Abstract
Objective
One of the major causes of diabetes and obesity is abnormality in glucose metabolism and glucose uptake in the muscle and adipose tissue based on an insufficient action of insulin. Therefore, many of the drug discovery programs are based on the concept of stimulating glucose uptake in these tissues. Improvement of glucose metabolism has been assessed based on blood parameters, but these merely reflect the systemic reaction to the drug administered. We have conducted basic studies to investigate the usefulness of glucose uptake measurement in various muscle and adipose tissues in pharmacological tests using disease-model animals.
Methods
A radiotracer for glucose, 18F-2-deoxy-2-fluoro-d-glucose (18F-FDG), was administered to Wistar fatty rats (type 2 diabetes model), DIO mouse (obese model), and the corresponding control animals, and the basal glucose uptake in the muscle and adipose (white and brown) tissues were compared using biodistribution method. Moreover, insulin and a β3 agonist (CL316,243), which are known to stimulate glucose uptake in the muscle and adipose tissues, were administered to assess their effect. 18F-FDG uptake in each tissue was measured as the radioactivity and the distribution was confirmed by autoradiography.
Results
In Wistar fatty rats, all the tissues measured showed a decrease in the basal level of glucose uptake when compared to Wistar lean rats. On the other hand, the same trend was observed only in the white adipose tissue in DIO mice, while brown adipose tissue showed increments in the basal glucose uptake in this model. Insulin administration stimulated glucose uptake in both Wistar lean and fatty rats, although the responses were inhibited in Wistar fatty rats. The same tendency was shown also in control mice, but clear increments in glucose uptake were not observed in the muscle and brown adipose tissue of DIO mice after insulin administration. β3 agonist administration showed the similar trend in Wistar lean and fatty rats as insulin, while the responses were inhibited in the adipose tissues of Wistar fatty rats.
Conclusion
A system to monitor tissue glucose uptake with 18F-FDG enabled us to detect clear differences in basal glucose uptake between disease-model animals and their corresponding controls. The responses in the tissues to insulin or β3 agonist could be identified. Taken as a whole, the biodistribution method with 18F-FDG was confirmed to be useful for pharmacological evaluation of anti-diabetic or anti-obesity drugs using disease-model animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus type 2 (T2DM) is a metabolic disease which is characterized by consistent high blood glucose levels caused by insufficient amounts of the antihyperglycemic hormone insulin or its action [1, 2]. Since chronic hyperglycemia causes serious complications to the blood vessels, heart, eyes, kidneys, and nerves, most anti-diabetes drugs are designed to control blood sugar levels in diverse mechanisms of action. T2DM is a complex disease and supposed to be caused by a combination of lifestyle and genetic factors [3, 4]. Among them, obesity and associated chronic inflammation are known to induce a state called “insulin resistance,” which is characterized by abnormalities in glucose metabolism in diverse organs [5]. Skeletal muscles and adipose tissues, major key players in glucose metabolism, have been recognized as the main targets of the anti-diabetes drugs [6]. Glucose transport is a rate-limiting step for insulin-stimulated glucose utilization in skeletal muscles [7, 8] and adipose tissues [9, 10], and acceleration of this process is one of the anticipated mechanisms of action of anti-diabetic drugs. Therefore, a method to measure the in vivo glucose uptake in those tissues is important in pharmacological research for anti-diabetic drugs.
Glucose transport and phosphorylation have been extensively measured in vivo [11] and 14C-2-deoxyglucose has been used as radiotracer [12]. However, a long half-life radiotracer is not suitable for kinetic studies, especially in in vivo settings. Recently, positron emission tomography (PET) imaging was used to monitor regional uptake of the glucose analog, 2-deoxy-2-18F-fluoro-d-glucose (18F-FDG), which is a short half-life radiotracer with better penetration depth in the tissues. This technology enables us to monitor glucose uptake in situ in a non-invasive way [13–15]. To date, there are many reports on glucose uptake measurement in muscle and adipose tissues by 18F-FDG-PET [9–11] in clinical practice; however, there are fewer reports in the preclinical area. One of the reasons for this would be that 18F-FDG-PET and its various applications are already established as standard clinical measurement tools. On the other hand, application to preclinical studies would need specialized instruments for laboratory animals and optimization of protocols especially for pharmacological studies on metabolic diseases.
