Abstract
Background
HPV-associated oral cavity squamous cell carcinoma (SCC) is not well-characterized in the literature, and also has a clinical significance that is poorly understood.
Methods
We gathered a cohort of oral cavity (OC) SCC with nonkeratinizing morphology, either in the invasive or in situ carcinoma (or both), tested for p16 by immunohistochemistry and high risk HPV E6/E7 mRNA by RTPCR (reference standard for transcriptionally-active high risk HPV) and gathered detailed morphologic and clinicopathologic data.
Results
Thirteen patients from two institutions were proven to be HPV-associated by combined p16 and high risk HPV mRNA positivity. All 13 patients (100%) were males, all were heavy smokers (average 57 pack/year), and most were active drinkers (9/11 or 81.8%). All 13 (100%) involved the tongue and/or floor of mouth. All had nonkeratinizing features, but maturing squamous differentiation varied widely (0–90%; mean 37.3%). Nonkeratinizing areas had high N:C ratios and larger nests, frequently with pushing borders, and minimal (or no) stromal desmoplasia. The carcinoma in situ, when present, was Bowenoid/nonkeratinizing with cells with high N:C ratios, full thickness loss of maturation, and abundant apoptosis and mitosis. HPV was type 16 in 11 patients (84.6%) and type 33 in two (15.4%). Nine patients had treatment data available. These underwent primary surgical resection with tumors ranging from 1.6 to 5.2 cm. Most had bone invasion (6/9–66.7% were T4a tumors), and most (6/9–66.7%) had extensive SCC in situ with all 6 of these patients having final margins positive for in situ carcinoma.
Conclusions
HPV-associated OCSCC is an uncommon entity that shows certain distinct clinical and pathologic features. Recognition of these features may help pathologic diagnosis and could potentially help guide clinical management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The significance of transcriptionally-active high risk human papillomavirus (HPV) in oropharyngeal squamous cell carcinoma (SCC) patients is well established [1,2,3,4]. Patients with HPV-associated oropharyngeal SCC have better prognosis [1] and most have a distinct nonkeratinizing morphology [5,6,7]. The rate of transcriptionally-active high risk HPV is much lower at other anatomic subsites [8, 9]. In the oral cavity, several large, well performed retrospective studies have shown a low attributable fraction of HPV-associated tumors of approximately 3 to 5% [10, 11]. Morphology is not well-described in most of these studies [10, 11] but some, for example Lingen et al. [10] described the HPV mRNA positive oral cavity SCC tumors in their series as “basaloid” while Rooper et al. [12], who examined morphology in HPV-positive SCCs from prospective clinical trials, showed that many (and perhaps most) non-oropharyngeal HPV-associated SCCs can have similarly distinct nonkeratinizing features. We gathered a cohort of oral cavity SCC patients with nonkeratinizing morphology in the invasive tumors, the in situ component, or both, and described their morphology and clinical features in detail.
Materials and Methods
Patients and Samples
With institutional review board approval at two different institutions, we gathered oral cavity SCC patients with tumors showing nonkeratinizing morphology in their invasive tumors akin to that seen in oropharyngeal SCC [7, 13]. We also included patients where the SCC in situ had nonkeratinizing, basaloid, or Bowenoid morphology as described by other authors [14, 15]. Patients were identified from three sources: (1) a large oral tongue SCC cohort from Vanderbilt University Medical Center with 220 in house surgical pathology specimens from 2000 to 2017 (2) The author’s (JSL) pathology practice at Vanderbilt University Medical Center from 2016 to 2021 and (3) An oral cavity biopsy service of a co-author (MHS) with cases from 2013 to 2021. Detailed clinical information was gathered from retrospective electronic chart review at both institutions.
