Abstract
Objectives
This study investigated the prognostic value of preoperative breast-specific gamma imaging (BSGI) uptake measured by a semi-quantitative method in invasive ductal carcinoma (IDC).
Methods
One hundred and sixty-two women with IDC who underwent preoperative BSGI were retrospectively enrolled. The tumor-to-normal tissue ratio (TNR) was measured on BSGI and correlated with histologic prognostic factors. The prognostic impact of TNR was tested with regard to progression-free survival (PFS) and compared with established prognostic factors.
Results
High TNR was significantly correlated with tumor size >2 cm (p < 0.001), high nuclear grade (p = 0.04), high histologic grade (p = 0.01), axillary node positivity (p = 0.04), ER negativity (p = 0.03), HER2 positivity (p = 0.01), and high MIB-1 index (p = 0.001). Among 162 patients, 14 experienced recurrence during mean follow-up time of 34.7 ± 14.9 months. In Kaplan–Meier survival analyses, high TNR (p < 0.001), high nuclear grade (p = 0.02), high histologic grade (p = 0.007), ER/PR negativity (p = 0.003 and p < 0.001, respectively), HER2 positivity (p = 0.01), triple negativity (p = 0.02), and high MIB-1 index (p = 0.02) showed a significant relationship with poor prognosis. Among them, high TNR was an independent poor prognostic factor in a multivariate regression analysis (p = 0.01).
Conclusions
High BSGI uptake measured by a semi-quantitative method was correlated with diverse poor histologic prognostic factors and was an independent poor prognostic factor in invasive ductal cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most frequent malignancy in women with approximately 226,870 newly diagnosed cases and 39,510 women dying from breast cancer in the United States alone in 2012 [1]. Despite adequate locoregional treatment, about 10–20 % of patients with operable breast cancer will develop local recurrence [2]. Therefore, an accurate prediction of prognosis is important for patient stratification.
Preoperative imaging modalities are expected to provide prognostic information for breast cancer. The prognostic value of functional parameters obtained from preoperative magnetic resonance imaging has been reported [3, 4]. Nuclear medicine imaging methods provide prognostic information for breast cancer that are based on suspicion of a tumor. F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) evaluates cellular glucose metabolism, and enhanced uptake of F-18 FDG is an indicator of tumor aggressiveness. Previous studies suggested that the standardized uptake value (SUV) of primary breast cancer measured from FDG PET is significantly correlated with prognosis [4, 5].
Tc-99m sestamibi breast-specific gamma imaging (BSGI) is another useful nuclear medicine imaging technique and its clinical demands for breast cancer imaging are increasing. BSGI contributes to the detection of high-risk lesions in negative or indeterminate mammography (MMG) as well as improvement of disease management compared with ultrasonography (US) [6]. Tc-99m sestamibi uptake of BSGI depends on abundant mitochondrial activity within tumor cells, which is a different mechanism than that utilized by FDG PET. Therefore, BSGI is expected to provide independent biological information on breast cancer. Biological information on breast cancer is important for predicting prognosis and is mainly estimated by pathologic procedures. A few studies have reported a correlation between Tc-99m sestamibi uptake and histologic prognostic factors of breast cancer [7, 8]. The prognostic value of Tc-99m sestamibi washout rate from breast cancer cells for predicting responses to neoadjuvant chemotherapy has been investigated based on its relationship with P-glycoprotein (Pgp) expression [9]. However, the prognostic value of preoperative BSGI for predicting recurrence in invasive breast cancer has not been investigated.
The purpose of the current study was to evaluate the prognostic value of semi-quantitative BSGI uptake for predicting recurrence based on its relationship with diverse histologic prognostic factors in breast cancer.
Materials and methods
Patients
Patients who were newly diagnosed with breast cancer and underwent preoperative BSGI were the primary subject group. The primary inclusion criteria were: (1) pathologically confirmed invasive ductal carcinoma; (2) absence of distant metastasis; (3) absence of neoadjuvant chemotherapy or radiotherapy before BSGI; and (4) pathologic tumor size ≥1 cm to avoid any partial volume effect. Despite the use of high-resolution equipment, the small-field-of-view of the breast-specific gamma camera and the small tumor size still remain as limitations [7]. Retrospective review of our database between March 2009 and September 2012 yielded 168 eligible patients. For staging work up, all patients underwent MMG as well as US and/or MRI. After staging work up, all patients were treated with breast-conserving surgery or mastectomy. There were six patients whose tumor foci were not visualized on BSGI due to their close proximity to the chest wall (Fig. 1). Excluding those six patients, 162 patients were referred for further statistical analysis. Our institutional review board approved the retrospective chart review, and all of the data were obtained from medical records.