Rodents have widely been used to mimic human diseases and test potential therapeutic interventions. The Wistar fatty rat is known to develop obesity with diabetic complications such as hyperglycemia, hyperinsulinemia, hyperlipoidemia, and glucose intolerance; therefore, it has become one of the standard models for studying obesity and insulin resistance [16]. In fact, this model has been used in evaluating various anti-diabetic drugs, including a biguanide [17], an α-glucosidase inhibitor [18], a thiazolidinedione [19], and a dipeptidyl peptide-4 (DPPIV) inhibitor [20]. Diet-induced obesity (DIO) mice, another obesity model with co-morbidities such as T2DM, hypertension, and hypercholesterolemia, have also been used in pharmacological studies for anti-obesity drugs [21–23]. Insulin resistance is generally measured as an important index to evaluate the efficacy of anti-diabetic drugs using a homeostasis model assessment of insulin resistance (HOMA-IR), glycohemoglobin (GHb), blood glucose, or insulin in preclinical studies [16–20]. Since these are blood parameters, they are not suitable for monitoring direct effects of the drugs on the target tissues, such as muscle and adipose tissues, in a real-time manner.
Insulin stimulates glucose uptake and consumption in these tissues via various mechanisms [24] including upregulation of glucose transporters (GLUT). In fact, the muscle and adipose tissues are characterized with their high expression of GLUT4, which is an insulin-responsive subtype [25]. Metabolic stimulation via β3-adrenergic receptor (β3-AR) is another mechanism to stimulate glucose uptake and consumption. The receptor is well known to be expressed predominantly in brown adipose tissue [26], but distributed in many other tissues. CL316,243, a selective β3-AR agonist, has already been shown to stimulate glucose uptake in brown adipose tissue by a 18F-FDG-PET study [27]. Therefore, insulin and CL316,243 are expected to be valuable tools to assess insulin resistance and a drug’s effects on glucose uptake in various muscle and adipose tissues of rat and mouse models using a 18F-FDG biodistribution method.
Materials and methods
Materials
(R,R)-5-[2-[2,3-(3-chlorophenyl)-2-hydroxyethyl-amino]propyl]-1,3-benzo-dioxole-2,2-dicarboxylate, disodium salt (CL316,243) was purchased from Tocris Bioscience (Ellisville, MO, USA). 18F-FDG was purchased from Nihon Medi-Physics Co., LTD (Kurume, Japan). All the other reagents were obtained from commercial suppliers.
Animals
Wistar fatty rats were established and bred in-house [28]. All animals used were male of 23 weeks old for insulin and 25 or 28 weeks old for CL316,243 studies. As a reference animal, male Wistar lean rats (heterozygote or wild-type, bred in-house) were used for both insulin (22 weeks old) and CL316,243 (27 or 29 weeks old) studies. DIO mice were prepared by feeding a high-fat diet (D12492, Research Diets, Inc., NJ, USA) to male C57BL/6J mice (Charles River Laboratories Japan, Inc., Yokohama, Japan) from 4 to 49 weeks. C57BL/6J mice fed with normal diet (11–12 weeks old) were used as controls. All the animals, except the DIO mouse, were fed with standard chow (CE2, Japan SCL, Inc., Hamamatsu, Japan) and water ad libitum and kept under environmentally controlled conditions (12 h normal light/dark cycles, 23 °C and 50% relative humidity). The Institutional Animal Care and Use Committees of Shonan Research Center in Takeda Pharmaceutical Company Limited approved all of the experiments.
Catheterization, dosing, and 18F-FDG injection
The experimental design, such as animal numbers, body weights, interventions, and dose (including 18F-FDG), are summarized as Table 1. The animals were fasted for at least 12 h, and then every animal was surgically catheterized into the right femoral vein under 2–5% isoflurane anesthesia. The animals were set on a heating pad (40 °C, KN-475, Natsume Seisakusho Co LTD. Tokyo, Japan), and the rectal temperature was monitored to maintain at more than 36 °C. Vehicle (saline), insulin (0.25 or 0.50 U/mL/kg for rats, 0.20 or 0.40 U/mL/kg for mice), or CL316,243 (0.7 or 2.0 mg/mL/kg) was administered through the catheter. Ten minutes after insulin or 30 min after CL316,243 administrations, 18F-FDG (3.7 MBq/300 µL for rats, 1.85 MBq/50 µL for mice) was injected via the vein catheter.
