Abstract
Background
The study aims to analyse the non-calcifying/Langerhans cell rich (NCLC) subtype of calcifying epithelial odontogenic tumour (CEOT).
Method
The features of cases of the NCLC subtype of CEOT noted in the English literature by PubMed as well as 3 new cases were reviewed.
Results
Overall, twenty-one cases were noted. Many were women in the fourth to sixth decades (male-to-female ratio =1 to 2). Radiologically, the lesion is often unilocular with resorption of the affected teeth. Nineteen of the 21 cases occurred in the maxilla, especially the anterior portion. On pathological examination, epithelial cells are noted in non-calcifying amyloid-rich fibrous stroma. The main differential diagnosis is the amyloid subtype of central odontogenic fibroma. Immunohistochemical studies revealed the tumour epithelial cells were positive for cytokeratins and p63 and contained CD1a, S-100, and langerin-positive Langerhans cells. On a median follow-up of 2 years, one patient had a recurrence one year after curettage.
Conclusion
The NCLC subtype of CEOT is unique as it contains significant numbers of Langerhans cells and has clinicopathological features distinctive from classic CEOT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A calcifying epithelial odontogenic tumour (CEOT) also known as the Pindborg tumour, is a benign odontogenic epithelial tumour that accounts for less than 1% of all odontogenic tumours. It was initially reported by Thoma and Goldman in 1946 [1]. The names used for the tumour were “ameloblastoma of unusual type with calcification,” “adenoid adamantoblastoma,” “cystic complex odontoma,” and “malignant odontoma” in the literature2. Pindborg described the tumour in detail by reporting three new cases and reviewing four cases in former literature in 1958 [2]. He suggested the division of the epithelial odontogenic tumours into ameloblastoma and CEOT [3]. In 1963, Shafer first applied the name “Pindborg tumour” for CEOT [4]. In 1971, CEOT was included in the first edition of the World Health Organisation (WHO) histological typing of odontogenic tumours, jaw cysts and allied lesions [5].
To date, approximately 430 cases of CEOT have been reported in the literature [6,7,8,9,10]. About two-thirds of all the reported cases were in the mandible, most often in the posterior areas. Radiographically, the tumour exhibits either a unilocular or a multilocular defect. The lesion is entirely radiolucent or contains calcified structures of varying size and density. The histological hallmarks of CEOT are discrete islands, strands, or sheets of polyhedral epithelial cells in a fibrous stoma. Also common are multiple concentric Liesegang ring calcifications and the deposits of amyloid-like material which are positive for Congo Red.
In addition to conventional CEOT, other variants have been reported successively, such as the clear cell variant [11], the non-calcifying Langerhans cell rich (NCLC) variant [12], cystic variant [13], and the pigmented variant [14]. In the section on odontogenic tumours of the latest 5th WHO classification of head and neck tumours published in 2022 [15], CEOT is divided into the clear cell, cystic/microcystic and NCLC subtypes. Lesions with mixed CEOT and adenomatoid odontogenic tumour (AOT) features should be classified as AOT subtypes [16].
Amongst the three subtypes of CEOT, only a small but not negligible number of case reports have been published on the NCLC subtype. The NCLC subtype of CEOT is rare and was first reported by Asano et al. in 1990 [17]. The unique clinical, imaging, and pathological manifestations of the CEOT, NCLC subtype distinguish it from the conventional CEOT. Furthermore, the pathological differentiation of CEOT, NCLC subtype from odontogenic fibromas, amyloid subtype is still controversial. The amyloid subtype of odontogenic fibroma is characterised by small dispersed epithelial nests embedded in a fibrous stroma, with amyloid deposits and Langerhans cells [15]. Thus, in this paper, we reported three original cases and critically reviewed all the cases with NCLC CEOT reported in the English literature to investigate their demographic characteristics, clinicopathological manifestations, and differentiation from the amyloid subtype of odontogenic fibroma for accurate diagnosis and management of patients with this entity.