Morphologic Review
All of the cases were reviewed by the lead study head and neck pathologist (JSL). This included all slides from biopsy specimens and, for those that underwent resection, all slides from the resection as well, including regional nodes. For a smaller subset of the cases, only the initial biopsy specimens were available for review. Tumors were evaluated using the descriptions put forth by Rooper et al. [12] for invasive carcinomas and by Woo et al. [14] for the precursor lesions. Namely, tumors were evaluated for nonkeratinizing morphology—defined by Rooper et al. [12] and Chernock et al. [13] as being composed of invasive sheets, nests, and/or lobules of basaloid cells with indistinct cell borders, lack of intercellular bridges, high nuclear to cytoplasmic ratios, and relatively uniform oval nuclei with hyperchromasia. Cytoplasmic keratinization was allowed, as long as parts of the invasive tumor had some nonkeratinizing areas. Despite basaloid cytomorphology, cases with the typical features of basaloid SCC, as defined by Wain et al. and the World Health Organization, such as jigsaw puzzle molding of the nests, hyalinized basement membrane material, and prominent comedonecrosis were excluded. Cases were also evaluated for precursor lesions—defined by Woo et al. [14] as lesions with brightly eosinophilic, compact ortho- and/or parakeratosis, epithelial hyperplasia with marked karyorrhexis and apoptosis throughout the epithelium, conventional features of dysplasia with increased nuclear to cytoplasmic ratios, hyperchromasia, and cellular and nuclear pleomorphism. For study purposes, the degree of maturing squamous differentiation in the invasive tumor regions was semiquantitated in 5% increments based on the surface area of the entire primary tumor that consisted of squamous cells with maturing differentiation with abundant, eosinophilic cytoplasm, frank keratinization, or both. For the surgically resected tumors, the pattern that the maturing squamous differentiation had (when present) with the nonkeratinizing component was also characterized qualitatively as “discrete” versus “intermixed”. The amount the keratinizing differentiation that was in the center of the tumor nests versus at their periphery was also estimated in 5% increments.
Immunohistochemistry and In Situ Hybridization
Immunohistochemistry was performed for p16, either in clinical practice or for research purposes, using the E6H4 antibody (Ventana Medical Systems, Inc.; predilute) on a Leica Bond automated instrument (Leica Biosystems, Inc.) with antigen retrieval consisting of 10 min in the ER1 proprietary antigen retrieval solution. Primary antibody solution was diluted using Leica’s BOND primary antibody diluent. The Bond Polymer Refine detection system was used for visualization. Slides were then dehydrated, cleared and coverslipped. Staining was interpreted using the CAP recommendations [16] (positive = nuclear and cytoplasmic positivity in > 70% of tumor cells of at least moderate to strong intensity).
In situ hybridization for high risk HPV E6/E7 mRNA was performed by Propath Laboratories (Dallas, TX) using the RNAscope® 2.5 HD—BROWN Manual Assay (Advanced Cell Diagnostics, Inc., Hayward, CA) targeting HPV-associated RNA in the nucleus and cytoplasm of the target cells. Tissue samples that previously stained positive for high-risk HPV, as well as some that were negative, were used as batch control tissues and reacted appropriately. The high-risk/intermediate-risk HPV RNAscope probe used at ProPath covers HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68.
Specimen Collection, Processing, DNA and RNA Isolation
Formalin fixed paraffin embedded (FFPE) tissue blocks were retrieved from surgical pathology archives and cut into 10 μm sections on glass slides. Tumor rich areas were circled on a representative H&E slide with a dotting pen by one of the head and neck pathologists (JSL or MHS) for macro-dissection. Then, total RNA was extracted from the identified tumor regions with the miRNeasy FFPE Kit (Qiagen Inc, Valencia, Calif) according to the manufacturer’s protocol.
Expression Profiling of HPV and Functional-Related Human Genes Using RT-PCR
HPV RT-qPCR assays were used to profile the expression of HPV E6 and E7 with total RNA extracted from the tumor blocks as previously published [17]. All oligo primers in the assays were purchased from Sigma-Aldrich. Reverse transcription (RT) reaction was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time PCR was then performed using Power SYBR Green PCR Master Mix (Applied Biosystems) and 500 nM HPV type-specific primers. HPV assays (profiling E6 and E7 transcripts separately from each of the 13 HPV types) were individually performed on a 384-well PCR plate. The PCR running protocol was 95 °C for 10 min, followed by 36 cycles of amplification (95 °C for 10 s, 58 °C for 15 s and 60 °C for 15 s).
