Abstract
Objective
Our study was to investigate the value of pretreatment 18F-FDG uptake heterogeneity to predict the prognosis of patients with locally recurrent nasopharyngeal carcinoma (LRNPC) treated by carbon ion radiotherapy (CIRT).
Methods
Twenty-nine LRNPC patients who underwent whole-body 18F-FDG PET/CT scanning before CIRT were enrolled. Heterogeneity index (HI)-based 18F-FDG uptake, and the PET/CT traditional parameters, including SUVmax, MTV, and TLG were assessed. Receiver operator characteristics (ROC) determined the best cutoff value, and local recurrence-free survival (LRFS) and progression-free survival (PFS) were evaluated by the Kaplan–Meier method and log-rank test. And the predictive ability was evaluated by the ROC curve. Cox analyses were performed on LRFS and PFS.
Results
In this study, univariate analysis showed that HI was a significant predictor of LRNPC treated by CIRT. HI could be used to predict LRFS and PFS. Patients with HI (≥ 0.81) had a significantly worse prognosis of LRFS (12.25 vs. NR, p = 0.008), and of PFS (10.58 vs. NR, p = 0.014). The AUC and its sensitivity and sensitivity and specificity were 0.75, 84.21% and 70.00% for LRFS and 0.82, 80.95% and 75.00% for PFS, respectively. Multivariate analysis showed that HI was an independent predictor for the LFRS of LRNPC with CIRT.
Conclusion
18F-FDG uptake heterogeneity may be useful for predicting the prognosis of patients with LRNPC treated by CIRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is rare in globally but common in its endemic regions, including southern Asia and North East India [1]. Radiotherapy (RT) is the only effective treatment for NPC [2], but its recurrence rate is still 20–40% [3]. At present, the main treatment methods for local recurrent nasopharyngeal carcinoma (LRNPC) are surgery, re-irradiation, and systemic treatment [4]. And intensity-modulated radiotherapy (IMRT), as a kind of radiotherapy, is the most effective method of them for LRNPC [2]. But there is a shortcoming in IMRT, which is a correlation between the dose of re irradiation with IMRT and the risk of radiotherapy complications, including mucosal necrosis and massive bleeding [2, 5,6,7]. Chemotherapy is an important means of tumor treatment, as well as for locally recurrent nasopharyngeal carcinoma. Chemotherapy may improve local tumor control by improving radio-sensitivity. However, it can also increase potential late toxicities [4]. Wai Tong Ng et al. consider that induction docetaxel, cisplatin, and fluorouracil followed by weekly docetaxel and cetuximab in concurrence with intensity modulated radiation therapy can improve the treatment outcome for patients with LRNPC, but the high rate of temporal lobe necrosis limits its clinical applicability [8]. Donald Poon et al. also consider that concurrent chemoradiotherapy is feasible in patients with locally recurrent nasopharyngeal carcinoma, but the risk of major late toxicity is significant [9].
Carbon ions are considered to be the ideal and most suitable for radiotherapy among various types of ion species [10]. Because carbon-ion beam with the Bragg peak and high-LET properties can help it to precisely deliver the maximum dosage/biological effectiveness to the target tumor while inflicting the minimum damage to the surrounding normal tissues [11, 12]. Carbon ion radiotherapy (CIRT) has been used to salvage treatment in patients with LRNPC with satisfactory results [2]. There is still individual variability in the therapeutic response among patients with LRNPC. Hence, the ability to accurately estimate prognosis for the individual patient with LRNPC has important implications for CIRT.
PET/CT, as a functional imaging, has been widely used in the clinical oncology field because it shows the response to treatment and recurrence in various cancers [13,14,15,16]. Some studies have already shown that 18F-FDG PET/CT has good predictive value to estimate the radiotherapy for nasopharyngeal carcinoma [17,18,19], and the prediction value of intratumoral heterogeneity is especially prominent [20, 21]. However, as far as we know, the prediction by 18F-FDG PET/CT imaging of the prognosis of CIRT in LRNPC has never been reported, and its predictive value is unclear.
Hence, in this study, we investigated whether 18F-FDG uptake heterogeneity index (HI) can be used to predict the prognosis of LRNPC after CIRT to provide reasonable suggestions for clinical treatment decision-making in LRNPC treated by CIRT.
