Abstract
Mucoepidermoid carcinoma (MEC) is the most common malignant salivary gland tumour in both adults and children. Histological grading of MEC is subjective, but plays an important role in predicting patient prognosis. Epithelial mucin (MUC) status may aid in establishing a more accurate grade. This study aimed to investigate the expression of various mucins (MUC1, MUC2, MUC4 and MUC5AC) in MECs to determine a possible correlation with tumour grade. Fifteen cases of each tumour grade (low-, intermediate-, and high-grade) were retrieved from the pathology archives of the Department of Oral Pathology and Oral Biology at the University of Pretoria. The patients included 23 men and 22 women, and ranged from 13 to 85 years (mean 49.8 years). Sections from formalin-fixed paraffin-embedded (FFPE) tissue were used for fluorescence in situ hybridization (FISH) for MAML2 rearrangements and MUC immunohistochemical analysis. The percentage immunohistochemical expression of the neoplastic mucous cells was evaluated first, followed by the overall percentage expression of all tumour cells. The results indicated that MUC1 overexpression may be a reliable marker of high-grade MECs, whereas MUC4 overexpression may be more indicative of low-grade tumours. MUC5AC expression was considered an unreliable marker in determining grade. MUC2 was only expressed in a single case of MEC and may be considered a useful marker to exclude MEC as a diagnostic possibility. This study demonstrates that MECs show an altered MUC expression pattern that can be used for diagnostic purposes and to aid in establishing a more accurate tumour grade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucoepidermoid carcinoma (MEC) is the most frequent primary salivary gland malignancy in both adults and children, representing 5% of all salivary gland tumours and approximately 20–30% of malignant variants [1, 2]. The 4th Edition of the World Health Organization (WHO) classification of head and neck tumours, defines mucoepidermoid carcinoma as “a distinctive salivary gland malignancy composed of mucinous, intermediate and squamoid tumour cells forming cystic and solid patterns” [2].
Approximately 60% of MECs arise in the major salivary glands, with the parotid gland being the predominant site [1,2,3,4]. MECs may also arise in minor salivary glands and ectopic salivary gland tissue, with the minor glands of the palate most commonly affected [1, 2, 4]. MEC has been documented as carcinoma ex pleomorphic adenoma, but is exceedingly rare in contrast to other salivary gland malignancies [5]. Occasionally, these tumours arise within the facial skeleton, where they are termed intraosseous or central MECs [2, 6].
Evidence has shown that up to 80% of MECs harbour gene fusions. Among such aberrations, the t(11;19)(q12;p13) translocation involving CRTC1 (CREB-regulated transcription coactivator 1) (formerly MECT1) and CRTC3/MAML2 (CREB-regulated transcription coactivator 3/Mastermind-like transcriptional coactivator 2) genes is unique to MEC [4, 7,8,9]. Several studies have utilised reverse transcriptase polymerase chain reaction (RT-PCR) and fluorescence in situ hybridization (FISH) to detect CRTC1-MAML2 fusion transcript, with positive rearrangements seen in 38% to 82% of MECs [9,10,11,12]. Although the MAML2 alteration is specific for MEC, MAML2 testing is generally regarded as unnecessary, as the diagnosis of MEC is usually straightforward on histological grounds alone [8]. Initial research suggested that MAML2 rearrangements correlate with lower histological grade and a better overall prognosis [7, 9,10,11]. These studies have not been uniformly reproduced and MAML2 rearrangements may still be present in high-grade MECs [10]. Hence, MAML2 rearrangement status is no longer believed to be a useful prognostic factor for patients with well-documented MECs [4, 8, 12,13,14,15].
MECs show a vast pattern of histological features, with the main cellular components comprising of mucous, intermediate and epidermoid cells. Traditionally, MECs are histologically classified via a 3-tier grading system into low-, intermediate- and high-grade, based on the relative proportion of cell types [2, 3, 16]. An intermediate grade was incorporated in order to account for the large spectrum of histological appearances [1]. Numerous grading systems exist, with the AFIP (Armed Forces Institute of Pathology) system of Goode, Auclair and Ellis [17], and the system proposed by Brandwein et al. [3] used most commonly in daily histopathology practice.