Blood glucose level and tissue uptake measurements
In case of insulin administration, blood glucose level was monitored using ACCU-CHEK Compact Plus (Roche Diagnostics K.K., Tokyo, Japan) just before insulin administration and at 10 min (18F-FDG injection) and 70 min. In case of CL316,243 administration, it was monitored just before CL316,243 administration and at 30 min (18F-FDG injection) and 90 min. Then all the animals were immediately sacrificed. Muscles (soleus, gastrocnemius, and femoral), white adipose (mesenteric and subcutaneous), and brown adipose tissues were excised and then their weights and radioactivity were measured. 18F-FDG uptake in each tissue was expressed as the percentage of the injected dose per gram of tissue (%ID/g), the generally used parameter for radioactivity accumulation in tissue, which was calculated as follows: %ID/g = radioactivity in tissue (Bq/g) divided by injected dose (Bq) multiplied by 100%.
Autoradiography (ARG)
The interscapular tissues, consisting of muscle, white, and brown adipose tissues, were excised with the same timing as above. The tissues were held between two OHP film sheets and set at 5 mm thickness and they were placed on an imaging plate BAS SR-2040 (Fujifilm Co., Ltd., Tokyo, Japan) and exposed for 1 h. The plates were scanned by FLA7000 (GE Healthcare Japan, Tokyo, Japan). The images were depicted using the same color range in order to compare the 18F-FDG distribution among the rats and mice. The exposed tissues were scanned as optical images.
Statistics
All values were shown as mean ± SD. Statistical differences between the groups in insulin and CL316,243 studies were analyzed with a one-tailed William’s test and statistically significant differences between control and disease models were analyzed by student’s t test using SAS system Version 8.2 (SAS Institute Inc., NC).
Results
Comparison of baseline level of 18F-FDG uptake between model and control animals
As an initial step to know the characteristics of each model, baseline levels of 18F-FDG uptake (vehicle group, 60 min after 18F-FDG) were compared with the corresponding controls. Wistar fatty rats (T2DM model) showed lower 18F-FDG uptake than the Wistar lean (control) rats in all the tissues measured (Fig. 1). Only femoral muscle showed no significant difference (P > 0.05), but its trend was the same as for the others. In the gastrocnemius muscle, both type 1 (slow twitch oxidative, Fig. 1a) and type 2 (fast twitch, data not shown) fiber-rich regions showed the same trends with statistical significance. On the other hand, there was no significant difference in the glucose uptake in muscles measured between the DIO (obesity model) and control mice (Fig. 2a). In the white adipose tissues (mesenteric and subcutaneous), the DIO mice showed lower glucose uptake than the controls (Fig. 2b); however, the brown adipose tissue showed the opposite result (Fig. 2c). These findings for the brown adipose tissues were also confirmed by ARG images of the interscapular region (Figs. 3, 4).
Evaluation of the effects of insulin on glucose uptake
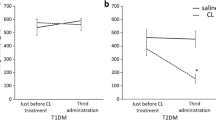
Insulin-dependent decreases in blood glucose were confirmed to continue until 60 min after 18F-FDG administration in control rats (0.50 U/mL/kg) and mice (0.40 U/mL/kg) (Table 2). In Wistar fatty rats and DIO mice with insulin resistance, the significant decreases in blood glucose were not shown in the lower insulin doses at the timing of 18F-FDG injection, which were clearly different from the responses in the corresponding control animals. Therefore, the effect of insulin on glucose uptake was evaluated at this time point. In both Wistar lean and fatty rats, all the muscles tested showed same trend in a dose-dependent increment of glucose uptake (Fig. 5a). Glucose uptake in the subcutaneous adipose tissue of Wistar fatty rats showed statistical significance in its increment (Fig. 5b), but the rates of change were less than those seen in the brown adipose tissue of both rats (Fig. 5c). The response to insulin in the brown adipose tissue of Wistar fatty rats reached plateau at a lower dose (0.25 U/mL/kg) in this study, but glucose uptake stayed equivalent to the basal level in Wistar lean rats. In DIO mice, insulin-stimulated glucose uptake was generally suppressed in all the muscles tested (Fig. 6a). Since the basal glucose uptake in the adipose tissue itself was lower in the DIO compared to the control mice, the increments stayed low even if they showed statistical significance (Fig. 6b). On the other hand, the brown adipose tissue levels remained high and unchanged regardless of the administration of insulin (Fig. 6c). These trends in the adipose tissues (white and brown) were also confirmed by ARG images of the interscapular region (Figs. 3, 4).