Materials and Methods
The keywords searched included “calcifying epithelial odontogenic tumour,” “Pindborg tumour,” “Langerhans cell,” “clear cell,” and “non-calcifying” in PubMed. In addition, recent literature on the related entity “amyloid subtype of odontogenic fibroma” was also searched. All the found literature and the references of the retrieved literature were browsed and filtered. If the same case occurs in different pieces of literature, we selected only one entry. Meanwhile, we included three original cases from the Hospital of Stomatology, Sun Yat-sen University, China obtained by searching the pathology database.
The included cases were reviewed in their clinical, pathological, and imaging data, as well as the follow-up and prognosis information. All statistical calculations were performed by SPSS software (29.0, International Business Machines Corporation [IBM], Armonk, NY, USA). A p-value of < 0.05 (two-tailed) was considered statistically significant.
Results
Epidemiology
Twelve articles have been published to date on this rare entity [7, 8, 12, 17,18,19,20,21,22,23,24,25,26]. The span of the years of publication ranges from 1990 to 2022. A total of twenty-one cases were identified, including 18 published cases plus three new ones. The cases were mainly from Asia, including China (including Taiwan) (n = 11), Japan (n = 2), South Korea (n = 2), and India (n = 1). A few cases were from North America, including the United States of America (n = 4) and Canada (n = 1). The NCLC subtype of CEOT was found in patients across a wide age range from 20 to 58 years (mean 43 years, median 41 years) (Fig. 1). Majority of the cases (except two cases) were discovered in the fourth to sixth decades of life. There was a female predilection (Fourteen females; seven males) with a male-to-female ratio of 1 to 2.
Clinical Features
The clinical features of NCLC CEOT cases are summarized in Table 1.
The chief complaints at the presentation were described in fourteen patients (67%, 14/21). Most presented as a slow-growing swelling with or without pain (57%, 8/14). The patient’s clinical symptoms came from the effects of the mass, including mucosal ulceration or indentation, tooth loosening and pain, and incomplete healing of the tooth extraction wound. Twelve patients described the time from the earliest onset of symptoms to visiting the doctor. The time duration was from one to 60 months (mean = 18 months, median = 9 months).
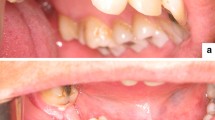
Physical examinations were revealed in fifteen patients (76%, 16/21). They presented swelling of the gingival or hard palate submucosally, localized depression of the mucosa, and teeth loosened or missing. Two extraosseous cases resembled a firm, well-circumscribed, ovoid-shaped nodule submucosally. Nineteen of the 21 cases occurred in the maxilla (, 90%). Eighteen cases were intraosseous (18/21, 85%). The most frequent clinical diagnosis was ameloblastoma. The other clinical diagnoses included odontogenic cysts and other odontogenic tumours.
Radiographically, many of the tumours exhibited a unilocular radiolucent defect (81%, 13/16), two cases showed multilocular radiolucent, while the remaining two showed a non-ossifying soft tissue mass. None of them were radiopaque. The margins of many of these defects were well-defined whereas four cases had an ill-defined periphery (25%, 4/16). The maximum dimension of the lesion ranged from 10 to 34 mm (mean = 21 mm, median = 20 mm). Teeth involvement was seen in 15 cases, a total of 63 teeth (mean = 4 teeth, median = 4 teeth). Six cases only involved the anterior tooth area of the maxilla, two cases were in the posterior tooth area of the mandible, one in the anterior mandible and ten cases involved both the anterior and posterior tooth area of the maxilla. Overall, 84% (16/19) affected the anterior maxilla. Root resorption in the lesion-involved area was described in 44% (7/16). In three cases, there was jaw resorption involving the maxillary sinus, nasal floor, and hard palate. In the three newly reported cases, we also observed the alveolar bone resorption in the affected area. Because of the lack of calcification, the lesions were originally diagnosed to be Langerhans cell histiocytosis, central giant cell granulomas, ameloblastoma or benign odontogenic cysts by radiologists.
