Abstract
Purpose
PET/CT has been considered limited for the evaluation of mucinous colorectal tumors due to low 18F-FDG uptake. The aim of our study was to compare PET/CT variables in mucinous (MC) and nonmucinous (NMC) rectal adenocarcinomas.
Methods
Consecutive patients with cT2-4N0-2M0 rectal cancer included in a prospective clinical trial were reviewed. PET/CT was performed for primary baseline staging. Visual and quantitative analysis included SUVmax and SUVmean, metabolic tumor volume (MTV) and total lesion glycolysis (TLG). PET/CT parameters were compared according to histological subtypes.
Results
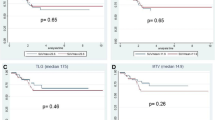
Overall, 73 patients were included (18 mucinous and 55 nonmucinous). SUVmax values were similar between MC and NMC (19.7 vs. 16.6; p = 0.5). MTV and TLG values were greater in the MC group (103.9 vs. 54.1; p = 0.007 and 892.5 vs. 358.8; p = 0.020) due to larger tumor volumes of MC.
Conclusions
Metabolic parameters at baseline PET/CT for patients with rectal cancer are similar in mucinous and nonmucinous histological subtypes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Integrated PET/CT with 18F-FDG is a well-established functional imaging modality that can detect active neoplastic tumor cells based on glucose metabolism status. Malignant cells express increased numbers of glucose transporter membrane proteins and have augmented levels of intracellular enzymes that promote glycolysis. Therefore, several types of malignant tumors avidly take up 18F-FDG [1].
18F-FDG PET/CT has a significant role in the assessment of patients with locally recurrent or metastatic colorectal cancer in final decision management. In addition, PET/CT may be helpful in assessing treatment response in various clinical settings. Particularly for rectal cancer, the observation of significant tumor regression after neoadjuvant chemoradiation (nCRT) therapy led surgeons to consider alternative surgical and non-surgical strategies in selected patients. In this setting, several studies have investigated the role of PET/CT in the assessment of treatment response to nCRT [2–5].
Mucinous adenocarcinoma (MC) represents 10–15 % of colorectal tumors [6]. By definition, MC is a subtype of colorectal cancer in which more than 50 % of the tumor consists of extracellular mucin [7]. A low or even absent 18F-FDG uptake by mucinous tumors has been attributed to the relative hypocellularity of these malignancies [8–10]. Therefore, in these patients, 18F-FDG PET/CT could result in false-negative findings and significantly limit its applicability in clinical practice. For these reasons, we decided to compare 18F-FDG PET/CT findings between rectal tumors classified as mucinous or nonmucinous (NMC) adenocarcinoma.
Materials and methods
Consecutive patients with clinical stage cT2-4N0-2M0 undergoing PET/CT imaging for primary baseline staging between January 2005 and February 2009 were eligible for the study. The original study was IRB approved and registered as a prospective trial under NCT 00254683. All subjects signed an informed consent form. All patients underwent nCRT as described elsewhere [4].
PET/CT
Whole-body PET/CT scans were obtained prior to nCRT, during baseline staging. Interval after 18F-FDG injections was approximately 60 min. Oral iodine contrast was administered to all patients. Delayed images of the pelvis were acquired approximately 180 min following 18F-FDG injection and immediately after intrarectal iodine contrast enema. All PET and CT images were retrieved from electronic media and reviewed using commercial software (AW Volume Share, ver. 5, GE Healthcare).
PET/CT metabolic parameters included maximum standard uptake value normalized by the body weight (SUVmax), mean standard uptake value (SUVmean), metabolic tumor volume (MTV) and total lesion glycolysis (TLG; defined as: SUVmean × MTV). Volumetric parameters (SUVmean, MTV and TLG) were measured from attenuation-corrected 60-min PET images. A predefined threshold of 20 % of the SUVmax was used to automatically generate volumes of interest.
