Cognitive-Behavioral Therapy (CBT) has a significant adjunctive effect in the treatment of Major Depressive Disorder (MDD), however its use as monotherapy in group-based approaches is less explored. We assessed the responses of distinct psychophysiological domains after a group-based CBT (gCBT, 16 weeks) intervention in drug-free patients with mild-moderate MDD (n = 20; women = 11) and compared them with a healthy control group (n = 25, women = 13). The treatment resulted in 65% of response and 55% of remission rates. Significant reductions in depressive and anxiety symptoms and increase in self-esteem and sleep quality were observed as gCBT responses. Moreover, after treatment, patients regulated their previously deregulated salivary cortisol awakening response and sleep quality toward healthy parameters. These improvements were correlated among themselves and dependent of remission outcome. Remitted patients showed larger improvements than non-remitted for all psychophysiological domains, except for serum cortisol that significantly changed only for no-remitted patients after gCBT but did not reached controls levels. Further, better baseline sleep quality was predictor of remission. The psychophysiological changes found support the use of gCBT as monotherapy treatment for mild-moderate MDD, corroborate the importance of the observation of the patients in theirs whole sociopsychophysiological condition since they are related to remission outcome and then stimulate further studies of validation of clinical protocols that work on all of these psychophysiological domains studied.
Trial Registration U1111–1215-4472. Registered 21 August 2018, http://www.ensaiosclinicos.gov.br/rg/RBR-3npbf8/
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The recognition and uses of alternative and complementary therapies of pharmacological treatments, such as psychotherapy, physical exercise, nutraceutical and psychedelic substances, have been increasing in the management of depressive symptoms (Haller, Anheyer, Cramer, & Dobos, 2019). Despite antidepressants are still the most adopted treatment for Major Depressive Disorder (MDD), patients have shown a significant preference for psychotherapies over pharmacotherapy. It is probably due to the relative low efficacy of these drugs, which are not fully efficient to all patients, and due to the induction of undesirable side effects, which may imply in a low adherence to treatment (Cipriani et al., 2018). Consequently, the MDD prevalence has increased worldwide (Liu et al., 2020), mostly in those populations with a moderate-severe childhood maltreatment history who has increased risk to MDD, mainly in men (Pompili et al., 2014).
Among the psychotherapeutic approaches the Cognitive-Behavioral Therapy (CBT) is the most used modality for the treatment of MDD (Baardseth et al., 2013) and shows a significant adjunctive effect to pharmacological treatment, helping in the reduction of depressive symptoms and avoiding the MDD recurrence (Dunlop et al., 2019). However, studies analyzing CBT as monotherapy for the treatment of MDD are less conclusive and its efficacy has been related to MDD severity (Davey et al., 2019). Compared to individual CBT, the group CBT offers some advantages, such as reduced cost and sharing the experiences, which can help in more effective and faster acceptance of their own problems. However, this modality of CBT is less used and studied (Schaub et al., 2018).
The MDD cognitive theory points out that the patients have unfair thoughts and beliefs about themselves, an improper self-concept, lower self-esteem and feelings of shame. It is proposed that these cognitive aspects negatively modulate emotional, physiological, and behavioral reactions (J. S. Beck, 2011; Rosenberg, 1979). Therefore, the CBT aims to improve the thoughts on self-concept and self-esteem (Kolubinski, Frings, Nikčević, Lawrence, & Spada, 2018), which consequently contributes to the recovering of positive emotions and behaviors, as well as to the adjustment of physiological systems (J. S. Beck, 2011).
Now taking into account some physiological aspects; it is observed that a lower registration of sensory input is related to larger depressive symptoms (Serafini et al., 2017), and a positive feedback between depressive and anxious symptoms seems to be mediated by a dysfunctional hyperactivation of amygdala (He et al., 2019). Therefore, the simultaneous improvements in both symptoms may be critical to a satisfactory MDD treatment response, including in those MDD patients without a clinical diagnoses of anxiety comorbidities (de Azevedo Cardoso et al., 2014).
Moreover, although MDD diagnosis does not include pathophysiological characteristics (American Psychiatric Association, 2014), changes in the Hypothalamus-Pituitary-Adrenal (HPA) axis function and cortisol levels been observed in MDD patients (Dedovic & Ngiam, 2015). Cortisol changes seem to correlate with sleep disturbances, high anxiety levels and rumination thoughts in depressive patients, closing a dysfunctional positive feedback system (LeMoult & Joormann, 2014; Santiago et al., 2020). Therefore, the cortisol has been proposed as an important biomarker of MDD for diagnosis, prognosis, and follow-up (Kennis et al., 2020). However, studies that have measured the effects of CBT as a monotherapy on cortisol levels of MDD patients are few and inconsistent (Fischer, Strawbridge, Vives, & Cleare, 2017; Holland, Schatzberg, O’Hara, Marquett, & Gallagher-Thompson, 2013).
Therefore, considering the physiological pathways that link HPA axis, sleep disturbances, anxious and depressive symptoms (LeMoult & Joormann, 2014; Santiago et al., 2020), the potential of CBT to treat comorbidities symptoms (McEvoy, Burgess, & Nathan, 2014; Thimm & Antonsen, 2014), as well as its action on self-esteem (J. S. Beck, 2011; Kolubinski et al., 2018). Besides, the trend of the recent studies in the fields of personalized medicine and precision psychiatry that has suggested a whole sociopsychophysiological understanding of the patients (Fernandes et al., 2017), we assessed the potential responses to CBT through several psychophysiological domains, such as depression and anxiety symptoms, self-esteem, sleep quality and cortisol levels (salivary and serum), to 16 weeks of a group-based CBT monotherapy for treatment of major depression. Although the protocol used is based on MDD treatment, we expected to find a reduction of depressive and anxious symptoms, associated with increases in self-esteem and in sleep quality, as well as an adjustment of cortisol levels, especially in remitted patients.
