance of the focal and severe decrease in 99 mTc-MIBI uptake in approximately more than a quarter of the LV myocardium indicated less possibility of LV reverse remodeling. We experienced some DCM cases with a progression of LV defect extent during follow-up, which may indicate that the disease progression of DCM occurs not only homogenously but also focally (data not shown).
LGE on CMR and uptakes in 99m Tc-MIBI scintigraphy
Shiraki et al. reported that patients with LGE on CMR had higher perfusion abnormalities in QPS than those without LGE, and there was a significantly positive correlation between the summed severity score in QPS and the extent of LGE on CMR in DCM patients [17], which was consistent with our findings. No patient with LV defect extent ≥ 23%, which indicates a non-viable LV, achieved LV reverse remodeling regardless of the presence or absence of LGE on CMR. Moreover, no patient whose LV myocardium was evaluated to have a possibility of reverse remodeling using 99mTc-MIBI scintigraphy had an LGE. This may be because the defect image on 99mTc-MIBI scintigraphy was shown earlier than LGE on CMR in the diseased myocardium or because the extent of LGE is sometimes underestimated in diffused myocardial damage [18].
Limitations
This study has several limitations. First, this was a single-center retrospective study, which might diminish the power of our statistical inference. Second, with fewer first-visit patients and dominant return-visit patients and by choosing only patients who underwent 99 m-MIBI scintigraphy, there was a bias in the patient population. Third, we did not have CMR data of half of the study patients and did not assess T1 mapping, which can detect diffuse fibrosis. Finally, optimization of medical therapies was not uniform in this study given the nature of the observational study. The cut-off values for identifying myocardial viability may have varied depending on the treatments used.
Conclusion
In summary, the optimal cut-off of 99mTc-MIBI %uptake for identifying non-viable LV myocardium was approximately 40% in DCM patients. The minimum %uptake, SRS, and LV defect extent, which reflect the severity or expansion of decreased perfusion, will be helpful in identifying the non-viable myocardium.
References
Dorbala S, Ananthasubramaniam K, Armstrong IS, Chareonthaitawee P, DePuey EG, Einstein AJ, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol. 2018;25:1784–846.
Udelson JE, Coleman PS, Metherall J, Pandian NG, Gomez AR, Griffith JL, et al. Predicting recovery of severe regional ventricular dysfunction. Comparison of resting scintigraphy with 201Tl and 99mTc-sestamibi. Circulation. 1994;89:2552–61.
Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903.
Germano G, Kavanagh PB, Waechter P, Areeda J, Van Kriekinge S, Sharir T, et al. A new algorithm for the quantitation of myocardial perfusion SPECT. I: technical principles and reproducibility. J NucI Med. 2000;41:712–9.
Kang X, Berman DS, Van Train KF, Amanullah AM, Areeda J, Friedman JD, et al. Clinical validation of automatic quantitative defect size in rest technetium-99m-sestamibi myocardial perfusion SPECT. J Nucl Med. 1997;38:1441–6.
Berman DS, Abidov A, Kang X, Hayes SW, Friedman JD, Sciammarella MG, et al. Prognostic validation of a 17-segment score derived from a 20-segment score for myocardial perfusion SPECT interpretation. J Nucl Cardiol. 2004;11:414–23.
Garcia EV, Van Train K, Maddahi J, Prigent F, Friedman J, Areeda J, et al. Quantification of rotational thallium-201 myocardial tomography. J Nucl Med. 1985;26:17–26.
Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, et al. Report of the 1995 world health organization/international society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–2.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14.
Nakajima K, Kumita S, Ishida Y, Momose M, Hashimoto J, Morita K, et al. Creation and characterization of Japanese standards for myocardial perfusion SPECT: database from the Japanese Society of Nuclear Medicine Working Group. Ann Nucl Med. 2007;21:505–11.
Wilcox JE, Fang JC, Margulies KB, Mann DL. Heart failure with recovered left ventricular ejection fraction: JACC Scientific Expert Panel. J Am Coll Cardiol. 2020;76:719–34.
Medrano R, Lowry RW, Young JB, Weilbaecher DG, Michael LH, Afridi I, et al. Assessment of myocardial viability with 99mTc sestamibi in patients undergoing cardiac transplantation. A scintigraphic/pathological study Circulation. 1996;94:1010–7.
Sciagrà R, Giaccardi M, Porciani MC, Colella A, Michelucci A, Pieragnoli P, et al. Myocardial perfusion imaging using gated SPECT in heart failure patients undergoing cardiac resynchronization therapy. J Nucl Med. 2004;45:164–8.
Ypenburg C, Schalij MJ, Bleeker GB, Steendijk P, Boersma E, Dibbets-Schneider P, et al. Impact of viability and scar tissue on response to cardiac resynchronization therapy in ischaemic heart failure patients. Eur Heart J. 2007;28:33–41.
Fukuchi K, Yasumura Y, Kiso K, Hayashida K, Miyatake K, Ishida Y. Gated myocardial SPECT to predict response to beta-blocker therapy in patients with idiopathic dilated cardiomyopathy. J Nucl Med. 2004;45:527–31.
Morishima I, Okumura K, Tsuboi H, Morita Y, Takagi K, Yoshida R, et al. Impact of basal inferolateral scar burden determined by automatic analysis of 99mTc-MIBI myocardial perfusion SPECT on the long-term prognosis of cardiac resynchronization therapy. Europace. 2017;19:573–80.
Shiraki K, Satoh H, Saitoh T, Saotome M, Urushida T, Katoh H, et al. Comparison of global and regional abnormalities in 99mTc-sestamibi and cardiac magnetic resonance imaging in dilated cardiomyopathy. J Card Fail. 2010;16:641–8.
Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–80.
Acknowledgements
The authors are grateful to Takashi Kamiya for his technical assistance. We would like to thank Editage (www.editage.com) for English language editing. This work was supported by a Health Labor Sciences Research Grant (Program Grant Number: JPMH20FC1051) and JSPS KAKENHI (Grant Number: JP18K08035). The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chimura, M., Ohtani, T., Sera, F. et al. Focal severe decrease in myocardial technetium-99 m sestamibi uptake indicates ventricular irreversibility in patients with dilated cardiomyopathy. Ann Nucl Med 35, 881–888 (2021). https://doi.org/10.1007/s12149-021-01625-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01625-4