Results
The clinical and pathologic features of all 13 tumors are shown in Table 1. Patients were all men with an average age of 65.1 (range 62–70). All of them were active smokers, and all were, by any standard definition, heavy smokers with, for the patients with quantitative data available, an average of 57.4 pack years of smoking. Of the 12 patients with available data, 11 (91.7%) were drinkers as well. Nine of the 11 (81.8%) were active drinkers and only one patient was a lifetime non-drinker. All tumors arose in the floor of mouth or tongue, particularly the ventral tongue (Fig. 1).
Morphologically, the invasive tumors all, by definition, had some component of nonkeratinizing SCC, either in situ, invasive, or both (Figs. 2, 3 and 4). Maturing squamous differentiation was highly variable, ranging from 0 to 90% (mean 37.3%). Tumor cell multinucleation was frequent. In situ carcinoma was present in 6/9 resected tumors (66.7%) and 8/13 tumors overall (61.5%). Further, among the 6 patients with SCC in situ in resection specimens, all had final margins positive for the in situ disease suggesting that the dysplastic epithelium was difficult to appreciate clinically. The dysplasia/carcinoma in situ was morphologically typical of that described by Woo et al. and of the entity HPV-associated oral dysplasia, which has been incorporated into the 5th edition head and neck World Health Organization tumor classification [18]. The SCC in situ frequently had a dense, brightly eosinophilic parakeratotic surface layer and consisted of basaloid cells with high N:C ratios, brisk mitotic activity, frequent apoptosis with mitosoid bodies, and bulbous rete ridges (Figs. 2, 3 and 4). The invasive tumors varied substantially in appearance. The key features that pointed to nonkeratinizing SCC, and thus to HPV-association, were the areas with high N:C ratios, spindled and hyperchromatic nuclei, brisk apoptosis and mitosis, large nests with smooth edges, lack of classic stromal desmoplasia, and aberrant patterns of maturing squamous differentiation where it may be present at the periphery of the nests or scattered throughout the nests in a haphazard manner (Figs. 2, 3 and 4). For the eight surgically resected tumors that also had a component of keratinizing squamous differentiation, the areas of maturing squamous differentiation were interspersed with the nonkeratinizing components in seven patients (7/8 87.5%) (Fig. 3) and discrete in one (1/8 12.5%). (Fig. 4). Most of the maturing squamous differentiation was located centrally in the nests (range 30 to 100%, average 74.3%). Other than frequent tumor cell multinucleation, none of the tumors demonstrated features that Rooper et al. [12] described as “warty” in their description of HPV-associated SCCs from clinical trials.
Morphologic features of an HPV-associated SCC with in situ and invasive carcinoma. A On low power, the tumor shows many large, rounded nests of blue cells with high N:C ratios, smooth borders, and lack of stromal desmoplasia. B On medium power, the nests are large and smooth and have minimal maturing squamous differentiation. C On high power, the cells of the invasive carcinoma have round to oval nuclei, minimal cytoplasm, nuclear pleomorphism, and brisk mitotic activity with a focus of necrosis. D This SCC in situ had the typical dense and brightly eosinophilic parakeratin and cells with high N:C ratios
Morphologic features of another case of invasive HPV-associated SCC with SCC in situ. A SCC in situ showing brightly eosinophilic parakeratin, bulbous rete, and cells with high N:C ratios throughout. B On higher power, there are prominent mitotic figures, apoptosis, and a “mitosoid” apoptotic body. C The invasive SCC in this case consisted of large, irregular nests with stromal desmoplasia, but the nests showed a mixture of higher N:C ratio areas interspersed with the keratinizing areas, the latter being haphazardly arranged. D On high power, the invasive carcinoma shows some nonkeratinizing features but also extensive maturing squamous differentiation
Morphologic features of another invasive HPV-associated SCC with SCC in situ. A The in situ carcinoma shows the typical features of HPV-associated dysplasia compared to the adjacent more normal epithelium (superior). B Low power view of the invasive tumor shows very large, irregular nests, some of which are very blue with high N:C ratio cells and others which are more keratinized with abundant, eosinophilic cytoplasm. C High power view of the blue areas of invasive tumor shows the typical features of nonkeratinizing SCC
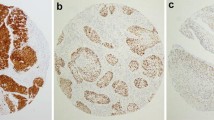
By definition, all were positive for p16 by immunohistochemistry (Fig. 5) and for transcriptionally-active high risk HPV by RTPCR. HPV16 was the most common type (11/13 84.6%) followed by HPV33 (2/13 15.4%). p16 was positive in both the in situ and invasive components (when present). HPV RNA in situ hybridization was also performed on the 9 patients with resected tumors and it was positive in 7 (77.8%) (Fig. 5).