Currently, the prognosis of patients with MEC is based largely on tumour stage, histological classification and differentiation, as well as the patient’s individual clinical characteristics [18]. Unfortunately, significant grading disparities exist among pathologists, with different criteria used in various studies [3, 12, 19, 20]. Typical low- and high-grade MECs are usually easily classified, but intermediate-grade tumours can share features of both low- and high-grade tumours [19]. Pathologists need to embrace a standardised grading system that is easy to use, reproducible, accurate and consistent in grading these tumours.
Surgical resection is the standard treatment for all grades of MEC [19, 21]. Local resection with clear margins is usually considered sufficient for low- and intermediate-grade tumours. High-grade tumours are usually treated with surgical excision with wide margins followed by postoperative radiotherapy. Neck dissection is indicated when there is clinical evidence of regional metastasis, high TNM stage or high histological grade [19, 21].
Epithelial mucins (MUCs) are large glycoproteins, mainly expressed by specialised epithelial cells of the aerodigestive tract [22,23,24]. All MUCs share an elevated number of sequences repeated in tandem, which defer slightly, characterising a specific MUC subtype [24,25,26]. Two main structural classes exist, including transmembrane (e.g. MUC1 and MUC4) and secreted mucins (e.g. MUC2, MUC5AC, MUC5B, MUC6, MUC7) [22, 23, 25]. These serve multiple roles in normal tissues such as providing a physical barrier against microorganisms, maintaining homeostasis, and aiding in communication between the extracellular environment and the epithelial cells [22, 25, 26]. Both MUC1 and MUC4 are expressed in normal salivary glands of all types [25]. In total, nineteen unique human mucin genes have been identified, with much of the work examining the association of these genes with carcinomas of the breast, lungs, colorectal region and prostate [24, 25]. Although the characteristic patterns of mucin expression for each organ can be maintained during neoplastic transformation, mucin expression may be altered in carcinomas compared to normal tissues [22, 23]. Mucins may therefore serve as potential markers aiding in the diagnosis of various carcinomas, as well as a predictor in terms of tumour recurrence and prognosis [23].
To date, few studies have evaluated mucin gene expression in head and neck cancers [25]. Recently, mucin gene expression was investigated in MECs with a possible link to overall prognosis, although results have been mixed. Initial studies utilised immunohistochemistry (IHC) to access expression of membrane-bound mucins (in particular MUC1 and MUC4) in MECs. These studies were limited, as several mucin genes had not been characterised at the time of research inception [27].
Due to the subjectivity of various qualitative and quantitative histological grading systems for MEC, epithelial mucin status may aid in establishing a more accurate grade, as well as predicting the biological behaviour of the tumour. The aim of this study is to investigate the expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2 and MUC5AC) in mucoepidermoid carcinomas to determine a possible correlation with tumour grade.
Materials and Methods
Case Selection
Major and minor salivary gland tumours, diagnosed as mucoepidermoid carcinoma, were retrieved from the pathology archives of the Department of Oral Pathology and Oral Biology, University of Pretoria. Two senior oral and maxillofacial pathologists confirmed the diagnosis in all of the cases. Cases were excluded from the study for reasons such as: missing formalin-fixed paraffin-embedded (FFPE) tissue block, inadequacy of tumoral tissue for further analysis, and cases where the tissue was placed in a decalcification medium prior to processing, to avoid complications with immunohistochemical stains. Histologically, the tumours were graded into three groups: low-, intermediate-, and high-grade, according to the modified grading system proposed by Brandwein et al. The use of this grading system is currently supported by the latest edition of the WHO classification of head and neck tumours. Cases where the tumour was either not graded or graded using another classification, were reassessed and graded by the system proposed by Brandwein et al. Fifteen cases of each tumour grade (low-, intermediate-, and high-grade) were selected, resulting in a total of 45 cases for further assessment.