Evaluation of the effects of β3-AR agonist on glucose uptake
Since β3-AR agonists are known to stimulate both lipolysis in white adipose and thermogenesis in brown adipose tissues, CL316,243, a selective β3-AR agonist, did not affect the blood glucose (Table 3) also in Wistar lean and fatty rats and the direct effects on glucose uptake in the tissues were explored. It has already been reported to stimulate glucose uptake in brown adipose tissue by 18F-FDG-PET study [27], and this was also confirmed in this study using Wistar lean rats (Fig. 7c). Among the adipose tissues measured, the mesenteric adipose tissue showed similar trends to those in the brown adipose but the subcutaneous adipose tissue did not (Fig. 7b). These results were well matched with the effect of β3-AR agonists on lipolysis in different adipose tissues [29]. In Wistar fatty rats, both brown and white adipose tissues showed similar responses to CL316,243 in proportion to the lower basal level than that of Wistar lean rats (Fig. 7b, c). Glucose uptake was stimulated by CL316,243 in all the muscle tissues measured in both Wistar lean and fatty rats (Fig. 7a).
Discussion
In this study, we have successfully detected the differences in glucose uptake between disease models and corresponding control animals and investigated a drug’s effect on them using 18F-FDG biodistribution methodology. Although the resolution of the analysis was limited to the tissue level, higher throughput has been achieved than with imaging analysis. PET scanning with 18F-FDG has already been applied to murine models of various diseases, and we have also utilized it in the evaluation of the anti-cancer drug TAK-733, an inhibitor of mitogen-activated protein kinase [30]. However, the application of PET to preclinical studies needs customized instruments for small animals, and this would generally hamper its establishment as a widely prevalent method. Therefore, we believe that this study would be an important base for the characterization of the metabolic status of diabetic and obese animal models which could be easily followed by any standard laboratory.
In the preclinical research of the representative drugs for diabetes and obesity, Wistar fatty rats and DIO mice were frequently used. Wistar fatty rat is one of the standard disease models of obesity and insulin resistance [16] and has been used in various anti-diabetic drugs such as a biguanide [17], an α-glucosidase inhibitor [18], a thiazolidinedione [19], and a dipeptidyl peptide-4 (DPPIV) inhibitor [20]. DIO mice have also been used in pharmacological studies for anti-obesity drugs [21–23]. There was no report about the difference of the glucose uptake in various muscle and adipose tissues affected by insulin in Wistar fatty rats and DIO mice and the present study could provide important basic information. In diabetes and obesity research, the drug effects have been usually evaluated by the systemic parameters such as glucose, HbA1, and insulin concentration in the blood, or the food intake or the body weight change. However, the drug research has become complicated and recently the target molecule expressed in a specific tissue has been focused. For example, salt-inducible kinase 2 (SIK2) is abundant in adipose tissue and regulate insulin signaling, and glucose uptake therefore has been reported to be a protective factor for obesity-induced insulin resistance [31, 32]. Skeletal muscle mitochondrial function has become a possible target for whole-body insulin resistance and sensitivity [33]. Therefore, the glucose uptake should be differentiated among the tissues in order to evaluate the effects of the new type drugs using the disease models. 18F-FDG is translatable PET tracer and the biodistribution method would provide the useful information in the drug research and development.
18F-FDG biodistribution study in rats and mice needs the precise setting of the experimental condition because it is affected by a variety of physiological parameters and conditions such as blood glucose levels, body temperature, feeding, and anesthesia condition. Especially in the present study, the experimental condition would lead an underestimation or overestimation of the insulin and CL316,243 effects. Firstly, blood glucose level could affect the 18F-FDG uptake. Some reports suggested the methodologies to measure the glucose uptake in the tissues. Ferre et al. reported that glucose clamping was performed to make a steady state of blood glucose concentration [34], but this method is complicated because the glucose infusion and the serial blood glucose measuring with the serial blood sampling are necessary. On the other hand, Virtanen et al. used a simple methodology without the glucose clamp [35], which was applied in the present study for seeking a high-throughput investigation. Secondly, the adjustment of the room temperature and body temperature is important in the 18F-FDG biodistribution study. It has been reported that the cold-stimulated condition increased the glucose uptake in the brown adipose tissue because it is a thermoregulatory organ and easily activated by a cold induction [36, 37]. The animals were anesthetized with isoflurane, which decreased the body temperature and then could give the underestimation of the insulin and CL316,243 effects because the basal uptake especially in the brown adipose tissue would increase; therefore, the animals were kept warm on the heating pad and the rectal temperature was maintained at the steady (>36 °C) in the present study. Thirdly, feeding condition could affect the 18F-FDG uptake; it reduced 18F-FDG uptake in the brown adipose tissue [37] but did not give a big difference in the muscle [38] compared with fasting condition. However, it usually has high blood glucose concentration, which leads the inhibition of 18F-FDG uptake in the tissues. Therefore, the effects of insulin or CL316,243 could be overestimated in case the basal uptake would reduce although the detailed investigation is needed. In the present study, fasting was performed to uniform the blood glucose concentration among the animal groups. Lastly, anesthesia is essential in preclinical studies to measure the glucose uptake especially in the muscle because the movement under conscious condition during the 18F-FDG injection could affect the tracer uptake [37]. However, it has been reported that the anesthesia itself affected the glucose uptake in the tissues [39]. In our preliminary study, the 18F-FDG uptake was fluctuated in the muscle in case that the animals could move freely; therefore, the isoflurane anesthesia was used to uniform the experimental conditions among the animals in the present study. In limitation, the animal strains were different from the previous reports; therefore, further investigation will be needed to clarify the effects of experimental conditions on 18F-FDG uptake in the tissues of Wistar fatty rats and DIO mice.