Methods
This open-label trial was conducted at the Federal University of Rio Grande do Norte (UFRN) between 2018 and 2019. It has been approved by the UFRN Human Ethics Committee (2,628,202) and registered at http://clinicaltrials.gov (U1111–1215-4472). The procedures of this study comply with the ethical standards of the relevant national and institutional committees for human experimentation and with the Declaration of Helsinki of 1975, revised in 2008. All subjects provided written informed consent prior to participation and it was ensured freedom to withdraw from the study at any time and without any prejudice. All study’s information has been kept confidential.
Volunteers
The recruitment of volunteers was performed by advertising on radio, social and academic media. All subjects have undergone a full clinical evaluation performed by a single trained psychiatrist including anamnesis and mental health evaluation assessed by Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) and the Hamilton Depression Rating Scale (HAM-D), which was used to assess the MDD diagnosis. After screening, the volunteers were grouped as follows:
-
Patients group (PG): 30 volunteers (16 women and 14 men) diagnosed with mild to moderate MDD without other mental or physical (metabolic and inflammatory) comorbidities were included in this group and selected to start the treatment. Other exclusion criteria were the previous use of antidepressant and current use of antidepressants, anxiolytic, corticoids, or drugs with action on neurovegetative functions, mood, and cognition. Therefore, the patients were drug-free during this study.
-
Control group (CG): 25 healthy volunteers (13 women and 12 men) with similar socio-demographic characteristics of the patients group, without history or current diagnosis of physical (metabolic and inflammatory), neurological or mental disease, and no use of regular medication with action on neurovegetative functions, mood and cognition. This group did not receive treatment or intervention.
Study Design
All volunteers (PG and CG) slept, individually, one night at the Laboratory of Neurobiology and biological rhythms of UFRN, when the Pittsburgh Sleep Quality Index (PSQI) (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) was assessed. In the following morning, around 6 a.m., the saliva and blood were collected to measure the salivary cortisol awakening response (CAR) and serum cortisol, respectively. Prior the patients started the treatment, the psychometric scales were assessed: Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI) and Rosenberg Self-Esteem Scale (RSE). Over the following 16 weeks, patients underwent the treatment. After that, up to one week from the end of treatment, patients slept once again in the sleep lab. Sleep quality was assessed by PSQI and blood and saliva were collected in the following morning. A new clinical evaluation with the same psychiatrist was conducted and the psychometric scales (BDI, BAI and RSE) and Hamilton Depression Rating Scale were assessed.
Treatment
Patients were treated with group Cognitive-Behavioral Therapy (gCBT) in monotherapy for 16 weeks; 12 weekly sessions and two fortnightly sessions (reinforcement and closing sessions). The duration of each session was about 2 h and patients comprised 3 therapeutic groups with 10 individuals per group, in average. The therapeutic protocol used was adapted from (Bieling, McCabe, & Antony, 2006). The main adaptation made was shortening the original protocol; however, the contents and the frequency of the sessions were maintained. The therapy was applied by a team headed by a trained psychologist with expertise in the cognitive-behavioral approach, a co-therapist, and an observer.
As an ethical consideration, individual sessions were held in case of decompensating or risk of suicide. On average, there was one individual session per patient. At the end of the study patients that had not achieved remission were included as outpatients in Psychology Service or/and psychiatry at Hospital Universitário Onofre Lopes, both from UFRN.
Instruments
Hamilton Depression Rating Scale (Hamilton, 1960) was used to assess the MDD diagnosis, its severity and the remission at the end of treatment. The HAM-D is a semi-structured interview for identification of frequency and intensity of depressive symptoms performed by a trained psychiatrist. It is one of the most usual tools to assess depressive symptoms and, because of that, was adopted as the primary outcome in this trial (Howland, 2008). A single psychiatrist did the evaluation on each patient, at the beginning and the end of the study. The HAM-D has 4 categories of classification: a) mild major depression 10 < scores < 13; b) moderate: 14 < scores < 17 and c) severe: scores >17. The Beck Depression Inventory (Beck, Steer, & Brown, 1996) is a 21 items, self-reported instrument that assesses depressive symptoms during the last week and proposes their classification in four levels: “Minimal” (0–11 scores), “mild” (12–19 scores), “moderate” (20–35 scores) and “severe” symptoms (36–63 scores). It was validated for adult Brazilian clinical population (Gomes-Oliveira, Gorenstein, Neto, Andrade, & Wang, 2012) (α = 0.93). The Beck Anxiety Inventory (Beck, Epstein, Brown, & Steer, 1988) was used to assess the anxiety state. It is a 21- question, self-reporting scale, which measures somatically, affectively and cognitively the anxiety level during the last week and proposes its classification into 4 levels as following: “minimum” (0–10 scores), “mild” (11–19 scores), “moderate” (20–30 scores) and “severe” (31–63 scores). This instrument was validated to adult Brazilian population and was proved suitable for use in clinical population (α = 0.83–0.92) (Cunha, 2001). Rosenberg Self-Esteem Scale (Rosenberg, 1979) is a 10 items self-completion instrument to measure self-concept traits. The total score ranges from 0 to 40. It was validated to adult Brazilian population (α = 0.90) (Hutz & Zanon, 2011). In this study conventional or non-inverted correction was adopted. Pittsburgh Sleep Quality Index (PSQI) is a self-assessment instrument that has 7 components, and it is used to measure sleep quality. The overall score ranges from 0 to 21 points, in which can be categorized into good sleep quality (0–4 points), poor sleep quality (5–10 points) and suggestive of sleep disorder (greater than 10 points). This instrument was validated to adult Brazilian population (Bertolazi et al., 2011) (α = 0.82).