Fluorescence In Situ Hybridization (FISH) Analysis
Haematoxylin and eosin–stained slides were re-examined for determination of areas for cell counting. Three-µm sections were cut from the FFPE tissue blocks and baked overnight at 58 °C. FISH for MAML2 rearrangement was performed using the ZytoLight SPEC MAML2 Dual Color Break Apart Probe and ZytoLight FISH Tissue Implementation kit (ZytoVision, Bremerhaven, Germany), according to the manufacturer’s recommendations. After dewaxing and hydration, slides were incubated in prewarmed ZytoLight Heat Pre-treatment Solution Citric at 98 °C for 15 min, then washed and digested with ZytoLight Pepsin solution for 10 min at 37 °C. The slides were then washed, dried and coverslipped with ZytoLight FISH MAML2 probe. Co-denaturation for 10 min at 75 °C and overnight hybridization at 37 °C were performed in a Thermobrite tissue hybridizer. Following hybridization, a stringent buffer wash was performed, the slides were then dehydrated and coverslipped with ZytoLight DAPI/DuraTect counterstain. Slides were examined immediately using a Nikon 50i fluorescence microscope.
One hundred randomly selected non-overlapping tumour cell nuclei were counted. The presence of yellow (fused orange and green signals demonstrating no break-apart) or separated orange and green (demonstrating chromosomal break-apart) fluorescent signals were examined. FISH results were considered positive for a MAML2 rearrangement if > 10% of all nuclei showed a separation of the orange and green signals by ≥ 2 signal widths, or if a deletion of the orange or green signal occurred.
Immunohistochemistry
For each case, representative paraffin wax blocks were selected for immunohistochemical (IHC) evaluation. Three-µm sections were cut from the FFPE blocks and baked overnight at 56 °C. IHC studies to demonstrate MUC1 and MUC4 protein expression (Table 1) were performed using the Ventana Benchmark GX automated system (Ventana Medical Systems Inc., Roche Diagnostics). Mild epitope retrieval was performed in CC1 high pH retrieval (Ventana Medical Systems Inc.), followed by incubation in an appropriately diluted primary antibody. The OptiView DAB multimer kit (Ventana Medical Systems Inc.) was used to detect antibody binding. Antibody retrieval for MUC2 and MUC5AC protein expression (Table 1) were performed in a TRIS high pH buffer using a Pascal Retrieval Unit (Dako CytoMation California Inc). Sections were incubated in appropriately diluted MUC2 and MUC5AC antibodies at room temperature. Detection was performed using the Novolink™ Polymer Detection System (Leica Biosystems Newcastle Ltd.). All IHC stains were counterstained in hematoxylin, dehydrated, cleared and mounted with a permanent mountant (DPX).
Two observers evaluated the immunohistochemical results independently by counting the percentage of positive cells. Cases without unanimous independent agreement were resolved with a consensus discussion. First, only the percentage expression of neoplastic mucous cells was evaluated. Next, the overall percentage expression of all tumour cells was calculated. MUC expression was quantified with a visual grading system whereby the intensity was categorised as negative, weak, moderate or strong. Neoplastic cells with a complete absence of expression were considered negative; weak expression as < 25%; moderate expression as 25–75%, and strong expression as > 75%. Staining intensity was not assessed as all cases showed a similar pattern (completely negative or strong).
Statistical Analysis
The clinicopathological characteristics, fluorescence in situ hybridization (FISH) and mucin (MUC) immunohistochemical expression were evaluated dichotomously. The results were recorded using Microsoft Excel Version 2016. Subsequent analysis of the categorical data was performed using SPSS software 26.0 (IBM Corporation, New York, NY). A univariate frequency table was constructed for each categorical variable, showing the percentage breakdown and distribution of the cases according to the variable parameters. Additionally, multivariate 2 × 3 and 3 × 3 tables were constructed in order to highlight the interaction of categorical variables prior to determining the statistical significance thereof. The association between independent, categorical, variables was evaluated using the Pearson’s Chi-squared test, along with the Fishers Exact test being used as a reasonability check. Correlations with a 2-sided Asymptotic Significance (p-value) of less than 0.05 were considered to be statistically significant. Where a statistical significant relationship was found to exist, the effect size was determined in order to measure the relative strength of the relationship between the two categorical variables.