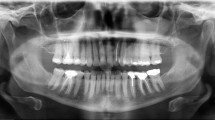
Figure 2 is the imaging appearance of case 21. The patient is a 32-year-old male. Panoramic radiograph showed an unilocular radiolucent involved in the left maxilla, approximately 17.6 × 12.7 × 10.4 mm in size and locally lobulated (Fig. 2A). Tooth resorptions were seen in 22, 23, 24. Cone beam computed tomography (CBCT) showed a cystic lesion with a scalloped margin and well-defined (Fig. 2B, 2C). In this case, the surgeon and radiologist made the clinical diagnosis of ameloblastoma.
Imaging presentation of NCLC-CEOT (case 21). The patient is a 32-year-old male. A Panoramic radiograph appearance of the unilocular radiolucent involving left maxilla, approximately 17.6 × 12.7 × 10.4 mm in size and locally lobulated. Root resorptions were seen in teeth 22, 23, and 24. B and C CBCT illustrate a cystic lesion with a scalloped margin and well-defined located between 22–24, caused resorption of alveolar crest bone and tooth root
Pathology and Diagnosis
Microscopically, the histopathological presentation of the NCLC subtype of CEOT is unique compared to the conventional CEOT. The NCLC subtype of CEOT has discrete small nests or islands of polyhedral epithelial cells in an abundant fibrous stroma. The eosinophilic cytoplasm and intercellular bridges can be noted (Fig. 3A). Meanwhile, clear cells constitute a portion of some epithelial components (Fig. 3B). There is mild to moderate infiltration of chronic inflammatory cells in the fibrous stroma (Fig. 3C). Abundant amyloid are seen in the fibrous connective tissue, which shows a homogeneous, red-stained appearance but lack calcification. A portion of the amyloid material shows a concentric ring-like appearance, but no identified calcifying mass (Fig. 3D).
Pathology features of NCLC-CEOT (Case 19). A Small, scattered tumour epithelial masses (insert) and abundant amyloid materials are seen within the fibrous connective tissue (40 ×). B Cells with clear cytoplasm (insert) can be noted in epithelium component (40 ×) C Mild to moderate chronic inflammatory cell infiltration in the fibrous stroma (20 ×) with higher magnification (insert) showing the lymphocytes. D Amyloid material with concentric rings (insert) (40 ×). E Tumour epithelia were positive for pan-CK (40 ×). F Langerhans cells were positive for langerin (40 ×). G Langerhans cells were positive for S-100 (40 ×). H Langerhans cells were positive for CD1a (40 ×). I The amyloid materials were brick-red by Congo red staining (40 ×)
Immunohistochemistry staining of pan-cytokeratin (CK), p63, cytokeratin (CK) 5/6 or β-catenin was done to identify odontogenic epithelial cells. The odontogenic epithelial cells in all the cases were strongly positive for one of the antibodies. Case 21 was positive for CK5/6, pan-CK and p63. Cases 19, and 20 were positive for pan-CK (Fig. 3E). Immunohistochemistry staining of S-100, CD1a or langerin was used to detect the clears cells in nineteen cases. The clear cells were positive for at least one of those antibodies. As shown in Fig. 3, scattered langerin, S-100 or CD1a-positive Langerhans cells were present in the tumour stroma (Fig. 3F, 3G, 3H). Amyloid was confirmed with Congo red staining ( brick red under light microscopy and an apple-green when viewed with birefringence). (Fig. 3I).
Treatment and Prognosis
Treatment information was available in eighteen cases. Three cases of extraosseous NCLC subtype of CEOT were completely resected with adequate margin. In the sixteen cases of intraosseous NCLC subtype of CEOT, nine cases were performed conservative local resection, seven cases were treated with curettage. Follow-up records were available in 16 cases. The time scope of follow-up was from one month to 120 months (mean 35.2 months, median 24 months). From the pooled data of the literature, only one lesion was reported to have recurred one year after curettage. The overall prognosis appears good. No malignant transformation or regional and distant metastasis had been reported. The pathological features and prognosis information of the NCLC CEOT cases were summarized in Table 2.