An example of whose tumor was not visualized on BSGI due to its close proximity to the chest wall. The maximum intensity projection images of MRI (a coronal view, b sagittal view) demonstrate a highly enhancing mass with a speculated margin in the far periphery of the lower inner of the left breast (arrows), while BSGI (c LCC view, d LMLO view) did not
BSGI imaging protocol
For BSGI, patients were injected with 555–925 MBq of Tc-99m sestamibi via an upper-extremity vein on the side opposite to the breast cancer. After 10 min, imaging was started and patients were seated during the entire scanning. Right craniocaudal (RCC), left craniocaudal (LCC), right mediolateral oblique (RMLO), and left mediolateral oblique (LMLO) images were obtained using a high-resolution breast-specific gamma camera (6800 Gamma Camera; Dilon Technologies, Newport News, VA, USA) with a systemic energy window centered over the 140-keV photo-peak. At least 150,000 counts were acquired for each planar image, and the acquisition time for each image ranged from 5 to 10 min.
Image analysis
All resultant images were transferred to a picture archiving and communication system (PACS) for analysis. All images were reviewed by two experienced nuclear medicine physicians (H.J.Y. and B.S.K., both board certified nuclear medicine specialists) and analyzed in consensus. For accurate tumor site localization, other anatomical imaging results, including MMG, US, and MRI, were reviewed together for reference during the analysis. However, the readers were strictly blinded to the patient’s pathologic report and final outcome.
For each patient, BSGI uptake by the tumor and normal tissue were measured as a value of uptake counts. A circular region of interest (ROI) overlaying the tumor lesion was placed on each planar image, and maximal pixel counts of the ROI were measured. For the normal breast, three standardized ROIs 1 cm in diameter were placed over normal breast parenchyma remote from the tumor site, and mean pixel counts of the three ROIs were averaged into a single value. The tumor-to-normal ratio (TNR) was generated as (maximal pixel counts of tumor)/(average of three mean pixel counts of normal parenchyma) in each BSGI image. Between CC and MLO projections, the higher TNR was selected for further analysis [10].
Pathologic assessment
All surgical specimens were prepared and evaluated by our institution’s pathologist using a standard protocol. Pathologic tumor size was measured at its greatest dimension and the cutoff point was ≤2 cm. The presence of any lymph node metastasis was considered as positive axillary node status (ANS). No internal mammary lymph node metastasis was present among enrolled patients. Histologic and nuclear grade were scored by the modified Bloom–Richardson Grading system.
A re-cut section of formalin-fixed, paraffin-embedded tissue was processed with Bound™ Automated Immunohistochemistry (Vision Biosystems Inc., Mount Waverley, Victoria, Australia) and a bound polymer detection system with counterstaining (Vision Biosystems Inc.). Commercial antibodies for estrogen receptor (ER, 1:300; Novocastra, Newcastle, UK), progesterone receptor (PR, 1:600; Novocastra), c-erbB-2 (1:1800; DAKO, Glostrup, Denmark), p53 (1:800; Novocastra), and MIB-1 (1:200; Novocastra) were used.
Positivity of ER and PR was defined as showing moderate or high intensity nuclear staining in at least 10 % of tumor cells, according to the Allred scoring system. Positivity of p53 was defined as having moderate or high intensity nuclear staining in at least 5 % of tumor cells, while positivity of MIB-1 was defined as having nuclear staining in at least 10 % of tumor cells. Positivity for c-erbB-2 was defined as showing high intensity cell membrane staining in at least 10 % of tumor cells. Triple negativity indicated all negative findings for ER, PR, and c-erbB-2.
Statistics
Data are expressed as mean ± SD and 95 % confidence interval (CI). p values less than 0.05 were regarded as statistically significant. All statistical analyses were performed using the MedCalc software package (Ver. 9.5, MedCalc Software, Mariakerke, Belgium).
Correlations between TNR and variable clinicopathologic factors were evaluated; the independent t test was used for bimodal variables, while the ANOVA test was used for trimodal variables. Then, multivariate regression analysis was performed using variables with p values less than 0.05 in the univariate analysis to determine independent significant factors.