Sampling and Dosage of Biological Material
The salivary cortisol awakening response is a feedfoward mechanism that prepares the individual to daily activities; therefore, it undergoes less modulation due to acute stressors. CAR changes have been associated with dysfunction of the HPA axis, observed in some mental illnesses (Wüst et al., 2000). On the other hand, the serum cortisol is highly influenced by acute and chronic stressors as well as by circadian changes, thus it is a measure that has an acute value. Therefore, since that these two measures provide distinct information, they were both selected and analyzed aiming to help in the consolidation of cortisol as biomarker of MDD (Kennis et al., 2020).
To account for the circadian oscillation in cortisol levels, both saliva and blood were always collected about 6 a.m. Volunteers were fasting for approximately 8 h. The samplings were made using Salivette® devices (Sarstedt Numbrecht, Germany), two saliva samples were collected by the volunteers under the researcher supervision. The 1st sampling was done immediately at awakening and the 2nd was done after 30 min. During the sampling participants were restricted to the bed and were instructed to rest and not eat or drink. The blood collections were performed, using disposable perforating material (needle and syringe), immediately after saliva collections by trained laboratory technicians or researcher. Salivary cortisol was measured by DRG® Salivary Cortisol ELISA kit SLV-4635 and Serum cortisol by DRG® Cortisol ELISA Kit 1887.
Statistical Analysis
Depressive (BDI) and anxiety symptoms (BAI), sleep quality (PSQI), self-esteem (RSE), serum cortisol (SC) and salivary cortisol awakening response (CAR) were the quantitative dependent variables evaluated in this study. Groups (PG and CG) and patient’s remission (remitted: R and non-remitted: NR) were considered categorical independent variables. Sociodemographic characteristics, such as gender, age, education, and income were investigated as covariates. The CAR was calculated from the change (%) in salivary cortisol between 0 and 30 min after awakening (Wüst et al., 2000).
The effect of sociodemographic characteristics was assessed by Wilcoxon sum rank test (for age) and chi-square test (for gender, education level and income). The effect of intervention was assessed by Wilcoxon sign rank test both considering the patients group (PG) as a whole, as well as stratifying it by remission condition (remitted patients [R] and non-remitted [NR]). In order to check whether the changes achieved with the intervention were comparable with a healthy pattern, we performed a Wilcoxon sum rank test comparing the baseline and post-treatment values to a healthy control. The effect sizes (r) and its bootstrapped confidence interval (CI 95%, 1000 resamples) are reported as r [lower limit, upper limit], and were obtained from the rcompanion package. The correlations between biomarkers after the treatment were assessed for remitted and non-remitted patients using Spearman’s correlation (⍴). Finally, a binary logistic regression was performed to find potential baseline predictors of remission, using non-remitted patients as reference (NR = 0; R = 1). Nagelkerke’s pseudo-R2 and OR is reported as importance measures of the prediction.
All analyzes were performed using R (4.0.2), assuming a significance level of p < 0.05.
Results
Sample Characteristics and Study Workflow
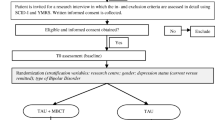
From the total of 385 volunteers, 55 were selected in the screening phase (CG: 25 and PG: 30). All volunteers were Brazilian young adults. We observed a slight bias towards women in the groups (PG = 55%, CG = 52%), further, most part of both groups was undergraduate students and had a low income (Supplementary Information Table S1). Sociodemographic characteristics showed no difference between groups (GC and PG) (Supplementary Information Table S1). Patients showed in average a mild level of major depression (HAM-D = 12.8 ± 3.55) before the treatment (Supplementary Information 1) and from the 30 patients who started the treatment, 20 completed the 16 weeks proposed. The consolidated standards for clinical trial reports (CONSORT) are shown in Fig. 1.
Overall Effect of Group Cognitive Behavioral Therapy
The group cognitive behavioral therapy induced large changes, with 65% of response (that means reduction in HAM-D > 50%) and 55% of remission rate. After treatment, patients showed a moderate to large significant decrease in depressive symptoms (HAM-D: V = 127, p = 0.002, r = −0.682 [CI -0.818, −0.445]; BDI: V = 209, p <0.0001, r = −0.868 [CI -0.879, −0.827]), moderate reductions in anxiety (BAI: V = 199.5, p < .0001, r = −0.789 [CI -0.879, −0.590]) and in the PSQI total scores, that means a moderate improvement of sleep quality (PSQI: V = 147, p = 0.008, r = −0.602 [CI -0.816, −0.304]), further also was observed a moderate increase in self-esteem (RSE: V = 15.5, p = 0.002, r = 0.682 [CI 0.427, 0.841]) (Fig. 1a and Supplementary Information Table S2). No changes were found for serum (SC: V = 130, p = 0.368) and salivary cortisol awakening response (CAR (%): V = 142, p = 0.173) (Fig. 2a and Supplementary Information Table S2).