Methods
Study population
Between June 2015 to January 2017, LRNPC patients who received 18F-FDG PET/CT scan 2 weeks prior to CIRT were enrolled in this study. Inclusion criteria were: (1) aged 18–80 years; (2) pathology confirmed WHO type I/II/III primary nasopharyngeal carcinoma; (3) the definitive course of the first radiotherapy to a total dose of ≥ 66 Gy was completed at least 6 months ago; (4) no distant metastasis; (5) no contraindication to MRI scan. (6) no other types of local treatment had been received after recurrence; (7) Karnofsky performance score ≥ 70; (8) women with reproductive potential are willing to accept appropriate contraceptive measures; (9) willing to sign written informed consent forms; (10) all patients achieved complete response (CR) after the initial treatment. CR with the first therapy was proved by regular imaging examination and nasopharyngoscopy for every patient within at least 6 months. Exclusion criteria: patients that received concurrent chemotherapy. All enrolled patients were restaged according to the American Joint Committee on Cancer (AJCC) staging system (7th edition). This study was approved by the institutional review board of Shanghai Proton and Heavy Ion center. Considering its retrospective nature, a waiver of informed consent was granted.
Treatment and follow-up
All patients received CIRT at a cumulative dose of 50–65 GyE (at 2 GyE/daily fraction to 3 GyE/daily fraction). After completion of radiotherapy, physical examination, MRI examination, and nasopharyngoscopy were performed every 3 months in the first 2 years, then every 6 months in the third to fifth year and once a year thereafter. CT, PET/CT and SPECT/CT are also performed as needed. The treatment efficacy was assessed using 1-year local recurrence-free survival (LRFS) and progression‐free survival (PFS). LRFS was defined as the time from the date of CIRT initiation to the first local recurrence, PFS was defined as the time from the date of CIRT initiation to the first disease progression, relapse or death. Local progression was determined by MRI using the Response Evaluation Criteria in Solid Tumors (RECIST) (version 1.1).
18F-FDG PET/CT imaging
18F-FDG PET/CT was performed using a Siemens biograph 16HR PET/CT scanner (Knoxville, Tennessee, USA). 18F-FDG was generated automatically by the cyclotron (CTI RDS Eclipse ST, Siemens, Knoxville, TN). The radiochemical purity of 18F-FDG was over 95%. All patients fasted for at least 6 h, and their blood glucose levels were confirmed to under 140 mg/dL. All patients lay still for 1 h after intravenous injection of FDG at a dose of 7.4 MBq/kg. Then all patients had a 18F-FDG PET/CT scan from the groin to skull base.
The PET/CT acquisition parameters were as follows: CT scanning was first performed, from the proximal thighs to head, with 120 kV, 80–250 mA, pitch 3.6 mm, tube rotation time 0.5 ms. Immediately after CT scanning, a PET emission scan that covered the identical transverse field of view was obtained. Acquisition time was 2–3 min per table position. PET image data sets were reconstructed iteratively by applying the CT data for attenuation correction, and coregistered images were displayed on a workstation.
Imaging interpretation
A multimodality computer platform (Syngo; Siemens) was used for image review and manipulation. Two board-certified experienced nuclear medicine physicians evaluated the images independently. The reviewers reached a consensus in cases of discrepancy. Quantification of glucose metabolic activity was obtained using the SUV normalized to body weight.
The maximum SUV (SUVmax) for recurrent lesions were evaluated by manually placing an individual region of interest on coregistered and fused transaxial PET/CT images. The boundaries were drawn large enough to include the lesion in the axial, coronal, and sagittal PET images. A connecting outline of the volume of interest was set using a cutoff value of 2.5, 3.0, 40% SUVmax, 50% SUVmax, 60% SUVmax and 70% SUVmax, and the contour around the target lesion inside the boundaries was automatically produced. MTV was defined as the sum of the metabolic volume above the threshold of the above cutoff values, and TLG was defined as the product of the SUVmean and the MTV. Heterogeneity index (HI) was defined as SUVmean divided by SUVmax[22].