Results
The main clinicopathological characteristics of the 45 tumours included in the study are summarised in Table 2. Figures 1, 2 and 3 show representative photomicrographs of one example of each tumour grade. The average age of patients was 49.8 years, with a wide age range (13 to 85 years). The gender distribution was essentially equal, with 23 cases in males and 22 cases in females. Twenty-nine tumours (65%) originated in the minor salivary glands and 16 (35%) in the major glands. The most common minor salivary gland sites included the palate (8 cases), followed by the buccal mucosa (7 cases), retromolar pad (5 cases), alveolar ridge (4 cases), floor of mouth (3 cases), and tongue and lip (1 case each). The parotid gland was the most commonly involved major salivary gland (11 cases), followed by the sublingual gland (3 cases) and the submandibular gland (2 cases).
Fluorescence In Situ Hybridization (FISH) Analysis
FISH was performed successfully on all 45 tumours. The results are summarised in Table 3. Of the 45 successful cases, 25 (55.6%) were positive for MAML2 rearrangement (Fig. 4) and 20 (44.4%) were negative. Of the 25 fusion-positive cases, 9 (36%) were high-grade, 9 (36%) were intermediate-grade and 7 (28%) were low-grade. No statistically significant association was found between MAML2 rearrangement status and tumour grade at a 95% confidence level (p = 0.698).
Mucin (MUC) Immunohistochemical Expression
MUC1
Mucous Cells
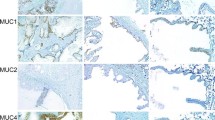
All MECs showed some degree of MUC1 expression in mucous cells, with 21 tumours (46.7%) showing strong expression, 13 tumours (28.9%) showing moderate expression and 11 tumours (24.4%) showing weak expression. The results are summarised in Table 4. High-grade tumours had the highest incidence of strong expression (73.3%) in mucous cells (Fig. 5a), intermediate-grade tumours had the highest incidence of moderate expression (40%) in mucous cells, and low-grade tumours had the highest incidence of weak expression (46.7%) in mucous cells. A statistically significant association was found between MUC1 expression in mucous cells and tumour grade at a 95% confidence level (p = 0.024).
Overall Tumour
All MECs showed some degree of MUC1 expression, with 24 tumours (53.3%) showing weak expression, 15 tumours (33.3%) showing moderate expression and 6 tumours (13.3%) showing strong expression. The results are summarised in Table 5. High-grade tumours had the highest incidence of strong expression (26.7%) (Fig. 5b), in comparison to 0% and 13.3% in intermediate-grade and low-grade tumours respectively. No statistically significant association was found between MUC1 expression in the overall tumour and tumour grade at a 95% confidence level (p = 0.272).
MUC2
Of the 45 MECs, only a single tumour (low-grade) showed MUC2 expression in mucous cells, and hence the overall tumour. The mucous cells showed a strong expression, whereas the overall tumour was scored as weak. As only one MEC showed MUC2 expression, no further statistical analysis was performed.
MUC4
Mucous Cells
Only two high-grade MECs (4.4%) showed no MUC4 expression in mucous cells, with all remaining tumours showing some degree of expression in mucous cells. The results are summarised in Table 6. Most MECs (75.6%) showed strong expression in mucous cells. Low-grade tumours had the highest incidence of strong expression (93.3%) in mucous cells (Fig. 6a), in comparison to 80% and 53.3% in intermediate-grade and high-grade tumours respectively. No statistically significant association was found between MUC4 expression in mucous cells and tumour grade at a 95% confidence level (p = 0.145).
Overall Tumour
Only two high-grade MECs (4.4%) showed no MUC4 expression, with all remaining tumours showing some degree of expression. The results are summarised in Table 7. Most MECs (42.2%) showed moderate expression. Low-grade tumours had the highest incidence of strong expression (53.3%) (Fig. 6b), in comparison to 33.3% and 0% in intermediate-grade and high-grade tumours respectively. A statistically significant association was found between MUC4 expression in the overall tumour and tumour grade at a 95% confidence level (p = 0.01).