For survival analysis, Kaplan–Meier analysis and Cox proportional regression analysis were performed with respect to disease progression. To compare the survival curve of each group, the log-rank test was used. A patient’s progression-free survival (PFS) time was determined from the date of the BSGI scan to the date of recurrence. The presence of recurrence was confirmed by tissue biopsy or at least two imaging studies such as US, MRI, CT, or FDG PET/CT.
Results
Clinicopathologic features and outcomes
One hundred and sixty-two women with breast cancer were included in the analysis. The average age was 49 ± 11 (range 26–82) years. The mean tumor size was 2.3 ± 1.3 (range 1–9.7) cm. One hundred and nineteen patients received breast-conserving surgery, while 43 received mastectomy. On BSGI, the overall TNR was 3.6 ± 1.5 (range 1.4–10.4). Other characteristics of the 162 patients are summarized in Table 1.
Correlation between TNR and histologic prognostic factors
Correlations between TNR and histologic prognostic factors are listed in Table 2. Among the histologic factors, tumor size, ANS, nuclear grade, histologic grade, ER, HER2, and MIB-1 index showed significant correlations with TNR. TNR of the large-sized tumor (>2 cm) group showed a significantly higher value compared to that of the small-sized tumor (≤2 cm) group (p < 0.001). Positive ANS showed a significant correlation with higher TNR value (p = 0.04). Nuclear grade and histologic grade showed positive correlations with TNR values (p = 0.04 and p = 0.01, respectively). The ER-negative group had a significantly higher TNR than the ER-positive group (p = 0.03). The HER2-positive group had a significantly higher TNR than the HER2-negative group (p = 0.01). Higher MIB-1 index was significantly associated with higher TNR (p = 0.001).
Among the significant variables, MIB-1 index (p = 0.03 and regression coefficient = 0.623) and tumor size (p < 0.001 and regression coefficient = 0.859) remained independent factors for TNR in the multivariate analysis.
Prognosis prediction
During the follow-up period of 34.7 ± 14.9 months (range 3.5–62.5 months), 14 (8.6 %) patients had disease progression. Disease progression, that is recurrence, was proven by either tissue biopsy (n = 5) or imaging studies (n = 9).
Receiver-operating characteristic (ROC) curve analysis determined the optimal cutoff value of TNR as 3.34 for Kaplan–Meier survival analysis. The PFS of the high TNR group was significantly lower than the low TNR group (p < 0.001). The survival curves of each TNR group are shown in Fig. 2. Among the histologic factors, high nuclear grade (p = 0.02), high histologic grade (p = 0.007), ER negativity (p = 0.003), PR negativity (p < 0.001), HER2 positivity (p = 0.01), triple negativity (p = 0.02), and high MIB-1 index (p = 0.02) were significant poor prognostic factors for disease progression. When the significant prognostic factors were tested by multivariate Cox proportional regression analysis, TNR was the only independent prognostic factor (p = 0.01 and regression coefficient = 2.767). The results of univariate and multivariate survival analyses are summarized in Table 3. Representative cases of low TNR (Fig. 3) and high TNR (Fig. 4) with different biologic features and prognosis are demonstrated.
A 68-year-old patient with invasive ductal carcinoma in the upper center of the left breast. BSGI (a LCC view, b LMLO view) demonstrates faint uptake in the periareolar area (arrows). The calculated TNR was 1.6. Histopathologic features were pT1a (1.1 cm at greatest dimension), nuclear grade 2, histologic grade 1, negative ANS, positive ER, negative HER2, and negative MIB-1 (positive in 5 % of cells). This patient had no recurrence during the follow-up period of 24.9 months
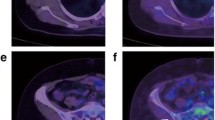
A 42-year-old patient with invasive ductal carcinoma in the lower inner area of the right breast. BSGI (a RCC view, b RMLO view) demonstrates a definite focal uptake in the lower inner area (arrows). The calculated TNR was 6.1. Histopathologic features were pT2 (2.3 cm at greatest dimension), nuclear grade 3, histologic grade 3, positive ANS, negative ER, negative HER2, and positive MIB-1 (positive in 20 % of cells). This patient had a recurrence in lung and lymph nodes after 6.8 months
Discussion
The present study demonstrated that TNR on BSGI has significant prognostic impact in predicting recurrence of operable invasive ductal breast cancer.
The prognosis of breast cancer depends on the biological behavior of the tumor, which is mainly determined by histologic biomarkers. Some authors reported significant correlations between the level of BSGI uptake and histologic biomarkers [8, 11–14]. Our results also demonstrated positive correlations for TNR with diverse histologic factors. These relationships could be an explanation for the strong prognostic power of TNR on BSGI.