The 18F-FDG uptakes by Wistar fatty rats were lower than those of Wistar lean rats in most of the muscle and adipose tissues tested (Fig. 1). These differences could be due to insulin resistance in the Wistar fatty rats [16], but the degrees of reduction were variable between the tissues tested (Fig. 1). The Wistar fatty rat was the first rat model for of T2DM established on the basis of the hypothesis that both environment factors and genetic background for diabetes were needed to develop diabetes [28]. Thereafter, the causative gene for obesity in Wistar fatty rat was reported to be the ‘fatty’ (Glu269Pro) leptin receptor mutation and it was believed to be related to its genetic background [40]. However, the pathophysiological pathway from obese to insulin resistance in the Wistar fatty rat is still unclear. The Zucker fatty (ZF) and Zucker diabetic fatty (ZDF) are other rat models to have the same mutation, being obese and insulin resistant but not diabetic at a young age and then progressively developing hyperglycemia with aging [41]. 18F-FDG uptake measurement was also applied to ZF and lean rats [35], but the results could not feasibly be directly compared with our results due to the different administration protocol.
The DIO model has been developed to study non-genetic obesity and its co-morbidities, and the animals become obese, hyperglycemic, and develop impaired glucose tolerance. The 18F-FDG uptake in the muscle tissues of DIO mice was almost similar to that of the control mice, but that of the mesenteric and subcutaneous adipose tissues of the DIO mice was quite lower than in the control mice (Fig. 2a, b). Moreover, glucose uptake was upregulated in the brown adipose tissue compared with the control mice (Fig. 2c), and these phenotypes were considered to be induced simply by nutritional excess and not to be genetic in origin. It has been reported that central GLP-1 receptor signaling, whose effects on white adipose tissue is blunted in the DIO mice, still increased sympathetic outflow towards brown adipose tissue to increase its glucose uptake [42]. Our approach was simply to measure basal glucose uptake, but the results matched well with these findings.
The major effects of insulin on muscle and adipose tissue are the regulation of carbohydrate, lipid, and protein metabolism [24]. In more detail concerning the carbohydrate metabolism, insulin increases the rate of glucose transport and glycolysis, stimulates the rate of glycogen synthesis, and decreases its utilization. In Wistar fatty and lean rats, insulin-stimulated glucose uptake in all the muscle and adipose tissues tested but the rate of change varied (Fig. 5). On the other hand, all the tissues tested in the DIO mice showed suppression of the response to insulin when compared with the control animals (Fig. 6), and these would be one of the typical differences between diabetic status and obese-induced insulin resistance. Brown adipose tissue is a specialized thermoregulatory organ that has a critical role in the regulation of energy metabolism [43], and our system also highlighted its distinct status, the difference in the response to insulin, between these two models (Figs. 5c, 6c). Detailed pharmacological analysis would be required to clarify their mechanism, but some of them might be able to be explained by differential expression of their energy metabolism machineries, such as uncoupling proteins [44], and glucose transporters [45, 46]. CL316,243 is a murine-selective β3-adrenoceptor agonist and is reported to correct obesity and elevated blood glucose in diabetic rodents [26]. The significance of the β3-adrenoceptor agonist-induced brown adipose tissue activation in obesity was explored in ZF and corresponding lean rats with 18F-FDG-PET [47], and a similar trend was observed in Wistar fatty and lean rats (Fig. 7c). On the other hand, the effect of insulin was more prominent than CL316,243 in Wistar lean rats, but both of them were equivalent and remained low in Wistar fatty rats (Figs. 5c, 7c). Therefore, an appropriate combination of interventions would need to be utilized in subsequent studies on the physiological mechanisms of obesity and insulin resistance based on the glucose uptake as index.