MUC5AC
Mucous cells
Only two high-grade MECs (4.4%) showed no MUC5AC expression in mucous cells, with all remaining tumours showing some degree of expression in mucous cells. The results are summarised in Table 8. Most MECs (62.2%) showed strong expression in mucous cells. Intermediate-grade tumours had the highest incidence of strong expression (80%) in mucous cells (Fig. 7a), in comparison to 53.3% in both high-grade and low-grade tumours. No statistically significant association was found between MUC5AC expression in mucous cells and tumour grade at a 95% confidence level (p = 0.326).
Overall Tumour
Only two high-grade MECs (4.4%) showed no MUC5AC expression, with all remaining tumours all showing some degree of expression. The results are summarised in Table 9. Most MECs (84.4%) showed weak expression. Intermediate-grade tumours had the highest incidence of weak expression (93.3%) (Fig. 7b), in comparison to 80% in both high- and low-grade tumours. Only a single tumour (high-grade) showed strong expression. No statistically significant association was found between MUC5AC expression in the overall tumour and tumour grade at a 95% confidence level (p = 0.137).
MUC Expression and FISH Analysis
Additionally, the mucin expression of all MUCs performed in this study was compared to the FISH MAML2 rearrangement results. No statistically significant association was found between MUC expression in both the mucous cells and the overall tumour, and the MAML2 rearrangement status at a 95% confidence level.
Discussion
Primary epithelial salivary gland tumours are a rare, morphologically diverse group of neoplasms that often present considerable diagnostic challenges to the pathologist, and management conundra to surgeons and oncologists [28]. Histological grading of mucoepidermoid carcinoma plays an important role in predicting accurate tumour behaviour and patient prognosis [16]. It is therefore imperative that the grading of these tumours is done in an accurate, reproducible manner with limited interobserver variability. The current study assessed the expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2 and MUC5AC) in MECs via immunohistochemistry as a possible technique to aid in establishing a more accurate tumour grade. To the authors knowledge, this is the only study to first independently assess mucin expression in neoplastic mucous cells only, before assessing overall tumour expression. This was done to distinguish whether mucin expression differed in the mucous cells alone, compared to the overall tumour.
MUC1 overexpression reduces cell–matrix and cell–cell adhesion, favouring invasion of the tumour cells into the underlying stroma [25, 26]. MUC1 is therefore considered a promising biomarker, with several carcinomas showing high MUC1 expression compared with normal tissue [26]. Furthermore, MUC1 overexpression in papillary thyroid carcinoma, breast carcinoma and prostate carcinoma was found to be associated with aggressive tumour behaviour and poor clinical outcome [22, 29, 30]. Previous studies by Handra-Luca et al. [31] and Llupi and Qoku [22] failed to find any prognostic significance of MUC1 expression in MECs. In contrast, Alos et al. [25] found that MUC1 expression in greater than 50% of tumour cells was indeed associated with a higher histological grade, increased risk of metastasis and poorer prognosis. Siya et al. [32] were in agreement with the findings of Alos et al. [25], however their study suggested a MUC1 expression level of greater than 75% in tumour cells. Shemirani et al. [27] evaluated mucin expression using quantitative polymerase chain reaction (qPCR) techniques, finding that greater expression of MUC1 in tumour cells correlated with a less aggressive disease process and an increased survival rate. The current study found that high-grade MECs had the highest incidence of strong MUC1 expression in mucous cells, with intermediate- and low-grade tumours showing the highest incidence of moderate and weak expression respectively. These findings were in support of previous studies by Alos et al. [25] and Siya et al. [32], and demonstrated a statistically significant association between MUC1 expression in mucous cells and tumour grade. Regarding MUC1 expression in the overall tumour, both low- and intermediate-grade tumours showed a weaker overall MUC1 expression in comparison to high-grade tumours. These findings were once again in support of those by Alos et al. [25] and Siya et al. [32], however; in the current study the association was not statistically significant. These results indicate that MUC1 overexpression may be a reliable marker of high-grade MECs.