Pathology
Pathology was performed uniformly in a single center by an experienced gastrointestinal pathologist. MC was defined as the presence of more than 50 % of mucin in the primary tumor area [7]. Patients were divided into two groups (MC or NMC) based on histopathological features of the final surgical specimen. Patients managed non-operatively (complete clinical response or refusal to radical surgery) or with no residual cancer in the resected specimen (complete pathological response) were included if the mucinous component was explicitly reported in the primary baseline diagnostic biopsy. Final tumor size was defined as the greatest tumor diameter measured at pathological examination.
Statistical analysis
Statistical analysis was performed using SAS V9.4 (SAS Institute Inc, Cary NC). All results were expressed by mean ± SD. Differences were considered statistically significant for p < 0.05. Mann–Whitney nonparametric test was used to compare the mean values of each PET/CT variable between the MC and NMC groups.
Results
Overall, 73 patients had sufficient histological information, were included in the study and constitute our patient population.
Patients’ demographics and PET/CT variables are detailed in Table 1. Eighteen (24.7 %) patients were classified as MC and 55 (75.3 %) as NMC. All primary rectal tumors could be visually identified on PET/CT images regardless of the histological subtype, resulting in 0 % false-negative rate. Mean tumor sizes were similar between both groups (p = 0.765).
There were no statistical differences in SUVmax and SUVmean values between MC and NMC (19.7 ± 12.8 vs. 16.6 ± 7.8; p = 0.578 and 7.9 ± 5.4 vs. 7.0 ± 3.5; p = 0.808, respectively) (Table 1). Curiously, solid areas within mucinous cancers indicated by high attenuation in CT images appear to have increased avidity to 18F-FDG uptake as indicated in Fig. 1.
The metabolic tumor volumes (MTV) were statistically larger in the MC group (103.9 vs. 54.1; p = 0.007). Consequently, TLG values were also statistically higher in the MC group (892.5 vs. 358.8; p = 0.020) (Table 1).
Discussion
Mucinous colorectal adenocarcinomas have been considered a potential limitation to 18F-FDG PET due to the hypocellularity of these lesions and abundant mucin. This has been suggested as the main reason for lower glucose metabolic rate of these malignancies during PET/CT imaging [10–12]. However, there is little evidence to support that mucinous colorectal cancers have lower metabolic activity that could result in false-negative findings. Most of the available studies are small retrospective series, frequently using PET imaging (and not PET/CT) and often not providing exact false-negative rates.
Our study demonstrates that mucinous and nonmucinous rectal cancers have similar 18F-FDG uptake at PET/CT imaging. None of our patients had absent 18F-FDG uptake and SUV estimates (mean and maximal) were similar between tumors regardless of the histological subtype (mucinous or nonmucinous).
One study using PET/CT in 37 patients with colorectal adenocarcinoma failed to identify 5 tumors (13.5 %). Only one of these five undetected tumors was found to be mucinous. However, the overall number of mucinous tumors (of the 37 included) is not available [13]. Another study described PET/CT findings in 97 patients with rectal cancer at primary baseline staging. PET/CT did not show 18F-FDG uptake in only two rectal tumors. Only one of these tumors was mucinous [14]. The authors grouped together poorly differentiated and mucinous tumors and only one (out of the 26 patients poorly differentiated or mucinous) had no 18F-FDG uptake at PET/CT imaging. These same authors also estimated SUVmax of these tumors and, in line with our findings, showed similar values between mucinous and nonmucinous colorectal cancers.
Originally, disappointing findings of 18F-FDG uptake in mucinous colorectal cancers derive from studies using PET imaging (and not PET/CT). In one of these studies, dedicated PET showed absent or minimal 18F-FDG uptake on visual analysis in 13 out of 22 mucinous neoplasms even though SUVmax values were not available [9]. Another study suggested that 18F-FDG PET had a lower sensitivity for detecting local recurrence or metastases among mucinous colorectal cancers (58 vs. 92 %; p = 0.005) [8].