Statistical analyses
Quantitative data were shown as mean (range) or median (95%Cl). 1-year local recurrence-free survival (LRFS) and progression‐free survival (PFS) represent the treatment outcome. LRFS was defined as the time from the date of CIRT initiation to the first local recurrence, PFS was defined as the time from the date of CIRT initiation to the first disease progression, relapse or death. The optimal cut-off values of PET parameters were determined by receiver operating characteristic (ROC) analysis. The survival analyses were performed by Kaplan0Meier method. Log-rank tests and Cox proportional hazards model were used for univariate and multivariate analysis. And predictors were determined using the factors with significance of p value less than or close to 0.2 after univariate analyses, and the multivariate Cox regression model was developed with backward elimination. All analyses were performed using SPSS, version 19.0 (SPSS Inc., Chicago, IL, USA). A p value < 0.05 was statistically significant in this study.
Results
Patient and tumor characteristics
This analysis included 29 patients with LRNPC, who had 18F-FDG PET/CT scan in SPHIC before CIRT within 2 weeks. Patients and tumor characteristics were summarized in Table 1.
Predictive value of treatment outcome
In this study, 21 patients (72.41%) were documented as having tumor recurrence or disease progression, and 19 patients (65.52%) had local recurrence, and 2 (6.90%) had distant metastasis. Median progression-free survival (PFS) was 14.78 months (95% confidence interval [CI]: 9.30–20.27), and the median follow-up time was 37.36 months [95% confidence interval (CI): 34.82–39.89].
The predictive value of the conventional clinical risk factors and PET parameters were examined using the univariate analysis.
When the cut-off value was 70%SUVmax, the analysis results were shown in Table 2. We used the time-dependent ROC analysis which found the best cutoff value for LRFS and PFS. None of the conventional clinical risk factors had any predictive value for LRFS or PFS. For PET parameters, only HI was a significant predictor, which can predict not only LRFS but also PFS. With regard to LRFS, the median LRFS with high HI (> 0.81) was 12.25 months and the median LRFS with low ratio did not reach (NR). Hence, patients with high HI (> 0.81) have a significantly worse prognosis than patients with low HI (≤ 0.81, p = 0.008). And the correlation between survival time and HI is shown in Fig. 1. The survival curves of LRFS were shown in Fig. 2a. Similarly, patients with high HI (> 0.81) had a significantly worse prognosis for PFS (10.58 vs. NR, p = 0.014). The survival curves of PFS were shown in Fig. 2b.
SUVmax, MTV, and HI were analyzed by multivariate analysis. The result showed that only HI was an independent predictor for the LFRS of LRNPC with CIRT (Table 3). With HI > 0.81, the increased risk of local recurrence for LRNPC with CIRT was 4.04 -fold (p = 0.034).
Time‐dependent ROC analysis
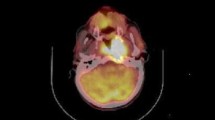
In this section, to further verify the predictive ability of the PET/CT parameters and clinical characteristics, time‐dependent ROC curves for censored and LRFS and PFS data and areas under the ROC curve (AUC) were estimated. With regard to LRFS, the AUC of ROC curves was 0.75, and the sensitivity and specificity were 84.21% and 70.00%, respectively, indicating good predictive value (was shown in Fig. 2c). For PFS, the AUC was 0.82, the sensitivity and the specificity were 80.95% and 75.00%, respectively, indicating a better predictive value, which was shown in Fig. 2d. Therefore, the results of our study indicate that HI might be a promising predictive indicator of carbon radiotherapy outcome. Typical PET/CT images are shown in Fig. 3.
Representative images. a A 53-year-old male patient with locally recurrent nasopharyngeal carcinoma (lrNPC) underwent 18F-FDG PET/CT scan. The recurrent lesion had 18F-FDG uptake, and the SUVmax = 10.34, SUVmean = 8.36, and so HI was 0.809. We followed up for 34.79 months, and neither locally recurrent-free survival (LRFS) nor progression-free survival (PFS) could be followed to the end. b A 59-year-old male patient with locally recurrent nasopharyngeal carcinoma (LRNPC) underwent 18F-FDG PET/CT scan. The recurrent lesion had 18F-FDG uptake, and the SUVmax = 7.25, SUVmean = 6.31, so HI was 0.870. He developed distant metastasis after 10.58 months of follow-up, and the PFS was 10.58 months and his LRFS was not reached
Other PET/CT parameters
When the cutoff values were 2.5, 3.0, 40% SUVmax, 50% SUVmax and 60% SUVmax, the data analysis was the same as above. The results show that only when the cutoff value was 60% SUVmax, the patients with high TLG (> 8.00) had a significantly worse prognosis for LRFS (12.26 vs. 25.43, p = 0.023), and the same results for PFS (16.76 vs. 18.07, p = 0.028) (Supp. ESM_1.pdf). and the AUC of ROC curves were 55.6% and 52.1%, respectively (Supp. ESM_2.pdf).