18F-FDG biodistribution method enables to measure the glucose uptake of the tissue directly and clarify the responses depending on the tissue types. These could be recognized as an advantage and applied to various drug researches. For example, a recent report said the brown adipose recruitment in white adipose tissue by β3 agonist administration [27] and the biodistribution study combined with the related factor measuring could be used to clarify the mechanism. In a translational aspect, the glucose uptake can be measured using 18F-FDG-PET in clinical study; therefore, the 18F-FDG preclinical biodistribution data could provide a translational path on drug research. In case a drug target expression or function in a tissue are different between animal and human, the preclinical biodistribution method can be used to predict the drug effects in human based on the animal data. Also in a technical aspect, 18F-FDG biodistribution study has practical advantages on preclinical pharmacological test. The direct measurement of 18F-FDG radioactivity in the tissues can provide a high-throughput preclinical pharmacological test, although 14C-2-deoxyglucose or 14C-FDG methodologies need a long time and complicated procedures in the whole experiments, especially in the solution of the tissue in the solvent buffer.
There are some limitations in this study. Firstly, this study did not measure the factors related to a pathway of glucose uptake and expenditure. Glucose transporter 4 (GLUT4) and uncoupling protein 1 (UCP1) measurement could add the more detailed information about drug effects on glucose uptake and expenditure. Secondly, there are other animal disease models for type 2 diabetes and obesity. Each model strain has different pathogenesis, genetic background, and disease characteristics. The present data can be applied to the study using the Wistar fatty rats and DIO mice. Further investigations are needed in case of other models and the comparison of naive glucose uptake among the models should provide valuable information for a selection of animal models suitable for the drug evaluation. Third, the present study did not include the results of CL316,243 effects in DIO mice. Some mechanisms of CL316,243 have been reported in diabetes mice [48, 49] and DIO mice are non-genetic disease model; therefore, it was not simple to compare the results with that of control mice. Further investigation will clarify these points.
Conclusion
We have successfully detected basal level and glucose uptake changes in insulin- and CL316,243-administered small animals by 18F-FDG biodistribution method, and identified the differences between the control and disease models. This study is expected to be valuable as a cue for following studies for the mechanistic analysis of obesity and insulin resistance in small animal models. Since glucose uptake and utilization represent the cellular physiological status, this methodology would be feasible to be applied to a variety of pharmacological studies for metabolic diseases such as diabetes and obesity.
References
Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322–35.
Han TS, Lean ME. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. J R Soc Med Cardiovasc Dis. 2016;5:1–13.
Abranches MV, Oliveira FC, Conceição LL, Peluzio MD. Obesity and diabetes: the link between adipose tissue dysfunction and glucose homeostasis. Nutr Res Rev. 2015;28:121–32.
Zhou K, Pedersen HK, Dawed AY, Pearson ER. Pharmacogenomics in diabetes mellitus: insights into drug action and drug discovery. Nat Rev Endocrinol. 2016;12:337–46.
Rasouli N. Adipose tissue hypoxia and insulin resistance. J Investig Med. 2016;64:830–2.
Kahn B. Facilitative glucose transporters: regulatory mechanisms and dysregulation in diabetes. J Clin Invest. 1992;89:1367–74.
Katz A, Nyomba BL, Bogardus C. No accumulation of glucose in human skeletal muscle during euglycemic hyperinsulinemia. Am J Physiol (Endocrinol Metab) 1988;255:E942–945.
Ziel FH, Venkatesan N, Davidson MB. Glucose transport is rate limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes. 1988;37:885–90.
Christen T, Sheikine Y, Rocha VZ, Hurwitz S, Goldfine AB, Di Carli M, et al. Increased glucose uptake in visceral versus subcutaneous adipose tissue revealed by PET imaging. JACC Cardiovasc Imaging. 2010;3:843–51.
Reichkendler MH, Auerbach P, Rosenkilde M, Christensen AN, Holm S, Petersen MB, et al. Exercise training favors increased insulin-stimulated glucose uptake in skeletal muscle in contrast to adipose tissue: a randomized study using FDG PET imaging. Am J Physiol Endocrinol Metab. 2013;305:E496–E506.