The role of MUC2 in carcinogenesis is incompletely understood. Several studies found MUC2 gene expression to be consistently positive in mucinous carcinomas of the colon, stomach, pancreas, breast and ovary, linking its expression to the so-called “mucinous pathway of carcinogenesis” [23, 33]. Other studies highlight the tumour suppressor nature of MUC2, indicating that overexpression of MUC2 by pancreatic and biliary tumours was associated with a low degree of invasiveness and better overall prognosis [26]. Only a single previous study by Alos et al. [25] assessed MUC2 expression in MECs. They found an overall lack of expression, suggesting that MECs develop along a different pathway from other mucinous tumours. In the current study, only a single tumour (low-grade) showed MUC2 expression in the mucous cells, and hence the overall tumour. All remaining tumours showed no expression of MUC2 in both the mucous cells and the overall tumour. These findings were in support of those by Alos et al. [25], further strengthening the concept that MECs possibly develop along a different pathway from other mucinous tumours. MUC2 may therefore serve as a useful marker to exclude MEC from the differential diagnosis when encountering a malignant salivary gland tumour.
MUC4 contains an epidermal growth factor (EGF)-like domain that interacts with ERBB2 leading to a decrease in apoptosis and an alteration in tumour adhesion [26, 34]. MUC4 overexpression has been reported in well-differentiated ovarian tumours. The opposite is true for adenocarcinomas of the breast and pancreas, where MUC4 overexpression has been related to overall tumour aggressiveness [26, 35]. Previous studies by Handra-Luca et al. [31], Alos et al. [25] and Llupi and Qoku [22] all found that the expression of MUC4 correlated with lower histological grade and an improved prognosis. The current study found MUC4 to be a good marker of neoplastic mucous cells. Low-grade MECs, with their high proportion of mucous cells, showed the highest incidence of strong MUC4 expression in mucous cells. These findings were in support of previous studies [22, 25, 31], but failed to show a statistically significant association between MUC4 expression in mucous cells and tumour grade. Statistical analysis did however reveal that based on the strength of the relationship, an increased sample size would likely result in a statistically significant association. Regarding MUC4 expression in the overall tumour, low-grade tumours showed the highest incidence of strong MUC4 expression. MUC4 expression in the overall tumour decreases as the tumour grade increases. This is likely due to the decreased mucous cell content in higher grade MECs. These findings were in support of previous studies [22, 25, 31] and demonstrated a statistically significant association between MUC4 expression in the overall tumour and tumour grade. These results indicate that MUC4 overexpression may be a reliable marker of low-grade MECs.
MUC5AC may suppress immune cells and produce potent anti-apoptotic effects, thereby playing an important role in creating a suitable environment for cancer cell growth [36]. MUC5AC expression has been observed in a variable percentage of carcinomas of pancreaticobiliary, gastrointestinal and endocervical origin [23, 24]. Several studies have reported MUC5AC expression as a good prognostic indicator in gastric, gallbladder and colon carcinomas, whereas others have reported a poor prognosis in pancreatic carcinoma [37]. Previous studies by Alos et al. [25] and Llupi and Qoku [22] found that MUC5AC expression was less intense in high-grade MECs compared to low-grade counterparts. The current study found that most MECs showed strong MUC5AC expression in mucous cells, with intermediate-grade tumours showing the highest incidence of strong expression. Regarding MUC5AC expression in the overall tumour, most tumours showed weak expression, with intermediate-grade tumours having the highest incidence of weak expression. Interestingly, only a single tumour (high-grade) showed strong overall expression. No statistically significant association was seen between MUC5AC expression in mucous cells or the overall tumour, and tumour grade. These findings were in contrast to those by Alos et al. [25] and Llupi and Qoku [22], and suggest that MUC5AC expression is an unreliable marker in determining tumour grade of MECs.
Conclusion
Mucoepidermoid carcinomas show an altered mucin expression pattern that can be used for diagnostic purposes, as well as a supplement to aid in establishing a more accurate grade. The results of this study indicate the MUC1 overexpression may be a reliable marker of high-grade MECs, whereas MUC4 overexpression may be more indicative of low-grade tumours. MUC5AC expression was found to be an unreliable marker in determining tumour grade. Only a single MEC in the study showed weak MUC2 expression, suggesting that MUC2 may be a useful marker to exclude MEC from the differential diagnosis when encountering a malignant salivary gland tumour. These findings shed new light on the biological process of MECs and aid in establishing a more accurate grade.