Discussion
As far as we know, this is the first study to evaluate the predictive ability and prognostic significance of 18F-FDG PET/CT in LRNPC treated by CIRT. And a novel PET/CT parameter-HI was confirmed to possibly be a useful index. HI, SUVmean/SUVmax ratio, was used to measure gray-level dispersion (global heterogeneity) [22].
Generally, the NPC stage affects the disease prognosis with radiotherapy. Patients with early-stage NPC have good 5-year local control rates, ranging from 70 to 90%, but 30% of patients have local recurrence and distant metastasis in the intermediate and advanced stage. Meanwhile, the discouraging prognosis of recurrent NPC presents a huge challenge for the oncologist [23]. At present, CIRT has been used in the treatment of LRNPC with encouraging results due to its physical and biological advantages [2]. First, the physical advantage shows a characteristic depth–dose profile, known as the Bragg curve [24], which allows the majority of energy to reach the target, and only less energy normal tissues around the target. Second, the biological advantage shows a high linear energy transfer (LET), which results in a high relative biological effectiveness (RBE) to destroy the DNA completely [25]. Therefore, CIRT has been also used to treat other tumors, including intracranial tumors [26], lung tumors [27], gastrointestinal tumors [28], genitourinary tumors [29], sarcoma [30], cutaneous tumors [31], breast cancer [32], gynecologic cancer [33], and pediatric cancer [34]. A large number of early data reported in the previous literature show that CIRT is both effective and safe for various tumors [35]. Meanwhile CIRT combined with immunotherapy has more theoretical advantages through various mechanisms [36]. However, there are two main limitations to CIRT. On the one hand, the “fragmentation tail” can increase the dose to the distal target, resulting in the uncertainty of energy distribution in the target; On the other hand, the exact calculation of RBE is still uncertain [35].
Therefore, there is an urgent need to predict accurately the prognosis of the individual patient with LRNPC treated by CIRT. 18F-FDG PET/CT imaging has been widely used to predict the prognosis of various cancers. Some traditional parameters of PET/CT, including SUVmax, MTV, and TLG, have potential prognostic value for NPC, but the results were varied and controversial [20]. Unfortunately, traditional parameters did not have any prognostic value in this study.
Individual variability in tumor therapeutic response has been attributed to factors such as tumor cell metabolism, proliferation, angiogenesis, necrosis, and tumor fibrosis, referred to as intratumoral heterogeneity [20]. However, it is difficult to quantify intratumoral heterogeneity. Similarly, the heterogeneity of 18F-FDG uptake within tumors has been attributed to a number of factors including cellularity, proliferation, angiogenesis, necrosis, and hypoxia [37]. Hence, the heterogeneity of 18F-FDG may represent intratumoral heterogeneity, which has been used in clinical research. The main methods to evaluate HI of 18F-FDG uptakes are textural analysis, cumulative SUV-volume histograms (CSH), fractal analysis, the coefficient of variance (COV), etc. [20], but they are all complex and difficult for clinicians to determine. Intra-tumor heterogeneity includes phenotypic diversity such as cell surface markers, genetic abnormality, growth rate, apoptosis and other hallmarks of cancer.