Reinhardt M, Beu M, Vosberg H, Herzog H, Hübinger A, Reinauer H, et al. Quantification of glucose transport and phosphorylation in human skeletal muscle using FDG PET. J Nucl Med. 1999;40:977–85.
Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol. 1994;76:979–85.
Fowler JS, Wolf AP. 2-Deoxy-2-[18F]fluoro-d-glucose for metabolic studies: current status. Appl Radiat Isot. 1986;37:663–8.
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18) 2-Fluoro-2-deoxy-d-glucose: validation of method. Ann Neurol. 1979;6:371–88.
Huang SC, Williams BA, Barrio JR, Krivokapich J, Nissenson C, Hoffman EJ, et al. Measurement of glucose and 2-deoxy-2 [18F]fluoro-d-glucose transport and phosphorylation rates in myocardium using dual-tracer kinetic experiments. FEBS Lett. 1987;216:128–32.
Ishii Y, Ohta T, Sasase T. Non-obese type 2 diabetes animals models. In: Chackrewarthy S, editor. Glucose tolerance. Rijeka: InTech; 2012. pp. 223–242. doi:10.5772/52712.
Suzuki M, Odaka H, Suzuki N, Sugiyama Y, Ikeda H. Effects of combined pioglitazone and metformin on diabetes and obesity in Wistar fatty rats. Clin Exp Pharmacol Physiol. 2002;29:269–74.
Yamamoto M, Otsuki M. Effect of inhibition of alpha-glucosidase on age-related glucose intolerance and pancreatic atrophy in rats. Metabolism. 2006;55:533–40.
Ikeda H, Sugiyama Y. Insulin resistance-reducing effect of a new thiazolidinedione derivative, pioglitazone. Nihon Yakurigaku Zasshi. 2001;117:335–42.
Feng J, Zhang Z, Wallace MB, Stafford JA, Kaldor SW, Kassel DB, et al. Discovery of alogliptin: a potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IV. J Med Chem. 2007;50:2297–300.
Goto T, Nakayama R, Yamanaka M, Takata M, Takazawa T, Watanabe K, et al. Effects of DSP-8658, a novel selective peroxisome proliferator-activated receptors a/γ modulator, on adipogenesis and glucose metabolism in diabetic obese mice. Exp Clin Endocrinol Diabetes. 2015;123:492–9.
Ito M, Fukuda S, Sakata S, Morinaga H, Ohta T. Pharmacological effects of JTT-551, a novel protein tyrosine phosphatase 1B inhibitor, in diet-induced obesity mice. J Diabetes Res. 2014;2014:680348.
Lee EY, Kim YW, Oh H, Choi CS, Ahn JH, Lee BW, et al. Anti-obesity effects of KR-66195, a synthetic DPP-IV inhibitor, in diet-induced obese mice and obese-diabetic ob/ob mice. Metabolism. 2014;63:793–9.
Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. 2011;93:S52–9.
Kanzaki M. Insulin receptor signals regulating GLUT4 translocation and actin dynamics. Endocr J. 2006;53:267–93.
de Souza CJ, Hirshman MF, Horton ES. CL-316,243, a beta3-specific adrenoceptor agonist, enhances insulin-stimulated glucose disposal in nonobese rats. Diabetes. 1997;46:1257–63.
Park JW, Jung KH, Lee JH, Quach CH, Moon SH, Cho YS, et al. 18F-FDG PET/CT monitoring of β3 agonist-stimulated brown adipocyte recruitment in white adipose tissue. J Nucl Med. 2015;56:153–8.
Ikeda H, Shino A, Matsuo T, Iwatsuka H, Suzuoki Z. A new genetically obese-hyperglycemic rat (Wistar fatty). Diabetes. 1981;30:1045–50.
Umekawa T, Yoshida T, Sakane N, Kondo M. Effect of CL316,243, a highly specific beta(3)-adrenoceptor agonist, on lipolysis of epididymal, mesenteric and subcutaneous adipocytes in rats. Endocr J. 1997;44:181–5.
Ishino S, Miyake H, Vincent P, Mori I. Evaluation of the therapeutic efficacy of a MEK inhibitor (TAK-733) using 18F-fluorodeoxyglucose-positron emission tomography in the human lung xenograft model A549. Ann Nucl Med. 2015;29:613–20.
Säll J, Pettersson AM, Björk C, Henriksson E, Wasserstrom S, Linder W, et al. Salt-inducible kinase 2 and—3 are downregulated in adipose tissue from obese or insulin-resistant individuals: implications for insulin signalling and glucose uptake in human adipocytes. Diabetologia. 2017;60:314–23.