Larger tumor size and axillary nodal involvement are poor prognostic factors for breast cancer and are accepted as variables on which to form the basis of TNM staging [15]. A positive correlation of TNR with tumor size indicates that high mitochondrial activity of a tumor is one of the important molecular mechanisms of tumor growth and local progression. A significant relationship between TNR and tumor size has been reported by other groups [8, 11]. High TNR was also significantly correlated with positive axillary nodal status. Cwikla et al. [12] also suggested a significant positive correlation between TNR and the presence of axillary node involvement. Preoperative lymph node prediction is still under investigation as various imaging modalities are developed and the current findings indicate the potential for BSGI to be used for this purpose [16].
Higher nuclear and histologic grade reflects the “aggressive potential” of the tumor. Our results suggested that higher tumor grade is significantly associated with higher TNR. Similarly, several studies also reported a positive correlation of tumor-to-background ratio with tumor grade [8, 12].
HER2 overexpression is a strong prognostic factor for disease recurrence and poor overall survival, particularly for node-positive patients [17]. The HER2-positive group showed significantly higher TNR compared to the HER2-negative group in this study. Meanwhile, the presence of hormonal receptors has an inverse correlation with HER2 overexpression [18]. Hormonal receptor negativity is known as a poor prognostic factor [19]. In this study, hormonal receptor status showed an inverse correlation with TNR. This inverse correlation between hormone receptor status and TNR has also been demonstrated by another group [11, 12].
MIB-1 monoclonal antibody is an indicator of cell proliferation and MIB-1 positivity is highly correlated with worse survival [20, 21]. According to our results, a higher MIB-1 index was significantly associated with higher TNR. A positive correlation between TNR and cell proliferation has also been reported in previous studies [13, 14]. This is considered to be the mechanism underlying how MIBI uptake may reflect the participation of mitochondria in breast cancer cell proliferation.
The relationship between histologic biomarkers and F-18 FDG uptake on PET has also been investigated in a previous study [5], in which tumor size, hormone receptor status, and nuclear grade were found to be independent factors for F-18 FDG uptake on PET, whereas MIB-1 proliferation index and tumor size were independent factors for Tc-99m MIBI uptake on BSGI in our study. The differences in the cellular uptake mechanisms of PET and BSGI may contribute to these results.
Despite previous reports regarding the correlation between BSGI uptake and histologic prognostic factors of breast cancer, the prognostic value of BSGI uptake has not been evaluated directly. When the prognostic value of semi-quantitative TNR measured on preoperative BSGI was tested with respect to recurrence, the PFS rate of the high TNR group was significantly lower than that of the low TNR group. In addition, high nuclear grade, high histologic grade, ER and PR negativity, HER 2 positivity, triple negativity, and high MIB-1 were significantly associated with poor prognosis in this study. Although tumor size is a known important prognostic factor and has high correlation with BSGI uptake, the PFS rate of the large-sized tumor group did not show a significant difference with the small-sized tumor group. The relatively small pathologic size of most tumors in this study, which were less than 5 cm, might influence the small difference of the PFS rate between the large-sized tumor and small-sized tumor groups. The prognostic value of ANS showed a considerable trend toward significance (p = 0.09). When significant factors in the univariate analysis were tested simultaneously by Cox proportional analysis, TNR was the only independent prognostic factor for the prediction of recurrence. Considering the significant relationships between TNR and diverse poor prognostic factors, the result was reasonable. Preoperative BSGI is expected to help prognosis prediction, in addition to detecting breast cancer.
One of the limitations of the current study was that the prognostic value of BSGI uptake regarding overall survival was not available. No occurrence of death in this study prevented the investigation of overall survival. Despite a sufficient follow-up period, exclusion of patients with distant metastasis might contribute to no occurrence of death. A low number of events per independent variable is a second limitation. For cases with a low number of events, the regression coefficients can be biased; therefore, the current results should be interpreted with caution. Third, we did not consider tumor markers as variables (e.g., CEA and CA15-3), and they can be important prognostic factors in breast cancer. A previous study reported a positive relationship between tumor markers and tumor-to-background ratio (TBR) on BSGI [8], and we expected similar results.
The current study limited enrollment to a single pathologic subtype of IDC with a size larger than 1 cm to minimize possible confounding effects. However, restricting the study group to a single IDC subtype could be a disadvantage as well. An extended application of the current results to whole breast cancer will be limited even though IDC subtype comprises the majority of breast cancers.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Freedman G, Fowble B. Local recurrence after mastectomy or breast-conserving surgery and radiation. Oncology (Williston Park). 2000;14:1561–81.