An easy and feasible measurement of heterogeneity of 18F-FDG uptake was proposed [22]. This method of measurement has been successfully applied to predict PFS and LRFS of LRNPC treated by CIRT in this study. And their AUCs representing predictive prognostic ability were 0.75 for LRFS and 0.82 for PFS, respectively. Previously, some traditional parameters of PET/CT, including SUVmax, MTV, and TLG, have been used to evaluate the prognosis of CIRT. Katsuyuki Shirai, and others have proposed that SUVmax can predict PFS of early stage non-small cell lung cancer treated by CIRT (p = 0.01), but cannot predict LRFS [38]. And Suman Shrestha, and others suggested that MTV can predict PFS of early-stage non-small cell lung cancer treated by CIRT (p < 0.02). The predictive ability of MTV was evaluated by ROC curve, and the AUC was 0.72 (p < 0.04) [39]. They both suggest that TLG is not important for the prognosis [38, 39]. Hence, it is possible that HI of 18F-FDG uptake is of greater value in predicting carbon ion radiotherapy outcome than traditional parameters of PET/CT imaging.
MTV and TLG are important predictors of prognosis, but were not related to the prognosis in our study. MTV and TLG reflect, respectively, the metabolism of a tumor and the malignant degree of the tumor itself. And they are both predictors of tumor prognosis. Tumor heterogeneity of FDG uptake is a part of radiomics, as Alexander J Lin have stated with the potential to enhance the treatment of radiation-resistant tumors [40]. All patients with locally recurrent nasopharyngeal carcinoma included in this study may have radiotherapy resistance. Radiotherapy resistance is a poor prognostic factor. Therefore, we suppose that tumor heterogeneity may be related to radiation resistance, but more study is needed to confirm this.
As far as we know, this study was the first to investigate the predictive prognostic value of HI of 18F-FDG uptake in patients with LRNPC treated by CIRT. And the results showed that HI was not only an independent predictor of local control, but also has good prediction ability for LRNPC treated by CIRT, which suggested that HI may support potentially a more aggressive systemic therapy for LRNPC patients at high risk of CIRT failure.
This study still has some limitations. First, a small sample size was the main limiting factor. Second, the EBV DNA level is very important to the patients with NPC, and was demonstrated to have a significant prognostic impact in patients with NPC from a meta-analysis of 8128 cases [41], but there is no discussion about it here due to the lack of clinical data. Meanwhile, the prognostic performance of HI also needs to be further confirmed by large, prospective clinical research.
Conclusion
Our preliminary study showed that HI based on 18F-FDG uptake may predict the prognosis of LRNPC with CIRT. It could be useful to identify patients with LRNPC who would benefit from CIRT and provide theoretical support for individualized treatment.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Paul P, Deka H, Malakar AK, Halder B, Chakraborty S. Nasopharyngeal carcinoma: understanding its molecular biology at a fine scale. Eur J Cancer Prev. 2018;27(1):33–41.
Hu J, Bao C, Gao J, Guan X, Hu W, Yang J, et al. Salvage treatment using carbon ion radiation in patients with locoregionally recurrent nasopharyngeal carcinoma: initial results. Cancer. 2018;124(11):2427–37.
Kong F, Zhou J, Du C, He X, Kong L, Hu C, et al. Long-term survival and late complications of intensity-modulated radiotherapy for recurrent nasopharyngeal carcinoma. BMC Cancer. 2018;18(1):1139.
Lee AWM, Ng WT, Chan JYW, Corry J, Mäkitie A, Mendenhall WM, et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat Rev. 2019;79:101890.
Hua YJ, Han F, Lu LX, Mai HQ, Guo X, Hong MH, et al. Long-term treatment outcome of recurrent nasopharyngeal carcinoma treated with salvage intensity modulated radiotherapy. Eur J Cancer. 2012;48(18):3422–8.
Han F, Zhao C, Huang SM, Lu LX, Huang Y, Deng XW, et al. Long-term outcomes and prognostic factors of re-irradiation for locally recurrent nasopharyngeal carcinoma using intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol). 2012;24(8):569–76.
Qiu S, Lin S, Tham IW, Pan J, Lu J, Lu JJ. Intensity-modulated radiation therapy in the salvage of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2012;83(2):676–83.
Ng WT, Ngan RKC, Kwong DLW, Tung SY, Yuen KT, Kam MKM, et al. Prospective, multicenter, phase 2 trial of induction chemotherapy followed by bio-chemoradiotherapy for locally advanced recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2018;100(3):630–8.
Poon D, Yap SP, Wong ZW, Cheung YB, Leong SS, Wee J, et al. Concurrent chemoradiotherapy in locoregionally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2004;59(5):1312–8.