p16 immunohistochemistry and high risk HPV RNA in situ hybridization. A p16 immunohistochemistry on the invasive SCC showing diffuse strong nuclear and cytoplasmic staining. B p16 immunohistochemistry on the in situ SCC showing diffuse strong nuclear and cytoplasmic staining. C RNA in situ hybridization on the invasive SCC showing granular, brown positive staining of the cytoplasm. D RNA in situ hybridization on the in situ SCC showing granular, brown positive staining of the cytoplasm
Discussion
While HPV-associated oropharyngeal SCC is established as a biologically, clinically, and morphologically distinct form of head and neck cancer, now even with its own staging system, HPV in SCC from other head and neck anatomic subsites and its clinical significance has remained aloof. The rate of transcriptionally-active HPV-associated oral cavity SCC is now well established from large, retrospective studies. Lingen et al. [10] studied a cohort of 409 patients from four major academic institutions in the United States and found, using RT-PCR, an etiologic fraction of 5.9%. They did not report outcome data [10]. In another very large study from the Netherlands, Nauta et al. [11], using DNA PCR with E6 mRNA confirmation by RT-PCR, found, among 940 patients with oral cavity SCC, 21 (2.2%) to be positive. Overall and disease free survival were no different between the HPV positive and negative patients. The baseline seems to be 2 to 6% of all oral cavity SCC patients with transcriptionally-active high risk HPV. Rooper et al. took a different approach in order to better address the morphology of the tumors, reviewing all patients in clinical trial specimens with tumors positive for high risk HPV mRNA by either RNA in situ hybridization or RT-PCR [12]. They found that only 10 to 20% of all HPV mRNA positive non-oropharyngeal SCC cases had conventional keratinizing morphology. This suggests that most truly HPV-associated OCSCC have either nonkeratinizing, or what they described as “warty” morphology. In another way of trying to address this question (although not a systematic assessment), from a 220 patient oral tongue SCC cohort at VUMC from 2000 to 2017, we found that 4/220 (1.8%) had tumors that would be classified as nonkeratinizing SCC based on features of oropharyngeal SCC (all 4 of these patients are represented in the current study).
The morphology of HPV-associated pre-cancers has also been studied [19,20,21]. Woo et al. [14], in a series of 20 HPV-associated oral dysplasias based on p16 immunohistochemistry plus DNA in situ hybridization for high risk HPV, described pre-cancers predominating in the floor of mouth and ventral tongue with a characteristic nonkeratinizing or basaloid pattern with dense surface parakeratin and epithelium showing numerous apoptotic bodies and “mitosoid” apoptotic forms. McCord et al. and Khanal et al. studied p16 and high risk HPV in series of oral dysplasias and also emphasized the high N:C ratios and extensive apoptosis and mitosis in the HPV-associated forms [20, 21]. From these and other studies, the invasive tumors and pre-cancers appear to predilect for the ventral tongue and floor of mouth [19, 20] and most show, at least partially, the distinct, nonkeratinizing, basaloid, or “Bowenoid” morphology (terminology depending on the author) that is so prototypical of HPV-associated SCC of the oropharynx and other anatomic sites [10].
Our study, which gathered these tumors based on this characteristic morphology, shows the ventral tongue and floor of mouth localization. It also finds that these patients are not demographically the same as for oropharyngeal SCC. We did find patients to be almost exclusively Caucasian men, similar to oropharyngeal SCC, but differently, we found them to all be active, heavy smokers and most to have active (current) alcohol use (Table 1). Most of the patients had extensive nonkeratinizing SCC in situ which was difficult for surgeons to detect. Of the six patients with SCCis who underwent surgical resection, all six of them (100%) had positive final margins for in situ disease. While the morphologic associations with HPV status are interesting, the clinical importance, of course, should reside in management and outcomes. Knowing from a biopsy that shows characteristic nonkeratinizing morphology that the tumor is HPV-associated could help the surgeon to anticipate the possibility of wide field effect type SCC in situ around the visible mass lesion.