In fact, our findings with integrated PET/CT indicate that mucinous rectal cancers have similar 18F-FDG uptake estimated by SUV values. Even though the exact reasons for these findings may be unclear, it is worthwhile noticing that the solid components of the tumors appeared to be extremely 18F-FDG-avid and may have compensated for the lack of 18F-FDG uptake of the mucinous component (Fig. 1). A detailed analysis of tumor cellularity and FDG uptake within each of the tumors in our series would have been required to fully explore this possibility and constitutes a relevant limitation of our study. Precise anatomical delineation of these tumors with PET/CT technology allows for proper estimation of 18F-FDG uptake and the use of PET alone in these previous studies may have accounted for these differences. In addition, the greater MTV and TLG among MC in our series are possibly explained by the larger tumor volumes among this histological subtype. Even though final pathological measurement of tumor diameter was similar between groups, differences in volume estimation may have accounted for these differences since TLG is directly affected by MTV (TLG = MTV × SUVmean).
Finally, it has been suggested that metastatic liver tumors with different histologic subtypes (MC and NMC) may have distinct metabolic patterns at PET/CT imaging when comparing early and late scans (also known as dual time point imaging—DTPI). In a report of a single patient, an increase of 18F-FDG uptake by a metastatic nodule of mucinous subtype was exclusively detected on late images (3 h). In contrast, the nonmucinous nodule showed 18F-FDG uptake on early (60 min) images. Distinct patterns of 18F-FDG using dual time point imaging (DTPI) could have been explained by the mucinous histological phenotype [15]. In our series, early images (obtained at 60 min) revealed 18F-FDG uptake of the primary tumor in all patients (mucinous and nonmucinous) suggesting that distinct patterns of 18F-FDG uptake in DTPI may not be exclusively dependent on histological subtypes. Metabolic parameters of delayed images were not evaluated in our study because iodinated contrast enema was routinely used and attenuation correction errors could have influenced estimation of SUV values [16].
Additional limitations of our study include the retrospective design and a relatively limited sample size, resulting in a slightly higher incidence of mucinous rectal cancer. In addition, inclusion of locally advanced disease failed to provide evidence on small and early cancers in regards to mucinous histology and PET/CT imaging or 18F-FDG uptake. The inclusion of small tumors (1–2 cm) in our series could have affected estimation of SUV and MTV values by partial volume effect, as suggested in previous studies [17]. However, considering there were no differences in tumor size between groups, underestimation of SUV and overestimation of MTV of these tumors may have occurred in both groups evenly.
Conclusions
Mucinous rectal adenocarcinomas have considerably high 18F-FDG uptake at PET/CT imaging, similar to nonmucinous rectal cancers. These findings suggest that 18F-FDG PET/CT may be a useful tool in the assessment of locally advanced rectal tumors, even among mucinous subtypes.
References
Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan C-N, Wolf AP. Metabolic trapping as a principle of radiopharmaceutical design: some factors responsible for the biodistribution of [18F] 2-deoxy-2-fluoro-d-glucose. J Nucl Med. 1978;19(10):1154–61.
Martoni AA, Di Fabio F, Pinto C, Castellucci P, Pini S, Ceccarelli C, et al. Prospective study on the FDG-PET/CT predictive and prognostic values in patients treated with neoadjuvant chemoradiation therapy and radical surgery for locally advanced rectal cancer. Ann Oncol. 2011;22(3):650–6. doi:10.1093/annonc/mdq433.
Melton GB, Lavely WC, Jacene HA, Schulick RD, Choti MA, Wahl RL, et al. Efficacy of preoperative combined 18-fluorodeoxyglucose positron emission tomography and computed tomography for assessing primary rectal cancer response to neoadjuvant therapy. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2007;11(8):961–9. doi:10.1007/s11605-007-0170-7 (discussion 9).