Discussion
CEOT occurs over a wide age range with maximum prevalence in the 4th decade and no gender predisposition [15]. In contrast, on review of the literature, the NCLC subtype of CEOT was found mainly in the 4th to 6th decades of life and with a female predominance. Radiologically, classic CEOT often occurs in the posterior mandible, mostly associated with an impacted tooth and 30% were multilocular. In the NCLC subtype of CEOT, 90% occurred in the anterior maxilla and caused root resorption of the affected teeth. It mostly presents a well-defined unilocular radiolucent area on radiographs and without radiopaque. Therefore, the imaging diagnosis and clinical impression are often odontogenic cysts or ameloblastoma.
Classic CEOT consists of islands and sheets of polyhedral epithelial cells with eosinophilic homogenous amyloid substance, and calcified tissue. When it comes to the NCLC subtype of CEOT, its most distinctive microscopic feature is the existence of very small nests and cords of neoplastic cells containing a few clear cells, and the presence of abundant amyloid material without calcification. The presence of clear cells and the absence of calcification in the cases posed a diagnostic challenge.
Three articles revealed the ultrastructure of NCLC CEOT by electron microscopy [17,18,19]. The epithelial cells contained bundles of tonofilaments. Many interdigitating microvilli were present between tumour cells and desmosomes were occasionally found in adjacent cells. Some epithelial nests contained a small number of Langerhans cells containing indented nuclei and Birkbeck’s granules. No desmosomes were observed between them and neighbouring epithelial cells.
The significance of Langerhans cells in this neoplasm and their effects on tumour behaviour remains to be resolved. Langerhans cells derive from bone marrow and migrate into the skin and oral mucosa serving as antigen-presenting cells and are positive for CD1a, S-100 and langerin in immunohistochemistry staining. Since both oral and odontogenic epithelial cells originate from the same oral ectoderm, Langerhans cells may also migrate to tumorigenic odontogenic epithelial nests [20]. This speculation has been proved in some kinds of odontogenic cysts and tumours [27, 28]. Mello et al. found that CD1a-positive Langerhans cells were present in 55% of ameloblastomas, 78% of odontogenic keratotic cysts (OKC) and 100% of odontogenic calcified cysts [28].
Langerhans cells also can be seen in the conventional CEOT, with a ratio of Langerhans cells to epithelial tumour cells of 1.7:100 and 0.8:100, respectively, reported in two cases by Chen et al. [12]. However, in the NCLC subtype of CEOT, the number of Langerhans cells was significantly higher, with a ratio of 82.7:100 and 42.1:100 [12]. The authors believed the increased number of Langerhans cells might be associated with inflammation. The ratio of Langerhans cells to tumour epithelial cells in the three newly reported cases is similar (47:100, 43:100, 39:100). According to Lin et al., the antigenicity of amyloid stimulates Langerhans cell's migration from the bloodstream to odontogenic epithelial nests [29]. However, in classic CEOT, calcifications in tumours restrict the migration of Langerhans cells as mineralization in amyloid leads to a decrease or loss of its antigenicity [29]. At the periphery of the lesion, mild to moderate inflammatory cell infiltration was often noted. The infiltrating inflammatory cells may be caused by abrasion, for the mucosal swelling due to intraosseous tumour. No matter whether the increase of Langerhans cells is driven by antigenicity of amyloid materials or other reason(s), it is interesting when accompanied by the absence or decrease in calcification.
The eosinophilic homogeneous material of amyloid has been shown to contain several ameloblast-associated proteins, the most consistently odontogenic ameloblast-associated protein (ODAM) [30]. In classic CEOT, calcifications develop within the amyloid materials and form concentric rings (Liesegang ring calcifications). These tend to fuse and form large, complex masses. Krolls et al. speculated that the presence of calcification and amyloid-like material in CEOT probably indicates higher levels of cell differentiation and accounts for the more self-limiting behaviour of CEOT compared with ameloblastoma [31]. The reason for the absence of calcification in the NCLC subtype of CEOT has been suggested to be related to the appearance of Langerhans cells [18]. Thus, CEOT without calcification may be in its early stage of maturation and amyloid material may calcify as the lesion progresses. This is because small epithelial masses can also be observed located in the connective tissue in the marginal areas of the classic CEOT. Owing to the paucity of this subtype of CEOT, the consequence of a non-calcifying lesion needs further investigation.