When we analyzed the patient group by the condition of remission after gCBT, the remitted patients showed significant large improvements in depressive (HAM-D: V = 45, p = 0.009, r = −0.805 [CI -0.889, −0.666]; BDI: V = 66, p = 0.001, r = −0.883 [CI -0.892, −0.886]) and anxiety symptoms (V = 65, p = 0.005, r = −0.859 [CI -0.892, −0.727]), self-esteem (V = 1, p = 0.008, r = 0.814 [CI 0.591, 0.889]) and sleep quality (V = 42.5, p = 0.020, r = −0.721 [CI -0.892, −0.395]), in addition to a marginal decrease in cortisol awakening response (V = 55, p = 0.056, r= −0.591 [CI -0.889, −0.134]). No changes were found for serum cortisol for remitted patients (V = 24, p = 0.465) (Fig. 1b, Supplementary Information Table 3). In contrast, the only changes showed by non-remitted patients were large decreases in serum cortisol (V = 43, p = 0.012, r = − 0.810 [CI -0.897, −0.533]) and self-perceived depressive symptoms (BDI V = 44, p = 0.008, r = −0.850 [CI -0.897, −0.653]). No changes were found for depressive symptoms (HAM-D: V = 21, p = 0.270), self-perceived anxiety symptoms (V = 37.5, p = 0.085), sleep quality (V = 37, p = 0.096), self-esteem (V = 9, p = 0.232) and CAR (V = 27, p = 0.652) (Fig. 2b, Supplementary Information Table 3).
Effect size (r) and 95% bootstrapped confidence interval of psychophysiological outcomes in (a) patients’ group as a whole after a group-based Cognitive-Behavioral Therapy (gCBT), and in the (b) patients’ group stratified in remitted (R: blue) / non-remitted (NR: red) after gCBT. Hamilton Depression Rating Scale (HAM-D), Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI), Rosenberg self-esteem scale (RSE), Pittsburgh Sleep Quality Index (PSQI), Serum cortisol (SC; μg/dL), Salivary cortisol awakening response (CAR; %). When any limit of the confidence interval crosses the 0 (r = 0.00), it means that no statistical significance is found (p > 0.05)
After treatment, for remitted patients depressive scores (HAM-D) assessed by the psychiatrist was correlated with the self-perceived depressive symptoms (BDI) (⍴ = 0.69, p = 0.029). The lower depressive symptoms were correlated with lower self-perceived anxiety symptoms (HAM-D ⍴ = 0.76, p = 0.007 and BDI p = 0.72, p = 0.015) and higher self-esteem (HAM-D p = −0.69, p = 0.037 and BDI ⍴ = −0.74, p = 0.005). In addition, lower self-perceived depressive and anxiety symptoms were also correlated with better sleep quality (PSQI) (BDI ⍴ = 0.65, p = 0.028 and BAI ⍴ = 0.63, p = 0.029). Also, better sleep quality was correlated with higher self-esteem (p = −0.85, p = 0.013) (Fig. 3).
Correlations (Spearman’s ⍴) between psychophysiological outcomes in non-remitted (NR, light red grid at the lower left panel) and remitted (R, light blue grid at the upper right panel) patients after a group-based Cognitive-Behavioral Therapy (16 weeks). Only significant correlations (p < 0.05) are shown. Colors of plotted ⍴ values denote the direction of correlation (red = negative; blue = positive). Hamilton Depression Rating Scale (HAM-D), Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI), Rosenberg self-esteem scale (RSE), Pittsburgh Sleep Quality Index (PSQI), Serum cortisol (SC; μg/dL), Salivary cortisol awakening response (CAR; %)
For non-remitted patients, significant correlations were restricted to BDI, BAI, and RSE. Specifically, larger RSE correlated with lower BDI (p = −0.86, p = 0.002) and BAI (p = −0.78, p = 0.012), and the two latter were positively correlated between them (p = 0.83, p = 0.01) (Fig. 3).
Comparisons with a Healthy Control Group
When compared to a healthy control group (CG), before the intervention, patients group (PG) scored higher in HAM-D (W = 0.00, p <0.0001, r = − 0.866 [CI - 0.881, − 0.817]), presented worse sleep quality (W = 80.5, p = 0.0001, r = − 0.580 [CI - 0.744, − 0.359]), high levels of serum cortisol (W = 130, p = 0.005, r = − 0.408 [CI - 0.635, − 0.119]) and reactivity of cortisol awakening response (W = 136, p = 0.009, r = − 0.389 [CI - 0.626, − 0.099]) (Supplementary Information 2, Fig. 3). Comparing the PG after the treatment with CG, the HAM-D (W = 66.5, p < 0.0001, r = − 0.644 [CI - 0.818, − 0.397]) and serum cortisol (W = 137, p = 0.01, r = − 0.385 [− 0.639, − 0.073]) remained significantly different. However, it must be highlighted that there was a 0.22-point decrease in HAM-D effect size, suggesting a gCBT-induced a satisfactory reduction on depressive symptoms although this intervention did not totally exclude them. Moreover, an adjustment of sleep quality (W = 169, p = 0.064) and in CAR reactivity (W = 194, p = 0.201) were observed and both reached healthy levels (Supplementary Information 2, Fig. 4).