Tsujii H, Kamada T. A review of update clinical results of carbon ion radiotherapy. Jpn J Clin Oncol. 2012;42(8):670–85.
Lomax AJ. Charged particle therapy: the physics of interaction. Cancer J. 2009;15(4):285–91.
Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25(8):953–64.
Lee JD, Yang WI, Park YN, Kim KS, Choi JS, Yun M, et al. Different glucose uptake and glycolytic mechanisms between hepatocellular carcinoma and intrahepatic mass-forming cholangiocarcinoma with increased 18F-FDG uptake. J Nucl Med. 2005;46(10):1753–9.
Gong C, Ma G, Hu X, Zhang Y, Wang Z, Zhang J, et al. Pretreatment 18F-FDG uptake heterogeneity predicts treatment outcome of first-line chemotherapy in patients with metastatic triple-negative breast cancer. Oncologist. 2018;23(10):1144–52.
Kang SR, Song HC, Byun BH, Oh JR, Kim HS, Hong SP, et al. Intratumoral metabolic heterogeneity for prediction of disease progression after concurrent chemoradiotherapy in patients with inoperable stage III non-small-cell lung cancer. Nucl Med Mol Imaging. 2014;48(1):16–25.
Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges JP, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52(3):369–78.
Huang Y, Feng M, He Q, Yin J, Xu P, Jiang Q, et al. Prognostic value of pretreatment 18F-FDG PET/CT for nasopharyngeal carcinoma patients. Medicine. 2017;96(17):e6721.
Mohandas A, Marcus C, Kang H, Truong MT, Subramaniam RM. FDG PET/CT in the management of nasopharyngeal carcinoma. AJR Am J Roentgenol. 2014;203(2):W146-157.
Alessi A, Lorenzoni A, Cavallo A, Padovano B, Iacovelli NA, Bossi P, et al. Role of pretreatment 18F-FDG PET/CT parameters in predicting outcome of non-endemic EBV DNA-related nasopharyngeal cancer (NPC) patients treated with IMRT and chemotherapy. Radiol Med (Torino). 2019;124(5):414–21.
Yang Z, Shi Q, Zhang Y, Pan H, Yao Z, Hu S, et al. Pretreatment 18F-FDG uptake heterogeneity can predict survival in patients with locally advanced nasopharyngeal carcinoma-a retrospective study. Radiat Oncol. 2015;10:4.
Gu B, Zhang J, Ma G, Song S, Shi L, Zhang Y, et al. Establishment and validation of a nomogram with intratumoral heterogeneity derived from 18F-FDG PET/CT for predicting individual conditional risk of 5-year recurrence before initial treatment of nasopharyngeal carcinoma. BMC Cancer. 2020;20(1):37.
Tello Galán MJ, García Vicente AM, Pérez Beteta J, Amo Salas M, Jiménez Londoño GA, Pena Pardo FJ, et al. Global heterogeneity assessed with 18F-FDG PET/CT Relation with biological variables and prognosis in locally advanced breast cancer. Rev Esp Med Nucl Imagen Mol. 2019;38(5):290–7.
Almobarak AA, Jebreel AB, Abu-Zaid A. Molecular targeted therapy in the management of recurrent and metastatic nasopharyngeal carcinoma: a comprehensive literature review. Cureus. 2019;11(3):e4210.
Otero J, Felis I, Ardid M, Herrero A. Acoustic localization of bragg peak proton beams for hadrontherapy monitoring. Sensors (Basel). 2019;19(9):1971.
Mohamad O, Sishc BJ, Saha J, Pompos A, Rahimi A, Story MD, et al. Carbon ion radiotherapy: a review of clinical experiences and preclinical research, with an emphasis on DNA damage/repair. Cancers (Basel). 2017;9(6):66.
Combs SE, Kessel K, Habermehl D, Haberer T, Jäkel O, Debus J. Proton and carbon ion radiotherapy for primary brain tumors and tumors of the skull base. Acta Oncol. 2013;52(7):1504–9.
Takahashi W, Nakajima M, Yamamoto N, Yamashita H, Nakagawa K, Miyamoto T, et al. A prospective nonrandomized phase I/II study of carbon ion radiotherapy in a favorable subset of locally advanced non-small cell lung cancer (NSCLC). Cancer. 2015;121(8):1321–7.