References
Ellis GL, Auclair PL. AFIP atlas of tumor pathology. Washington, DC: American Registry of Pathology in Collaboration with the Armed Forces Institute of Pathology; 2008.
El-Naggar AK, Chan JKC, Rubin-Grandis J, Takata T, Slootweg PJ, International Agency for Research on Cancer. World Health Organization classification of tumours. 4th ed. Lyon: International Agency for Research on Cancer; 2017.
Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25(7):835–45.
Griffith CC, Seethala RR. Mucoepidermoid carcinoma of minor salivary glands: potential pitfalls and prognostic challenges. Pathol Case Rev. 2011;16(4):145–50.
Klijanienko J, El-Naggar AK, Servois V, Rodriguez J, Validire P, Vielh P. Mucoepidermoid carcinoma ex pleomorphic adenoma: nonspecific preoperative cytologic findings in six cases. Cancer. 1998;84(4):231–4.
Waldron CA, Koh ML. Central mucoepidermoid carcinoma of the jaws: report of four cases with analysis of the literature and discussion of the relationship to mucoepidermoid, sialodontogenic, and glandular odontogenic cysts. J Oral Maxillofac Surg. 1990;48(8):871–7.
Nakayama T, Miyabe S, Okabe M, Sakuma H, Ijichi K, Hasegawa Y, et al. Clinicopathological significance of the CRTC3-MAML2 fusion transcript in mucoepidermoid carcinoma. Mod Pathol. 2009;22(12):1575–81.
Bishop JA, Cowan ML, Shum CH, Westra WH. MAML2 rearrangements in variant forms of mucoepidermoid carcinoma: ancillary diagnostic testing for the ciliated and warthin-like variants. Am J Surg Pathol. 2018;42(1):130–6.
Luk PP, Wykes J, Selinger CI, Ekmejian R, Tay J, Gao K, et al. Diagnostic and prognostic utility of Mastermind-like 2 (MAML2) gene rearrangement detection by fluorescent in situ hybridization (FISH) in mucoepidermoid carcinoma of the salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(5):530–41.
Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34(8):1106–21.
Behboudi A, Enlund F, Winnes M, Andren Y, Nordkvist A, Leivo I, et al. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosom Cancer. 2006;45(5):470–81.
Saade RE, Bell D, Garcia J, Roberts D, Weber R. Role of CRTC1/MAML2 translocation in the prognosis and clinical outcomes of mucoepidermoid carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142(3):234–40.
Luna MA. Salivary mucoepidermoid carcinoma: revisited. Adv Anat Pathol. 2006;13(6):293–307.
Seethala RR, Chiosea SI. MAML2 status in mucoepidermoid carcinoma can no longer be considered a prognostic marker. Am J Surg Pathol. 2016;40(8):1151–3.
Cipriani NA, Lusardi JJ, McElherne J, Pearson AT, Olivas AD, Fitzpatrick C, et al. Mucoepidermoid carcinoma: a comparison of histologic grading systems and relationship to MAML2 rearrangement and prognosis. Am J Surg Pathol. 2019;43(7):885–97.
Seethala RR. An update on grading of salivary gland carcinomas. Head Neck Pathol. 2009;3(1):69–77.
Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82(7):1217–24.
Gemryd P, Lundquist PG, Tytor M, Hellquist HB, Nordenskjold B. Prognostic significance of DNA ploidy in mucoepidermoid carcinoma. Eur Arch Otorhinolaryngol. 1997;254(4):180–5.
Pires FR, de Almeida OP, de Araujo VC, Kowalski LP. Prognostic factors in head and neck mucoepidermoid carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130(2):174–80.
Van Heerden WF, Raubenheimer EJ, Dreyer L. The role of DNA ploidy and Ki-67 in the grading of mucoepidermoid carcinomas. Anticancer Res. 2005;25(3c):2589–92.
Nance MA, Seethala RR, Wang Y, Chiosea SI, Myers EN, Johnson JT, et al. Treatment and survival outcomes based on histologic grading in patients with head and neck mucoepidermoid carcinoma. Cancer. 2008;113(8):2082–9.