Effect sizes (r) and bootstrapped 95% confidence intervals of psychophysiological outcomes at baseline (orange) and after (green) 16 weeks of group-based Cognitive-Behavioral Therapy (gCBT) of patients group (PG) in comparison to a healthy control group (CG). Hamilton Depression Rating Scale (HAM-D), Pittsburgh Sleep Quality Index (PSQI), Serum cortisol (SC; μg/dL), Salivary cortisol awakening response (CAR; %). When any limit of the confidence interval crosses the 0 (r=0.00), it means that no statistical significance is found (p > 0.05)
Predictors of Remission
Between psychophysiological outcomes, only the baseline sleep quality (B = −0.482, OR = 0.62, p = 0.039, Nagelkerke’s pseudo-R2 = 0.379) predicted remission after the 16 weeks of group-based Cognitive-Behavioral Therap. Lower values of PSQI before the intervention, which means better sleep quality, are associated with remission achievement (Fig. 5, Table 1). Moreover, the sociodemographic characteristics did not influence on remission outcome (Supplementary Information 1).
The baseline Pittsburgh Sleep Quality Index (PSQI) as predictor of remission outcome after 16 weeks of group-based Cognitive-Behavioral Therapy (gCBT). Lower values of PSQI before the intervention, which means better sleep quality, are associated with remission achievement. Shaded area represents the confidence interval (95%) of the predicted values (sigmoidal line). Non-remitted patients (NR) and remitted patients (R)
Discussion
In this study we observed a remission rate of 55% for patients diagnosed with mild-moderate major depression after 16 weeks of a group-based Cognitive-Behavioral therapy (gCBT) as monotherapy. This response was followed by reductions in self-perception of depressive symptoms and anxiety and increases in sleep quality and self-esteem. Moreover, after treatment, patients regulated their previous deregulated salivary cortisol awakening response and sleep quality to healthy parameters. A secondary analysis showed that these improvements were dependent of remission condition, and they were correlated among them. Remitted patients showed stronger responses of those outcomes (depressive and anxiety symptoms, sleep quality and self-esteem) plus a reduction of salivary cortisol awakening response, whereas non-remitted ones showed larger reduction in serum cortisol levels and self-perceived depressive symptoms. Moreover, a better sleep quality before treatment was predictive of remission achievement.
Distinct remission rates for CBT monotherapy as treatment for MDD are observed in the literature. Some factors can contribute to this discrepancy, such as patient age, the severity of disease, the previous use of antidepressants, the duration of CBT and the instruments used to measure the remission outcome (Davey et al., 2019; Li et al., 2018). In this study we had a remission rate slightly above the level usually seen in literature; we can speculate that this result was achieved due to the use of a group approach, as well as due to the homogeneity of groups. Those groups were composed of young undergraduate students; therefore, it could increase the identification among patients and the quality of their social support. The social support is seen as an active mechanism for resilience (Galvão-Coelho, Silva, & de Sousa, 2015) and it should be stimulated for management of MDD.
Moreover, 65% of patients showed a response to treatment and besides the moderate reduction in the depressive scores analyzed by a specialist; the self-reported depressive symptoms were significantly large reduced after gCBT as well, even for patients who did not achieve a complete remission. Moreover, after gCBT, the patients showed significant improvements in anxiety symptoms. The moderate anxiety levels found at baseline decreased to mild levels after intervention, especially in remitted patients. Strong positive correlations between improvements in depressive and anxious symptoms were found independent of remission condition. Similar to our findings, the anxiety relief is also perceived in other CBT protocols focused on the treatment of major depression (McEvoy et al., 2014; Thimm & Antonsen, 2014).
We also observed an increase of self-esteem in response to treatment, specifically in remitted patients, together with correlations between increases in self-esteem and reduction of depressive and anxious symptoms, which were observed independent of remission condition. Other studies also pointed out the importance of the improvement in self-esteem after cognitive psychotherapies (Kolubinski et al., 2018). Therefore, regardless of being low self-esteem the cause or the consequence of negative mood (Sowislo & Orth, 2013), the correlation between them must be considered and this domain better worked in psychotherapy approaches.
Before the treatment, patients disclosed worse sleep quality than the control group of healthy volunteers, showing suggestive sleep disturbance or poor sleep quality. After the intervention we noticed an improvement of sleep quality, when the patients were able to reach healthy patterns of sleep quality. However, after a secondary analysis this overall change observed to whole patient group seems to be due to the large decrease of PSQI score (which means an improvement of sleep quality) mainly found for remitted patients, in contrast to the marginal decrease found for non-remitted. Moreover, for those who achieved remission, it was observed a moderate correlation between improvements in sleep quality and depressive symptoms, corroborating the observation of different sleep responses to gCBT among patients with different remission results. We must also highlight that a better sleep quality before treatment was predictive of remission achievement. These results may contribute to the understanding of the relationship between changes in sleep quality and depressive mood (Sbarra & Allen, 2009). Sleep complaints tend to occur before the first depressive episode of MDD; in addition, its persistence after treatment is associated with higher rates of recurrence (Ohayon & Roth, 2003). Also, it is suggested that increased levels of anxiety are related to the presence of sleep disorders in young adult population with major depression (Nyer et al., 2013). Therefore, these results suggest the inclusion of tools to improve sleep quality in CBT treatment for major depression and highlight the importance of its measurements before treatment and at follow-ups.