Makishima H, Yasuda S, Isozaki Y, Kasuya G, Okada N, Miyazaki M, et al. Single fraction carbon ion radiotherapy for colorectal cancer liver metastasis: a dose escalation study. Cancer Sci. 2019;110(1):303–9.
Zhang Y, Li P, Yu Q, Wu S, Chen X, Zhang Q, et al. Preliminary exploration of clinical factors affecting acute toxicity and quality of life after carbon ion therapy for prostate cancer. Radiat Oncol. 2019;14(1):94.
Matsunobu A, Imai R, Kamada T, Imaizumi T, Tsuji H, Tsujii H, et al. Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer. 2012;118(18):4555–63.
Zhang H, Li S, Wang XH, Li Q, Wei SH, Gao LY, et al. Results of carbon ion radiotherapy for skin carcinomas in 45 patients. Br J Dermatol. 2012;166(5):1100–6.
Karasawa K, Omatsu T, Arakawa A, Yamamoto N, Ishikawa T, Saito M, et al. A phase I clinical trial of carbon ion radiotherapy for stage I breast cancer: clinical and pathological evaluation. J Radiat Res. 2019;60(3):342–7.
Irie D, Okonogi N, Wakatsuki M, Kato S, Ohno T, Karasawa K, et al. Carbon-ion radiotherapy for inoperable endometrial carcinoma. J Radiat Res. 2018;59(3):309–15.
Mohamad O, Imai R, Kamada T, Nitta Y, Araki N. Carbon ion radiotherapy for inoperable pediatric osteosarcoma. Oncotarget. 2018;9(33):22976–85.
Malouff TD, Mahajan A, Krishnan S, Beltran C, Seneviratne DS, Trifiletti DM. Carbon ion therapy: a modern review of an emerging technology. Front Oncol. 2020;10:82.
Helm A, Ebner DK, Tinganelli W, Simoniello P, Bisio A, Marchesano V, et al. Combining heavy-ion therapy with immunotherapy: an update on recent developments. Int J Part Ther. 2018;5(1):84–93.
Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJR. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging. 2013;40(1):133–40.
Shirai K, Abe T, Saitoh JI, Mizukami T, Irie D, Takakusagi Y, et al. Maximum standardized uptake value on FDG-PET predicts survival in stage I non-small cell lung cancer following carbon ion radiotherapy. Oncol Lett. 2017;13(6):4420–6.
Shrestha S, Higuchi T. Prognostic significance of semi-quantitative FDG-PET parameters in stage I non-small cell lung cancer treated with carbon-ion radiotherapy. Eur J Nucl Med Mol Imaging. 2020;47(5):1220–7.
Lin AJ, Dehdashti F, Grigsby PW. Molecular imaging for radiotherapy planning and response assessment for cervical cancer. Semin Nucl Med. 2019;49(6):493–500.
Qu H, Huang Y, Zhao S, Zhou Y, Lv W. Prognostic value of Epstein–Barr virus DNA level for nasopharyngeal carcinoma: a meta-analysis of 8128 cases. Eur Arch Otorhinolaryngol. 2020;277(1):9–18.
Acknowledgements
We appreciate the help of the members in the multidisciplinary team of Radiation Oncology, Shanghai Proton and Heavy Ion Center. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by grants from the National Key Research and Development Program of China (No. 2018YFC0115700) and the National Science and Technology Major Project (No. 2017ZX09304021).
Author information
Authors and Affiliations
Contributions
Conception and design: ZY, SS, and GM. Acquiring data, or analyzing and interpreting data: BG, JH, LK, JZ, ZL, YX, JL, JC, JC, and YZ. Drafting the manuscript: GM. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics statement
All procedures performed in studies involving humans were approved by the Institutional Review Board of Shanghai Proton and Heavy Ion Center and were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, G., Gu, B., Hu, J. et al. Pretreatment 18F-FDG uptake heterogeneity can predict treatment outcome of carbon ion radiotherapy in patients with locally recurrent nasopharyngeal carcinoma. Ann Nucl Med 35, 834–842 (2021). https://doi.org/10.1007/s12149-021-01621-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01621-8