Llupi M, Qoku R. Expression of mucins in normal salivary glands and mucoepidermoid carcinoma of salivary glands. 2013.
Lau SK, Weiss LM, Chu PG. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol. 2004;122(1):61–9.
Krishn SR, Ganguly K, Kaur S, Batra SK. Ramifications of secreted mucin MUC5AC in malignant journey: a holistic view. Carcinogenesis. 2018;39(5):633–51.
Alos L, Lujan B, Castillo M, Nadal A, Carreras M, Caballero M, et al. Expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC7) in mucoepidermoid carcinomas of salivary glands. Am J Surg Pathol. 2005;29(6):806–13.
Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4(1):45–60.
Shemirani N, Osipov V, Kolker A, Khampang P, Kerschner JE. Expression of mucin (MUC) genes in mucoepidermoid carcinoma. Laryngoscope. 2011;121(1):167–70.
Speight PM, Barrett AW. Salivary gland tumours: diagnostic challenges and an update on the latest WHO classification. Diagn Histopathol. 2020;26(4):147–58.
Rahn JJ, Dabbagh L, Pasdar M, Hugh JC. The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer. 2001;91(11):1973–82.
Wreesmann VB, Sieczka EM, Socci ND, Hezel M, Belbin TJ, Childs G, et al. Genome-wide profiling of papillary thyroid cancer identifies MUC1 as an independent prognostic marker. Cancer Res. 2004;64(11):3780–9.
Handra-Luca A, Lamas G, Bertrand JC, Fouret P. MUC1, MUC2, MUC4, and MUC5AC expression in salivary gland mucoepidermoid carcinoma: diagnostic and prognostic implications. Am J Surg Pathol. 2005;29(7):881–9.
Siyi L, Shengwen L, Min R, Wenjun Y, Lizheng W, Chenping Z. Increased expression of MUC-1 has close relation with patient survivor in high-grade salivary gland mucoepidermoid carcinoma. J Oral Pathol Med. 2014;43(8):579–84.
Copin MC, Devisme L, Buisine MP, Marquette CH, Wurtz A, Aubert JP, et al. From normal respiratory mucosa to epidermoid carcinoma: expression of human mucin genes. Int J Cancer. 2000;86(2):162–8.
Jepson S, Komatsu M, Haq B, Arango ME, Huang D, Carraway CA, et al. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27(kip), but does not activate mitogen-activated kinase or protein kinaseB/Akt pathways. Oncogene. 2002;21(49):7524–32.
Rachagani S, Torres MP, Moniaux N, Batra SK. Current status of mucins in the diagnosis and therapy of cancer. BioFactors. 2009;35(6):509–27.
Hoshi H, Sawada T, Uchida M, Iijima H, Kimura K, Hirakawa K, et al. MUC5AC protects pancreatic cancer cells from TRAIL-induced death pathways. Int J Oncol. 2013;42(3):887–93.
Kim SM, Kwon CH, Shin N, Park DY, Moon HJ, Kim GH, et al. Decreased Muc5AC expression is associated with poor prognosis in gastric cancer. Int J Cancer. 2014;134(1):114–24.
Funding
This study was funded in part by the National Research Foundation (NRF), South Africa.
Author information
Authors and Affiliations
Contributions
WvH, KH and LR all contributed to the study conception and design. The literature review was undertaken by LR with guidance from WvH. Material preparation, data collection and analysis were performed by WvH, KH and LR. Immunohistochemical stains and FISH analysis were performed by MvH. Statistical analysis was performed by JK-F. The first draft of the manuscript was written by LR, with all authors giving feedback and corrections. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the University of Pretoria, Faculty of Health Sciences Research Ethics Committee (Reference no.: 759/2019).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Robinson, L., van Heerden, M.B., Ker-Fox, J.G. et al. Expression of Mucins in Salivary Gland Mucoepidermoid Carcinoma. Head and Neck Pathol 15, 491–502 (2021). https://doi.org/10.1007/s12105-020-01226-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-020-01226-z