Moreover, at baseline patients showed hypercortisolemia and increased salivary cortisol awakening response when compared to healthy controls. Hyperactivity of HPA axis is an alteration usually seen in young MDD patients (Lopez-Duran, Kovacs, & George, 2009) and it is also a predictor of low response of CBT and disease recurrence (Holland et al., 2013), although we did not find this here. Some studies suggested that hypercortisolemia results from the impairment of the negative feedback loop of the HPA axis (Dean & Keshavan, 2017). An improvement in CAR was observed to remitted patients as response of treatment, while a reduction in serum cortisol was found for non-remitted. Despite this, only CAR reactivity was regulated to healthy control levels after the treatment, which is an interesting result since it is a stronger maker of dysfunction of the HPA axis and its change is associated to mental illnesses (Wüst et al., 2000). Although the regulated HPA axis function is critical for body-mind homeostasis, few studies analyze it and part of them failed in finding an adjustment of HPA axis after psychotherapy treatment for MDD (Holland et al., 2013). Furthermore, cortisol changes is not usually considered during antidepressant choice, so the mode of action of this drugs on the HPA axis is complex and depends on the type of antidepressant and the duration of treatment (Schüle, 2007).
Some limitations must be addressed, such as the modest sample size and a single data collection per subject before and after the treatment. Therefore, studies with larger populations of patients with major depression and design with sequential data measurements throughout the treatment are encouraged. Moreover, the lack of a placebo-controlled group is another limitation to be considered in our study. Some trials in psychology field have been using non-active or active placebo, such as the waiting list and attentional placebo or nonspecific treatment component control, respectively. However, some ethical concerns are observed for the use of placebo group. Moreover, the use of an active placebo must be done with caution for it does not include active therapeutic approaches (Guidi et al., 2018). Therefore, this issue still complex in psychological trials mainly with long interventions as that was adopted in this study.
Therefore, the positive results corroborate the importance of the observation of the patients in theirs whole sociopsychophysiological condition and the improvements observed on emotional, cognitive, and physiological domains after 16 weeks of a group-based CBT monotherapy stimulate it uses for treatment of mild-moderate major depression, mainly because it is a tool with low cost and no described side effects. In addition, as most part of current CBT approaches address one or two of those psychophysiological domains studied, and we found a relationship with remission outcome for most of them, these results support further studies that may explore the validation of clinical protocols that will simultaneously work on all these psychophysiological domains studied. For instance, adding some tools as mindfulness, sleep hygiene, self-concept and self-esteem, aiming to improve the treatment outcome.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
American Psychiatric Association. (2014). DSM-5: Manual diagnóstico e estatístico de transtornos mentais. Porto Alegre, RS: Artmed Editora.
Baardseth, T. P., Goldberg, S. B., Pace, B. T., Wislocki, A. P., Frost, N. D., Siddiqui, J. R., Lindemann, A. M., Kivlighan III, D. M., Laska, K. M., del Re, A. C., Minami, T., & Wampold, B. E. (2013). Cognitive-behavioral therapy versus other therapies: Redux. Clinical Psychology Review, 33(3), 395–405. https://doi.org/10.1016/j.cpr.2013.01.004.
Beck, J. (2011). Cognitive behavior therapy: Basics and beyond. New York, NY: The Guilford Press.
Beck, A. T., Epstein, N., Brown, G., & Steer, R. A. (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. https://doi.org/10.1037/0022-006X.56.6.893.
Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Manual for the Beck depression inventory-II. San Antonio, TX: Psychological Corporation.
Bertolazi, A. N., Fagondes, S. C., Hoff, L. S., Dartora, E. G., da Silva Miozzo, I. C., de Barba, M. E. F., & Menna Barreto, S. S. (2011). Validation of the Brazilian Portuguese version of the Pittsburgh sleep quality index. Sleep Medicine, 12(1), 70–75. https://doi.org/10.1016/j.sleep.2010.04.020.
Bieling, P. J., McCabe, R. E., & Antony, M. M. (2006). Cognitive-behavioral therapy in groups. New York, NY: The Guilford press.
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. https://doi.org/10.1016/0165-1781(89)90047-4.
Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., Leucht, S., Ruhe, H. G., Turner, E. H., Higgins, J. P. T., Egger, M., Takeshima, N., Hayasaka, Y., Imai, H., Shinohara, K., Tajika, A., Ioannidis, J. P. A., & Geddes, J. R. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. The Lancet, 391(10128), 1357–1366. https://doi.org/10.1016/S0140-6736(17)32802-7.
Cunha, J. A. (2001). Manual da versão em português das Escalas Beck. São Paulo, SP: Casa do Psicólogo.
Davey, C. G., Chanen, A. M., Hetrick, S. E., Cotton, S. M., Ratheesh, A., Amminger, G. P., Koutsogiannis, J., Phelan, M., Mullen, E., Harrison, B. J., Rice, S., Parker, A. G., Dean, O. M., Weller, A., Kerr, M., Quinn, A. L., Catania, L., Kazantzis, N., McGorry, P. D., & Berk, M. (2019). The addition of fluoxetine to cognitive behavioural therapy for youth depression (YoDA-C): A randomised, double-blind, placebo-controlled, multicentre clinical trial. The Lancet Psychiatry, 6(9), 735–744. https://doi.org/10.1016/S2215-0366(19)30215-9.
de Azevedo Cardoso, T., Mondin, T. C., Spessato, B. C., de Avila Quevedo, L., de Mattos Souza, L. D., da Silva, R. A., & Jansen, K. (2014). The impact of anxious symptoms in the remission of depressive symptoms in a clinical trial for depression: Follow-up of six months. Journal of Affective Disorders, 168, 331–336. https://doi.org/10.1016/j.jad.2014.03.034.
Dean, J., & Keshavan, M. (2017). The neurobiology of depression: An integrated view. Asian Journal of Psychiatry., 27, 101–111. https://doi.org/10.1016/j.ajp.2017.01.025.