Henriksson E, Säll J, Gormand A, Wasserstrom S, Morrice NA, Fritzen AM, et al. SIK2 regulates CRTCs, HDAC4 and glucose uptake in adipocytes. J Cell Sci. 2015;128:472–86.
Gordon JW, Dolinsky VW, Mughal W, Gordon GR, McGavock J. Targeting skeletal muscle mitochondria to prevent type 2 diabetes in youth. Biochem Cell Biol. 2015;93:452–65.
Ferré P, Leturque A, Burnol AF, Penicaud L, Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J. 1985;228:103–10.
Virtanen KA, Haaparanta M, Grönroos T, Bergman J, Solin O, Rouru J, et al. 2-[(18)F]fluoro-2-deoxy-d-glucose combined with microdialysis can be used for the comparison of tissue glucose metabolism in obese and lean rats. Diabetes Obes Metab. 2002;4:60–8.
Baba S, Engles JM, Huso DL, Ishimori T, Wahl RL. Comparison of uptake of multiple clinical radiotracers into brown adipose tissue under cold-stimulated and nonstimulated conditions. J Nucl Med. 2007;48:1715–23.
Fueger BJ, Czernin J, Hildebrandt I, Tran C, Halpern BS, Stout D, et al. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med. 2006;47:999–1006.
Wong KP, Sha W, Zhang X, Huang SC. Effects of administration route, dietary condition, and blood glucose level on kinetics and uptake of 18F-FDG in mice. J Nucl Med. 2011;52:800–7.
Toyama H, Ichise M, Liow JS, Vines DC, Seneca NM, Modell KJ, et al. Evaluation of anesthesia effects on [18F]FDG uptake in mouse brain and heart using small animal PET. Nucl Med Biol. 2004;31:251–6.
Schuller E, Patel N, Item C, Greber-Platzer S, Baran H, Patsch W, et al. The genetic background modifies the effects of the obesity mutation, ‘fatty’, on apolipoprotein gene regulation in rat liver. Int J Obes Relat Metab Disord. 2000;24:460–7.
Shiota M, Printz RL. Diabetes in Zucker diabetic fatty rat. Diabetes in Zucker diabetic fatty rat. Methods Mol Biol. 2012;933:103–23.
Kooijman S, Wang Y, Parlevliet ET, Boon MR, Edelschaap D, Snaterse G, et al. Central GLP-1 receptor signaling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia. 2015;58:2637–46.
Lee YH, Jung YS, Choi D. Recent advance in brown adipose physiology and its therapeutic potential. Exp Mol Med. 2014;46:e78.
Matsuda J, Hosoda K, Itoh H, Son C, Doi K, Hanaoka I, et al. Increased adipose expression of the uncoupling protein-3 gene by thiazolidinediones in Wistar fatty rats and in cultured adipocytes. Diabetes. 1998;47:1809–14.
Pereira LO, Lancha AH Jr. Effect of insulin and contraction up on glucose transport in skeletal muscle. Prog Biophys Mol Biol. 2004;84:1–27.
Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–52.
Schade KN, Baranwal A, Liang C, Mirbolooki MR, Mukherjee J. Preliminary evaluation of β3-adrenoceptor agonist-induced 18F-FDG metabolic activity of brown adipose tissue in obese Zucker rat. Nucl Med Biol. 2015;42:691–4.
MacPherson RE, Castellani L, Beaudoin MS, Wright DC. Evidence for fatty acids mediating CL 316,243-induced reductions in blood glucose in mice. Am J Physiol Endocrinol Metab. 2014;307:E563–70.
Kumar A, Shiloach J, Betenbaugh MJ, Gallagher EJ. The beta-3 adrenergic agonist (CL-316,243) restores the expression of down-regulated fatty acid oxidation genes in type 2 diabetic mice. Nutr Metab. 2015;12:8.
Acknowledgements
We appreciate Mr. Yoshiteru Yamashita (Technical Services for Animal Research, Takeda Rabics. Fujisawa, Japan) doing the rat vein catheterization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Rights and permissions
About this article
Cite this article
Ishino, S., Sugita, T., Kondo, Y. et al. Glucose uptake of the muscle and adipose tissues in diabetes and obesity disease models: evaluation of insulin and β3-adrenergic receptor agonist effects by 18F-FDG. Ann Nucl Med 31, 413–423 (2017). https://doi.org/10.1007/s12149-017-1169-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-017-1169-0