Since the NCLC subtype of CEOT occurs mostly in the anterior maxilla, which is like the site of central odontogenic fibroma, some researchers have suggested that the NCLC subtype of CEOT is an amyloid subtype of odontogenic fibroma [32]. Furthermore, smaller, scattered nests seen in this tumour are more consistent with central odontogenic fibroma versus the large sheets in CEOT [33, 34]. In addition, many have reported the presence of Langerhans cells within the epithelial nests of the amyloid subtype of odontogenic fibroma [35, 36]. Zhou et al. concluded that the percentage of Langerhans cells in epithelial nests of CEOT was no more than 2%, whereas it was approximately 40% in central odontogenic fibroma [37]. We examined immunohistochemical staining for Langerhans cells in the published literature and found that the proportion of Langerhans cells was greater than 2% in all cases. Nevertheless, whether the NCLC subtype of CEOT is the same disease as the amyloid subtype of central odontogenic fibroma is debated. More accumulative data are needed to further confirm this specific subtype. Besides, other odontogenic and maxillofacial bone tumours need to be considered in the differential diagnoses. The main differential diagnoses with the NCLC subtype of CEOT and their epidemiological and clinical features are listed in Table 3.
It is worth noting that some cases of CEOT reported in the literature were labelled as non-calcifying CEOT [38,39,40] as no calcium deposits could be demonstrated. However, in these cases, no Langerhans cells were demonstrated, and these cases were excluded from the analysis in the current study.
Pindborg initially suggested that CEOT originated from the reduced enamel epithelium of unerupted teeth [2]. Later, Chomette et al. found that the tumour cells bear a close morphological resemblance to the cells of the stratum intermedium of the enamel organ [41]. However, in half of the cases, CEOT was not associated with an unerupted tooth, and the existence of extraosseous cases, and other possible origins had to be considered. Philipsen suggested that the dental lamina complex or its remnants were the most possible candidate [42]. The disintegration of dental laminae gives rise to a countless number of epithelial remnants throughout the jaw bones and gingiva after the completion of odontogenesis. The three newly reported cases were also not related to impacted teeth and resulted in root resorption manifesting from the apical side. Therefore, the remnant of the dental lamina is a more reasonable source of the tumour.
The molecular pathology of CEOT is uncertain to date. However, a small number of articles have been published on the pathogenesis of CEOT. It proposed that ameloblastin (AMBN) gene alterations might be relevant to the pathogenesis of CEOT [43]. It found that the DNA sequencing was modified in an important domain of the AMBN in the CEOT. Urzua et al. detected amelogenin by immunohistochemical staining in different odontogenic tumours [44]. Amelogenin was positive in calcifying odontogenic cysts, compound and complex odontomas, and adenomatoid odontogenic tumours, but was negative in two CEOT. As calcifying odontogenic cyst, compound odontoma, complex odontoma and adenomatoid odontogenic tumour were noted to have low recurrence rates and good patients’ prognosis, the absence of amelogenin in CEOT might be responsible for its local aggressiveness [45]. De Sousa et al. found that the tumour suppressor genes (PTEN, CDKN2A), and oncogenes (JAK3, MET) mutations in CEOT, but the number of mutations was low, and it is unlikely that they were the driver genes [45]. Other CEOT-related studies also reported different gene mutations (PTCH1, ABMN, PTEN, CDKN2A, JAK3, MET), but they were not significantly related to the diagnosis and treatment of CEOT [16]. To date, no CEOT-specific gene has been identified. Nevertheless, identification of the gene specific to the NCLC subtype of CEOT could be a key to distinguishing the entity from the amyloid subtype of central odontogenic fibroma.