Dedovic, K., & Ngiam, J. (2015). The cortisol awakening response and major depression: Examining the evidence. Neuropsychiatric Disease and Treatment, 11, 1181–1189. https://doi.org/10.2147/NDT.S62289.
Dunlop, B. W., LoParo, D., Kinkead, B., Mletzko-Crowe, T., Cole, S. P., Nemeroff, C. B., Mayberg, H. S., & Craighead, W. E. (2019). Benefits of sequentially adding cognitive-behavioral therapy or antidepressant medication for adults with nonremitting depression. American Journal of Psychiatry, 176(4), 275–286. https://doi.org/10.1176/appi.ajp.2018.18091075.
Fernandes, B. S., Williams, L. M., Steiner, J., Leboyer, M., Carvalho, A. F., & Berk, M. (2017). The new field of ‘precision psychiatry’. BMC Medicine, 15(1), 80. https://doi.org/10.1186/s12916-017-0849-x.
Fischer, S., Strawbridge, R., Vives, A. H., & Cleare, A. J. (2017). Cortisol as a predictor of psychological therapy response in depressive disorders: Systematic review and meta-analysis. British Journal of Psychiatry, 210(2), 105–109. https://doi.org/10.1192/bjp.bp.115.180653.
Galvão-Coelho, N. L., Silva, H. P. A., & de Sousa, M. B. C. (2015). Resposta ao estresse: II. Resiliência e vulnerabilidade. Estudos de Psicologia, 20(2), 72–81. https://doi.org/10.5935/1678-4669.20150009.
Gomes-Oliveira, M. H., Gorenstein, C., Neto, F. L., Andrade, L. H., & Wang, Y. P. (2012). Validation of the Brazilian Portuguese version of the Beck depression inventory-II in a community sample. Revista Brasileira de Psiquiatria, 34(4), 389–394. https://doi.org/10.1016/j.rbp.2012.03.005.
Guidi, J., Brakemeier, E. L., Bockting, C. L. H., Cosci, F., Cuijpers, P., Jarrett, R. B., Linden, M., Marks, I., Peretti, C. S., Rafanelli, C., Rief, W., Schneider, S., Schnyder, U., Sensky, T., Tomba, E., Vazquez, C., Vieta, E., Zipfel, S., Wright, J. H., & Fava, G. A. (2018). Methodological recommendations for trials of psychological interventions. Psychotherapy and Psychosomatics. https://doi.org/10.1159/000490574.
Haller, H., Anheyer, D., Cramer, H., & Dobos, G. (2019). Complementary therapies for clinical depression: An overview of systematic reviews. BMJ Open, 9, e028527. https://doi.org/10.1136/bmjopen-2018-028527.
Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23(1), 56–62. https://doi.org/10.1136/jnnp.23.1.56.
He, C., Gong, L., Yin, Y., Yuan, Y., Zhang, H., Lv, L., Zhang, X., Soares, J. C., Zhang, H., Xie, C., & Zhang, Z. (2019). Amygdala connectivity mediates the association between anxiety and depression in patients with major depressive disorder. Brain Imaging and Behavior, 13(4), 1146–1159. https://doi.org/10.1007/s11682-018-9923-z.
Holland, J. M., Schatzberg, A. F., O’Hara, R., Marquett, R. M., & Gallagher-Thompson, D. (2013). Pretreatment cortisol levels predict posttreatment outcomes among older adults with depression in cognitive behavioral therapy. Psychiatry Research, 210(2), 444–450. https://doi.org/10.1016/j.psychres.2013.07.033.
Howland, R. H. (2008). Sequenced treatment alternatives to relieve depression (STAR*D). Journal of Psychosocial Nursing and Mental Health Services, 46(9), 21–24. https://doi.org/10.3928/02793695-20080901-06.
Hutz, C. S., & Zanon, C. (2011). Revision of the adaptation, validation, and normatization of the Roserberg self-esteem scale. Avaliação Psicológica, 10(1), 41–9.
Kennis, M., Gerritsen, L., van Dalen, M., Williams, A., Cuijpers, P., & Bockting, C. (2020). Prospective biomarkers of major depressive disorder: A systematic review and meta-analysis. Molecular Psychiatry, 25(2), 321–338. https://doi.org/10.1038/s41380-019-0585-z.
Kolubinski, D. C., Frings, D., Nikčević, A. V., Lawrence, J. A., & Spada, M. M. (2018). A systematic review and meta-analysis of CBT interventions based on the Fennell model of low self-esteem. Psychiatry Research, 267, 296–305. https://doi.org/10.1016/j.psychres.2018.06.025.
LeMoult, J., & Joormann, J. (2014). Depressive rumination alters cortisol decline in major depressive disorder. Biological Psychology, 100, 50–55. https://doi.org/10.1016/j.biopsycho.2014.05.001.
Li, J.-M., Zhang, Y., Su, W.-J., Liu, L.-L., Gong, H., Peng, W., & Jiang, C.-L. (2018). Cognitive behavioral therapy for treatment-resistant depression: A systematic review and meta-analysis. Psychiatry Research, 268, 243–250. https://doi.org/10.1016/j.psychres.2018.07.020.
Liu, Q., He, H., Yang, J., Feng, X., Zhao, F., & Lyu, J. (2020). Changes in the global burden of depression from 1990 to 2017: Findings from the global burden of disease study. Journal of Psychiatric Research, 126, 134–140. https://doi.org/10.1016/j.jpsychires.2019.08.002.