Although the current study does not have adequate numbers of patients or clinical outcome data to address the prognosis of patients with HPV-associated oral cavity SCC compared to patients with HPV-independent tumors, in the past decade and even more recently, several large retrospective studies provide strong evidence that outcomes are not more favorable. Nauta et al. studied 940 patients with oral cavity SCC in the Netherlands and found 21 (2.2%) to be HPV-associated (“transcriptionally active high risk HPV”) [11]. They found no difference in overall or disease free survival. In fact, the HPV-associated patients had slightly inferior survival. Even in Chung et al., who gathered non-oropharyngeal SCC patients from three prospective clinical trials with p16 and HPV specific testing and found that, across the entire cohort, p16 positive patients did statistically significantly better clinically, in subsite analysis, the 21 oral cavity SCC patients who were p16 or HPV positive (out of 80 total oral cavity SCC patients) did not show improved outcomes. Further, Bryant et al., in a study of Veterans Affairs patients with non-oropharyngeal SCC, where they found that the p16 positive patients (across all non-oropharyngeal sites together) had statistically significantly better survival than p16 negative, found in their subsite analysis that for the 42 (out of 128 or 33%) p16 positive oral cavity SCC patients, survival was improved slightly but did not quite reach statistical significance.
What might our findings mean for pathology practice then? The new WHO 5th edition Classification of Head and Neck Tumours [18] now has a specific diagnostic category for HPV-associated dysplasia. Although the risk of transformation to invasive cancer for HPV-associated dysplasia versus HPV-independent is not clear, they sought to identify this distinct form of pre-cancer clearly so that this can be determined in the future. Pathologists are expected to recognize and diagnose HPV-associated dysplasia and confirm it with p16 immunohistochemistry (70% nuclear and cytoplasmic cutoff) and, if positive, follow this with HPV-specific testing. Regarding frankly invasive SCC, although it is not indicated to test for p16/HPV for prognostic reasons, the strong association with extensive in situ carcinoma (and positive margins) may justify p16/HPV specific testing on biopsy specimens.it is enough to just recognize the distinct morphology in our clinical practices when we see it, not for prognosis but to understand the tumor, and how it is arising. This knowledge could potentially guide our surgeons to be cautious regarding the resection margins when performing primary surgery. One also expects, with time and increased vaccination, that HPV-associated oral cavity SCCs might be less common in the future.
In summary, we present a series of HPV-associated oral cavity SCC patients with distinct morphologic features and a strong predilection for the floor of mouth and ventral tongue. Nonkeratinizing or basaloid morphology is seen in both the invasive and in situ carcinomas, and there is frequently surrounding SCC in situ which is often difficult to recognize and clear surgically. In addition, patients are consistently heavy smokers and drinkers suggesting that the pathophysiology of cancer development may be different than for oropharyngeal SCC, where patients are less consistently smokers and have overall less tobacco exposure. Larger studies matching patients with HPV-independent tumors for morphology and outcome will help better solidify this as a unique type of oral cavity SCC.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9.
Gillison ML, Restighini C. Anticipation of the impact of human papillomavirus on clinical decision making for the head and neck cancer patient. Hematol/Oncol Clin North Am. 2015;29:1045–60.
Wagner S, Wittekindt C, Sharma SJ, et al. Human papillomavirus association is the most important predictor for surgically treated patients with oropharyngeal cancer. Br J Cancer. 2017;116:1604–11.
Chernock RD, El-Mofty SK, Thorstad WL, et al. HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3:186–94.
Lewis JS Jr, Khan RA, Masand RP, et al. Recognition of nonkeratinizing morphology in oropharyngeal squamous cell carcinoma—a prospective cohort and interobserver variability study. Histopathology. 2012;60:427–36.