Although it was originally believed that the CEOT had similar biological behaviour as the ameloblastoma, accumulating experience indicated that CEOT tends to be less aggressive. A recurrence rate of about 15% has been reported in CEOT. Among the reported cases of NCLC, only one case of recurrence occurred [21]. After one year of the initial conservative surgical management with curettage, its recurrence extended to the sinus. Therefore, the recurrence rate of the NCLC subtype of CEOT noted in the literature was 5%. Based on very limited information, the paucity of calcification seems not to be directly related to the prognosis of the tumour. The relationship between the prognosis of CEOT and the absence of calcification and the number of Langerhans cells should be documented by analysing data on long-term follow-up.3
Data Availability
All the data available in the study are in the paper.
References
Thoma KH, Goldman HM (1946) Odontogenic tumors: classification based on observations of the epithelial, mesenchymal, and mixed varieties. Am J Pathol. 22:433–471
Pindborg JJ (1958) A calcifying epithelial odontogenic tumor. Cancer 11:838–843
Pindborg JJ, Clausen F (1958) Classification on odontogenic tumours: a suggestion. Acta Odontol Scand 16:293–301
Shafer W, Hine M, Levy B (1963) A textbook of oral pathology, 2nd edn. Saunders, Philadelphia
Pindborg JJ, Kramer IR. 1971 International histological classification of tumours: No. 5. histological typing of odontogenic tumours, jaw cysts, and allied lesions. Geneva: World Health Organization.
Chrcanovic BR, Gomez RS (2017) Calcifying epithelial odontogenic tumor: an updated analysis of 339 cases reported in the literature. J Craniomaxillofac Surg 45:1117–1123
Ruddocks LA, Fitzpatrick SG, Bhattacharyya I, Cohen DM, Islam MN (2021) Calcifying epithelial odontogenic tumor: a case series spanning 25 years and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol 131:684–693
Li HL, Zhang L, Xia S, Chen S, Yang Y, Ye CJ, Huang XF (2022) Clinical pathologic analysis and review of literature on 11 cases of calcifying epithelial odontogenic tumor. Zhonghua Kou Qiang Yi Xue Za Zhi 57:1119–1127
Morais HGF, da Silva WR, Andrade ACM, Silva NSE, Xerez MC, Santos JWM, Germano AR, Costa ALL (2022) Pindborg tumor associated with a supernumerary tooth: a case report. Autops Case Rep 12:e2021358
McCloy R, Bacaj P, Bouquot JE, Qari H (2021) Thirteen Synchronous multifocal calcifying epithelial odontogenic tumors (CEOT): case report and review of the literature. J Oral Maxillofac Surg 79:2078–2085
Chen CY, Wu CW, Wang WC, Lin LM, Chen YK (2013) Clear-cell variant of calcifying epithelial odontogenic tumor (Pindborg tumor) in the mandible. Int J Oral Sci 5:115–119
Chen Y, Wang TT, Gao Y, Li TJ (2014) A clinicopathologic study on calcifying epithelial odontogenic tumor: with special reference to Langerhans cell variant. Diagn Pathol 9:37
Kamboj M, Yadav AB, Narwal A, Neera J (2020) Unusual cystic variant of calcifying epithelial odontogenic tumor. J Dent (Shiraz) 21:147–152
Priya S, Madanagopaal LR, Sarada V (2016) Pigmented Pindborg tumor of the maxilla: a case report. J Oral Maxillofac Pathol 20:548
WHO Classification of Tumours Editorial Board. Head and neck tumours . Odontogenic and maxillofacial bone tumours. Tilakarathne W M (ed), Lyon (France): International Agency for Research on Cancer; 2022, Chapter 8. WHO classification of tumours series, 5th edition.