Pickles MD, Manton DJ, Lowry M, Turnbull LW. Prognostic value of pre-treatment DCE-MRI parameters in predicting disease free and overall survival for breast cancer patients undergoing neoadjuvant chemotherapy. Eur J Radiol. 2009;71:498–505.
Nakajo M, Kajiya Y, Kaneko T, Kaneko Y, Takasaki T, Tani A, et al. FDG PET/CT and diffusion-weighted imaging for breast cancer: prognostic value of maximum standardized uptake values and apparent diffusion coefficient values of the primary lesion. Eur J Nucl Med Mol Imaging. 2010;37:2011–20.
Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38:250–8.
Weigert JM, Bertrand ML, Lanzkowsky L, Stern LH, Kieper DA. Results of a multicenter patient registry to determine the clinical impact of breast-specific gamma imaging, a molecular breast imaging technique. Am J Roentgenol. 2012;198:W69–75.
Tadwalkar R, Rapelyea J, Torrente J, Rechtman L, Teal C, Mcswain A, et al. Breast-specific gamma imaging as an adjunct modality for the diagnosis of invasive breast cancer with correlation to tumour size and grade. Br J Radiol. 2012;85:e212–6.
Lumachi F, Ermani M, Marzola M, Zucchetta P, Cecchin D, Basso S, et al. Relationship between prognostic factors of breast cancer and 99mTc-sestamibi uptake in patients who underwent scintimammography: multivariate analysis of causes of false-negative results. The Breast. 2006;15:130–4.
Wang J-H, Scollard DA, Teng S, Reilly RM, Piquette-Miller M. Detection of P-glycoprotein activity in endotoxemic rats by 99mTc-sestamibi imaging. J Nucl Med. 2005;46:1537–45.
Eo JS, Chun IK, Paeng JC, Kang KW, Lee SM, Han W, et al. Imaging sensitivity of dedicated positron emission mammography in relation to tumor size. Breast. 2012;21:66–71.
Papantoniou VJ, Souvatzoglou MA, Valotassiou VJ, Louvrou AN, Ambela C, Koutsikos J, et al. Relationship of cell proliferation (Ki-67) to 99mTc-(V) DMSA uptake in breast cancer. Breast Cancer Res. 2003;6:R56–62.
Cwikla J, Buscombe J, Kolasinska A, Parbhoo S, Thakrar D, Hilson A. Correlation between uptake of Tc-99m sestaMIBI and prognostic factors of breast cancer. Anticancer Res. 1999;19:2299–304.
Cutrone JA, Yospur LS, Khalkhali I, Tolmos J, Devito A, Diggles L, et al. Immunohistologic assessment of technetium-99m-MIBI uptake in benign and malignant breast lesions. J Nucl Med. 1998;39:449–53.
Mulero F, Nicolas F, Roca V, Castellón M, Claver M, de La Cruz P, et al. Usefulness of the quantification of (99M) Tc-MIBI uptake in breast neoplasms in the preoperative assessment of tumor aggressiveness. Rev Esp Med Nucl. 2000;19:263–9.
Utah A. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–7.
Abe H, Schmidt RA, Kulkarni K, Sennett CA, Mueller JS, Newstead GM. Axillary lymph nodes suspicious for breast cancer metastasis: sampling with US-guided 14-gauge core-needle biopsy—clinical experience in 100 patients. Radiology. 2009;250:41–9.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82.
Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95:142–53.
Bentzon N, Düring M, Rasmussen BB, Mouridsen H, Kroman N. Prognostic effect of estrogen receptor status across age in primary breast cancer. Int J Cancer. 2008;122:1089–94.
de Azambuja E, Cardoso F, de Castro G, Colozza M, Mano MS, Durbecq V, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12 155 patients. Br J Cancer. 2007;96:1504–13.
Stuart-Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast. 2008;17:323–34.
Acknowledgments
This research was supported by the Basic Science Research Program and the Bio and Medical Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2012R1A1A1012913 and 2012M3A9B6055379).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, HJ., Kim, Y., Chang, KT. et al. Prognostic value of semi-quantitative tumor uptake on Tc-99m sestamibi breast-specific gamma imaging in invasive ductal breast cancer. Ann Nucl Med 29, 553–560 (2015). https://doi.org/10.1007/s12149-015-0977-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-015-0977-3