Gondim DD, Haynes W, Wang X, et al. Histologic typing in oropharyngeal squamous cell carcinoma: a 4-year prospective practice study with p16 and high-risk HPV mRNA testing correlation. Am J Surg Pathol. 2016;40:1117–24.
Bryant AK, Sojourner EJ, Vitzthum LK, et al. Prognostic role of p16 in nonoropharyngeal head and neck cancer. J Natl Cancer Inst. 2018;110:1393–9.
Chung CH, Zhang Q, Kong CS, et al. p16 Protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32:3930–8.
Lingen MW, Xiao W, Schmitt A, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49:1–8.
Nauta IH, Heideman DAM, Brink A, et al. The unveiled reality of human papillomavirus as risk factor for oral cavity squamous cell carcinoma. Int J Cancer. 2021;149:420–30.
Rooper LM, Windon MJ, Hernandez T, et al. HPV-positive squamous cell carcinoma of the larynx, oral cavity, and hypopharynx: clinicopathologic characterization with recognition of a novel warty variant. Am J Surg Pathol. 2020;44:691–702.
Chernock RD. Morphologic features of conventional squamous cell carcinoma of the oropharynx: ‘keratinizing’ and ‘nonkeratinizing’ histologic types as the basis for a consistent classification system. Head Neck Pathol. 2012;6(Suppl 1):41–7.
Woo SB, Cashman EC, Lerman MA. Human papillomavirus-associated oral intraepithelial neoplasia. Mod Pathol. 2013;26:1288–97.
Lerman MA, Almazrooa S, Lindeman N, et al. HPV-16 in a distinct subset of oral epithelial dysplasia. Mod Pathol. 2017;30:1646–54.
Lewis JS Jr, Beadle B, Bishop JA, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the College of American Pathologists. Arch Pathol Lab Med. 2018;142:559–97.
Gao G, Chernock RD, Gay HA, et al. A novel RT-PCR method for quantification of human papillomavirus transcripts in archived tissues and its application in oropharyngeal cancer prognosis. Int J Cancer. 2013;132:882–90.
WHO Classification of Tumours Editorial Board. Head and neck tumours. Lyon: International Agency for Research on Cancer; 2022.
McCord C, Bradley G. Histopathologic features of high risk HPV-associated oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:120–1.
McCord C, Xu J, Xu W, et al. Association of high-risk human papillomavirus infection with oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:541–9.
Khanal S, Trainor PJ, Zahin M, et al. Histologic variation in high grade oral epithelial dysplasia when associated with high-risk human papillomavirus. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:566–85.
Funding
Research was performed using discretionary funds from the Department of Pathology, Microbiology, and Immunology. The work also utilized the Translational Pathology Shared Resource (TPSR) at Vanderbilt University Medical Center which is supported by NCI/NIH Cancer Center Support Grant 5P30 CA68485-19 and the Shared Instrumentation Grant S10 OD023475. This work was supported by funds from the NIH Grant R01DE026471 (Wang). This work was also supported by the National Cancer Institute (NCI) K07CA218247 (PI: Krystle Kuhs); Vanderbilt Clinical Oncology Research Career Development Program (K12 CA090625); and the Vanderbilt Institute for Clinical and Translational Research (UL1 TR000445 from NCATS/NIH). This work was supported by funds from the NIH Grant U24 DK059637-16.
Author information
Authors and Affiliations
Contributions
All authors whose names appear on the submission made substantial contributions to the conception or design of the work, to the acquisition, analysis, or interpretation of data, and/or drafted the work or revised it critically for important intellectual content including approving the version to be published. They agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest as it relates to this work.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was performed with approval of the respective institutional review boards of Vanderbilt University Medical Center and the University of Kentucky and complies with required ethical standards. Given the retrospective nature of the study, with consultation with the institutional review boards, it was determined that the study did not need ethical approval. Patients were never contacted, and we did not require informed consent or specific consent to publish.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lewis, J.S., Smith, M.H., Wang, X. et al. Human Papillomavirus-Associated Oral Cavity Squamous Cell Carcinoma: An Entity with Distinct Morphologic and Clinical Features. Head and Neck Pathol 16, 1073–1081 (2022). https://doi.org/10.1007/s12105-022-01467-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-022-01467-0