Vered M, Wright JM (2022) Update from the 5th edition of the World Health Organization Classification of Head and Neck Tumors Odontogenic and Maxillofacial Bone Tumours. Head Neck Pathol 16:63–75
Asano M, Takahashi T, Kusama K, Iwase T, Hori M, Yamanoi H, Tanaka H, Moro I (1990) A variant of calcifying epithelial odontogenic tumor with Langerhans cells. J Oral Pathol Med 19:430–434
Takata T, Ogawa I, Miyauchi M, Ijuhin N, Nikai H, Fujita M (1993) Non-calcifying Pindborg tumor with Langerhans cells. J Oral Pathol Med 22:378–383
Wang S, Chen X (2006) Langerhans cells containing calcifying epithelial odontogenic tumour: report of two cases and review of the literature. Oral Oncol Extra 42:144–146
Wang YP, Lee JJ, Wang JT, Liu BY, Yu CH, Kuo RC, Chiang CP (2007) Non-calcifying variant of calcifying epithelial odontogenic tumor with Langerhans cells. J Oral Pathol Med 36:436–439
Ganatra S, Castro H, Toporowski B, Hohn F, Peters E (2013) Non-calcifying langerhans cell -associated epithelial odontogenic tumor. Oral Surg Oral Med Oral Pathol Oral Radiol 116:507
Afroz N, Jain A, Maheshwari V, Ahmad SS (2013) Non-calcifying variant of calcifying epithelial odontogenic tumor with clear cells-first case report of an extraosseous (peripheral) presentation. European J Gen Dent 2(01):80–82
Tseng CH, Wang YP, Lee JJ, Chang JY (2015) Noncalcifying variant of calcifying epithelial odontogenic tumor with Langerhans cells. J Formos Med Assoc 114:781–782
Lee W, Myung NH, Kim CH (2016) Calcifying epithelial odontogenic tumor: report of three cases with immunohistochemical study. Int J Clin Exp Pathol 9:5733–5739
Santosh N, McNamara KK, Kalmar JR, Iwenofu OH (2019) Non-calcifying Langerhans cell-rich variant of calcifying epithelial odontogenic tumor: a distinct entity with predilection for anterior maxilla. Head Neck Pathol 13:718–721
Afrogheh A, Schneider J, Mohamed N, Hille J (2014) Calcifying epithelial odontogenic tumour with clear langerhans cells: a novel variant, report of a case and review of the literature. Head Neck Pathol 8:214–219
Chang CH, Wu YC, Wu YH, Sun A, Chen HM, Lin HP (2017) Langerhans cells in 60 odontogenic keratocysts. J Dent Sci 12:283–290
Mello LA, Figueiredo AL, Ramos EA, Gurgel CA, Martins MD, de Figueiredo CR, Cury PR, de Albuquerque Júnior RL, Ramalho LM, Santos JN (2013) CD1a-positive Langerhans cells and their relationship with E-cadherin in ameloblastomas and keratocystic odontogenic tumors. J Oral Pathol Med 42:454–461
Lin HP, Kuo YS, Wu YC, Wang YP, Chang JY, Chiang CP (2016) Non-calcifying and Langerhans cell-rich variant of calcifying epithelial odontogenic tumor. J Dent Sci 11:117–122
Crivelini MM, Felipini RC, Miyahara GI, de Sousa SC (2012) Expression of odontogenic ameloblast-associated protein, amelotin, ameloblastin, and amelogenin in odontogenic tumors: immunohistochemical analysis and pathogenetic considerations. J Oral Pathol Med 41:272–280
Krolls SO, Pindborg JJ (1974) Calcifying epithelial odontogenic tumor. a survey of 23 cases and discussion of histomorphologic variations. Arch Pathol 98:206–210
Tseng CH, Lu PH, Wang YP, Chiang CP, Cheng YL, Chang JYF (2021) Non-calcifying Langerhans cell rich variant of calcifying epithelial odontogenic tumor and amyloid rich variant of central odontogenic fibroma: a unique entity or a spectrum? Front Oral Health 2:767201
Ide F, Matsumoto N, Miyazaki Y, Kikuchi K, Kusama K (2019) What is the non-calcifying Langerhans cell-rich variant of calcifying epithelial odontogenic tumor? Head Neck Pathol 13:489–491