Lopez-Duran, N. L., Kovacs, M., & George, C. J. (2009). Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology, 34(9), 1272–1283. https://doi.org/10.1016/j.psyneuen.2009.03.016.
McEvoy, P. M., Burgess, M. M., & Nathan, P. (2014). The relationship between interpersonal problems, therapeutic alliance, and outcomes following group and individual cognitive behaviour therapy. Journal of Affective Disorders., 157, 25–32. https://doi.org/10.1016/j.jad.2013.12.038.
Nyer, M., Farabaugh, A., Fehling, K., Soskin, D., Holt, D., Papakostas, G. I., Pedrelli, P., Fava, M., Pisoni, A., Vitolo, O., & Mischoulon, D. (2013). Relationship between sleep disturbance and depression, anxiety, and functioning in college students. Depression and Anxiety., 30, 873–880. https://doi.org/10.1002/da.22064.
Ohayon, M. M., & Roth, T. (2003). Place of chronic insomnia in the course of depressive and anxiety disorders. Journal of Psychiatric Research, 37(1), 9–15. https://doi.org/10.1016/S0022-3956(02)00052-3.
Pompili, M., Innamorati, M., Lamis, D. A., Erbuto, D., Venturini, P., Ricci, F., Serafini, G., Amore, M., & Girardi, P. (2014). The associations among childhood maltreatment, “male depression” and suicide risk in psychiatric patients. Psychiatry Research, 220(1–2), 571–578. https://doi.org/10.1016/j.psychres.2014.07.056.
Rosenberg, M. (1979). Conceiving the self. New York, NY: Basic.
Santiago, G, T, P., de Menezes Galvão, A, C., de Almeida, R, N., Mota-Rolim, S, A., Palhano-Fontes, F., Maia-de-Oliveira, J, P., de Araújo D, B., Lobão-Soares B., Galvão-Coelho N, L. (2020). Changes in Cortisol but Not in Brain-Derived Neurotrophic Factor Modulate the Association Between Sleep Disturbances and Major Depression. Frontiers in Behavioral Neuroscience, 14. https://doi.org/10.3389/fnbeh.2020.00044
Sbarra, D. A., & Allen, J. J. B. (2009). Decomposing depression: On the prospective and reciprocal dynamics of mood and sleep disturbances. Journal of Abnormal Psychology, 118(1), 171–182. https://doi.org/10.1037/a0014375.
Schaub, A., Goldmann, U., Mueser, T. K., Goerigk, S., Hautzinger, M., Roth, E., Charypar, M., Engel, R., & Möller, H. J. (2018). Efficacy of extended clinical management, group CBT, and group plus individual CBT for major depression: Results of a two-year follow-up study. Journal of Affective Disorders, 238, 570–578. https://doi.org/10.1016/j.jad.2018.05.081.
Schüle, C. (2007). Neuroendocrinological mechanisms of actions of antidepressant drugs. Journal of Neuroendocrinology, 19(3), 213–226. https://doi.org/10.1111/j.1365-2826.2006.01516.x.
Serafini, G., Gonda, X., Canepa, G., Pompili, M., Rihmer, Z., Amore, M., & Engel-Yeger, B. (2017). Extreme sensory processing patterns show a complex association with depression, and impulsivity, alexithymia, and hopelessness. Journal of Affective Disorders., 210, 249–257. https://doi.org/10.1016/j.jad.2016.12.019.
Sowislo, J. F., & Orth, U. (2013). Does low self-esteem predict depression and anxiety? A meta-analysis of longitudinal studies. Psychological Bulletin, 139(1), 213–240. https://doi.org/10.1037/a0028931.
Thimm, J. C., & Antonsen, L. (2014). Effectiveness of cognitive behavioral group therapy for depression in routine practice. BMC Psychiatry, 14, 292. https://doi.org/10.1186/s12888-014-0292-x.
Wüst, S., Wolf, J., Hellhammer, D. H., Federenko, I., Schommer, N., & Kirschbaum, C. (2000). The cortisol awakening response-normal values and confounds. Noise and health, 2(7), 79.
Acknowledgments
The authors are thankful to all volunteers for this study and to Federal University of Rio Grande do Norte, Brazil, for institutional support.
Author Agreement Statement
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Funding
This study was funded by National Science and Technology Institute for Translational Medicine (INCT-TM Fapesp 2014/50891–1; CNPq 465458/2014–9). NLGC is supported by CAPES Foundation from Brazilian Ministry of Education (Research Fellowship 88887.466701/2019–00) National Science and Technology Institute for Translational Medicine (INCT-TM Fapesp 2014/50891–1; CNPq 465458/2014–9). The funder did not have any role in the design of the study, collection, analysis, and interpretation of data and in drafting the manuscript.
Author information
Authors and Affiliations
Contributions
NLGC, YMV and NGS planned the clinical trial; ACLL did volunteers screening; YMV conducted treatment; ACMG and GMSJ carried out statistical analysis; all authors contributed to the manuscript.
Corresponding author
Ethics declarations
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All procedures were approved by the ethical committee of the Federal University of Rio Grande do Norte, Brazil (#2628,202) and was in accordance with the 1964 Declaration of Helsinki, revised in 2008.
Consent to Participate
Participants became aware of the study procedures and provided informed written consent prior to participation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Varela, Y.M., de Almeida, R.N., Galvão, A.d. et al. Psychophysiological responses to group cognitive-behavioral therapy in depressive patients. Curr Psychol 42, 592–601 (2023). https://doi.org/10.1007/s12144-020-01324-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12144-020-01324-9