Perez RO, Habr-Gama A, Gama-Rodrigues J, Proscurshim I, Juliao GP, Lynn P, et al. Accuracy of positron emission tomography/computed tomography and clinical assessment in the detection of complete rectal tumor regression after neoadjuvant chemoradiation: long-term results of a prospective trial. Cancer. 2012;118(14):3501–11. doi:10.1002/cncr.26644 (National Clinical Trial 00254683).
Cascini GL, Avallone A, Delrio P, Guida C, Tatangelo F, Marone P, et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med. 2006;47(8):1241–8.
Hugen N, Brown G, Glynne-Jones R, de Wilt JH, Nagtegaal ID. Advances in the care of patients with mucinous colorectal cancer. Nat Rev Clin Oncol. 2015;. doi:10.1038/nrclinonc.2015.140.
Bosman FT. WHO classification of tumors of the digestive system. Lyon: IARC Press; 2010.
Whiteford MH, Whiteford HM, Yee LF, Ogunbiyi OA, Dehdashti F, Siegel BA, et al. Usefulness of FDG-PET scan in the assessment of suspected metastatic or recurrent adenocarcinoma of the colon and rectum. Dis Colon Rectum. 2000;43(6):759–67 (discussion 67–70).
Berger KL, Nicholson SA, Dehdashti F, Siegel BA. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol. 2000;174(4):1005–8. doi:10.2214/ajr.174.4.1741005.
Delbeke D, Martin WH. PET and PET-CT for evaluation of colorectal carcinoma. Semin Nucl Med. 2004;34(3):209–23. doi:10.1053/j.semnuclmed.2004.03.006.
O’Connor OJ, McDermott S, Slattery J, Sahani D, Blake MA. The use of PET-CT in the assessment of patients with colorectal carcinoma. Int J Surg Oncol. 2011;2011:846512. doi:10.1155/2011/846512.
Dewhurst C, Rosen MP, Blake MA, Baker ME, Cash BD, Fidler JL, et al. ACR appropriateness criteria pretreatment staging of colorectal cancer. J Am Coll Radiol. 2012;9(11):775–81. doi:10.1016/j.jacr.2012.07.025.
Gollub MJ, Grewal RK, Panu N, Thipphavong S, Sohn M, Zheng J, et al. Diagnostic accuracy of (1)(8)F-FDG PET/CT for detection of advanced colorectal adenoma. Clin Radiol. 2014;69(6):611–8. doi:10.1016/j.crad.2014.01.009.
Ozis SE, Soydal C, Akyol C, Can N, Kucuk ON, Yagci C, et al. The role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the primary staging of rectal cancer. World J Surg Oncol. 2014;12:26. doi:10.1186/1477-7819-12-26.
Yeh CL, Chen YK. Interesting image. Utility of FDG metabolism to differentiate synchronous metastatic liver lesions from synchronous colon cancer: nonmucinous versus mucinous adenocarcinoma. Clin Nucl Med. 2010;35(1):44–6. doi:10.1097/RLU.0b013e3181c361c4.
Ay MR, Zaidi H. Assessment of errors caused by X-ray scatter and use of contrast medium when using CT-based attenuation correction in PET. Eur J Nucl Med Mol Imaging. 2006;33(11):1301–13. doi:10.1007/s00259-006-0086-6.
Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48(6):932–45. doi:10.2967/jnumed.106.035774.
Acknowledgments
This is a retrospective study based on a prospectively collected data. The original trial was funded by two Brazilian research funding agencies: Conselho Nacional de Desenvolvimento Tecnológico e Científico (CNPq—Grant Number 483752/2006-1), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP—Grant Number 07/51069-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
dos Anjos, D.A., Habr-Gama, A., Vailati, B.B. et al. 18F-FDG uptake by rectal cancer is similar in mucinous and nonmucinous histological subtypes. Ann Nucl Med 30, 513–517 (2016). https://doi.org/10.1007/s12149-016-1089-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-016-1089-4