Eversole LR (2011) Odontogenic fibroma, including amyloid and ossifying variants. Head Neck Pathol 5:335–343
Roza ALOC, Sousa EM, Leite AA, Amaral-Silva GK, Morais TML, Wagner VP, Schuch LF, Vasconcelos ACU, de Arruda JAA, Mesquita RA, Fonseca FP, Abrahão AC, Agostini M, de Andrade BAB, da Silveira EJD, Martínez-Flores R, Rondanelli BM, Alberdi-Navarro J, Robinson L, Marin C, Assunção Júnior JNR, Valiati R, Fregnani ER, Santos-Silva AR, Lopes MA, Hunter KD, Khurram SA, Speight PM, Mosqueda-Taylor A, van Heerden WFP, Carlos R, Wright JM, de Almeida OP, Romañach MJ, Vargas PA (2021) Central odontogenic fibroma: an international multicentric study of 62 cases. Oral Surg Oral Med Oral Pathol Oral Radiol 131:549–557
Kakuguchi W, Nakamichi Y, Kitamura T (2020) Amyloid variant of central odontogenic fibroma in the mandible: a case report and literature review. Am J Case Rep 21:e925165
Zhou CX, Li TJ (2018) A clinicopathologic study on central odontogenic fibroma: with special reference to amyloid variant. Oral Surg Oral Med Oral Pathol Oral Radiol 126:513–520
Patankar S, Choudhari S, Sharma S, Dhumal S (2021) Noncalcifying clear-cell variant of calcifying epithelial odontogenic tumor: a case report and review. J Oral Maxillofac Pathol 25:204
Taneeru S, Guttikonda VR, Korlepara R, Gaddipati R, Kundoor VK (2017) Non calcifying type of calcifying epithelial odontogenic tumor: an unusual case report with special emphasis on histogenesis of calcifications. J Maxillofac Oral Surg 16:253–257
Kaushal S, Mathur SR, Vijay M, Rustagi A (2012) Calcifying epithelial odontogenic tumor (Pindborg tumor) without calcification: a rare entity. J Oral Maxillofac Pathol 16:110–112
Chomette G, Auriol M, Guilbert F (1984) Histoenzymological and ultrastructural study of a bifocal calcifying epithelial odontogenic tumor. Characteristics of epithelial cells and histogenesis of amyloid-like material. Virchows Arch A Pathol Anat Histopathol. 403:67–76
Philipsen HP, Reichart PA (2000) Calcifying epithelial odontogenic tumour: biological profile based on 181 cases from the literature. Oral Oncol 36:17–26
Perdigão PF, Carvalho VM, Marco DE, L, Gomez RS. (2009) Mutation of ameloblastin gene in calcifying epithelial odontogenic tumor. Anticancer Res 29:3065–3067
Urzúa B, Ahumada-Ossandón R, Casa-Weisser D, Franco-Martínez ME, Ortega-Pinto A (2021) Amelogenin in calcified matrices of odontogenic cysts and odontogenic tumors: an immunohistochemical study. J Dent Sci 16:7–14
de Sousa SF, Diniz MG, França JA, Fontes Pereira TDS, Moreira RG, Santos JND, Gomez RS, Gomes CC (2018) Cancer genes mutation profiling in calcifying epithelial odontogenic tumour. J Clin Pathol 71:279–283
Acknowledgements
The authors would like to thank the staff in the Department of Oral Pathology, Hospital of Stomatology, Sun Yat-sen University for preparing the histology sections.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
N.X. wrote the main script text Y.L, J.H, X.C contributes to figures A.L. concept and edit the main manuscript. All authors reviewed the manuscript
Corresponding author
Ethics declarations
Conflict of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, N., Chen, Z., Liu, Y. et al. Non-Calcifying/Langerhans Cell-Rich Calcifying Epithelial Odontogenic Tumour: A Critical Review of the Rare and Distinctive Entity. Head and Neck Pathol 17, 1011–1020 (2023). https://doi.org/10.1007/s12105-023-01602-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-023-01602-5