Abstract
We report a rare case of Cushing’s syndrome in a 59-year-old man who initially presented with concurrent acinic cell carcinoma of the parotid with high-grade transformation and co-existing papillary and medullary thyroid carcinomas, without noticeable cushinoid symptoms. Six-months later, he developed clinical features of Cushing’s syndrome which coincided with disease progression in the form of lung metastasis and mediastinal lymphadenopathy. Ectopic adrenocorticotropic hormone (ACTH) production and protein expression was limited to the high-grade transformed component of acinic cell carcinoma and in the lymph node metastasis, and was absent in the conventional acinic cell carcinoma as well as in the papillary and medullary thyroid carcinoma. He received adjuvant chemotherapy and supportive management with interval improvement for 8 months followed by disease progression with increasing serum cortisol levels and bone metastasis. He was offered palliative chemotherapy, however, declined further therapy and was lost to follow up. We discussed clinical and pathologic implications of ectopic ACTH production associated with acinic carcinoma and also reviewed the literature of this rare paraneoplastic syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cushing’s syndrome due to production and secretion of ectopic adrenocorticotropic hormone (ACTH) by non-pituitary solid malignant tumors is uncommon, accounting for 5–18% of ACTH-dependent Cushing’s syndrome in adults [1,2,3]. In patients with paraneoplastic Cushing’s syndrome, majority of cases are due to small cell carcinoma of the lung and bronchial carcinoids, while thymic carcinoids, pancreatic neuroendocrine tumor, pheochromocytoma and medullary thyroid carcinoma are responsible for remaining cases [2,3,4,5,6,7,8]. In rare instances, malignant salivary gland tumors such as acinic cell carcinoma and adenoid cystic carcinoma have been associated with ectopic ACTH production [9,10,11,12,13,14,15,16,17]. The pathogenesis of this condition has been attributed to the activation of the ACTH regulating proopiomelanocortin (POMC) gene in tumor cells [1]. The clinical manifestations of ectopic ACTH syndrome is related to the metabolic and electrolyte imbalances caused by the excessive production of cortisol beyond the neutralizing ability of the 11β-hydroxysteroid dehydrogenase enzyme in the renal tubules to convert cortisol to inactive cortisone [1, 18,19,20]. Patients with ectopic ACTH syndrome generally have early subtle clinical manifestations such as abdominal obesity, hypertension, psychiatric (depression, lethargy), orthopedic (osteoporosis), reproductive (menstrual dysregulation) and nonspecific systemic signs and symptoms that closely overlap with those of cancer-related morbidities, thereby precluding early detection. Unawareness and the nonspecific symptoms of paraneoplastic ACTH syndrome have invariably contributed to delayed diagnosis and prevented early therapeutic intervention of this condition [2,3,4, 9,10,11, 14].
Similar to other malignant tumors with paraneoplastic ACTH secretion, Cushing’s syndrome caused by ectopic ACTH production in patients with salivary gland carcinoma have been detected late during the course of progressive disease with fatal outcome [9,10,11,12,13,14,15,16,17]. The occurrence of this condition is rare. Only nine cases have been reported in the English literature, including acinic cell carcinoma (five cases), adenoid cystic carcinoma (three cases) and poorly differentiated adenocarcinoma (one case). All of these cases followed an aggressive clinical course including metastasis and/or high-grade transformation [9,10,11,12,13,14,15,16,17]. The restricted occurrence of this condition to certain tumor subtypes, exclusive of other more aggressive salivary gland malignant tumors, suggests that tumor context-dependent genetic factors or signaling might play a role in this clinical setting. We contend that early recognition and screening for ACTH expression in certain malignant salivary gland tumors, especially the ones with high-grade transformation, may have clinical and therapeutic implications with timely intervention.
We here present an intriguing case of Cushing’s syndrome in a patient with acinic cell carcinoma of the parotid with high-grade transformation and coexisting papillary and medullary thyroid carcinomas, who later on developed features of Cushing’s syndrome and tumor dissemination. We discuss the clinicopathologic manifestations and putative etiologic associations of the current case along with review of previously reported cases of acinic cell carcinoma associated with ectopic ACTH production (Table 1).
Case History
A 58-year-old non-smoker male presented at an outside institution in December 2014 with a right parotid mass that had reportedly grown from a pea-sized nodule to a size of a clementine over a period of 2 years. Positron emission tomography-computed tomography (PET-CT) from vertex of the head to thigh performed in December 2014 revealed a 4.3 cm 18F-fluorodeoxyglucose (FDG)-avid right parotid mass, as well as periparotid and right neck level II lymph nodes. Additionally, a 2.0 cm mass was noted in the thyroid isthmus with mild FDG uptake. No distant metastatic lesions were noted (cM0). Fine needle aspiration (FNA) was performed at the outside institution. The parotid mass was reported as poorly differentiated adenocarcinoma and the thyroid mass as suspicious for follicular neoplasm, respectively. In January 2015, he underwent right total parotidectomy with facial nerve preservation, right modified radical neck dissection, and right hemi-thyroidectomy. Pathology evaluation was reported as right parotid poorly-differentiated adenocarcinoma, 4.5 cm in greatest dimension with extra-parenchymal soft tissue extension, no perineural invasion, resection margins negative for tumor, and with lymph node metastasis in four out of forty-two lymph nodes (two periparotid and two right neck level II) (pT3, pN2b, AJCC 7th edn). The thyroid mass was diagnosed as adenomatoid nodule. Thereafter, he received 6 weeks of adjuvant radiotherapy, 35 fractions to the parotid tumor bed and right neck, in February–March 2015.
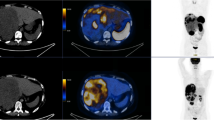
Six months following surgery, the patient presented with peripheral edema, abnormal weight gain (approximately 40 lb in 6 months), hypokalemia (serum K 3.1 mEq/L), hypertension (BP 187/112), muscle weakness and puffiness/fat deposits in the face and upper back, suggestive of Cushing’s syndrome, which was confirmed by increased morning serum cortisol (95.2 µg/dL; range 6.2–19.4) and serum ACTH (263 pg/mL; range 0–46) levels, suggesting an ectopic etiology. He was treated with furosemide, clonidine, KCl and ketoconazole. Re-staging PET-CT performed in July 2015 excluded pituitary adenoma, however, revealed multiple FDG-avid hypermetabolic supraclavicular (2.2 cm, max SUV 16.0), subcarinal (4.6 cm, max SUV 15.6), precarinal (1.7 cm, max SUV 14.4), mediastinal, and hilar lymph nodes as well as few scattered small bilateral lung nodules (3.0 to 5.0 mm, largest nodule was hypermetabolic). No FDG activity was noted in the head and neck tumor bed area or in the remaining thyroid. An endobronchial ultrasound-guided fine needle aspiration (EBUS-FNA) of the mediastinal node was performed and reported as poorly differentiated carcinoma, positive for pan-cytokeratin and EMA; and negative for CK7, CK20, S100, TTF-1, synaptophysin, melan A and p63, with cytomorphologic features similar to that of the parotid primary. He received first line systemic therapy with carboplatin and paclitaxel for 6 cycles until December 2015 with partial response. Two-months status-post therapy, an interval contrast CT scan of the chest, abdomen and pelvis showed improvement in subcarinal (3.7 cm, initially 4.6 cm), right precarinal (1.0 cm, initially 1.7 cm) and mediastinal lymph nodes with complete resolution of small bilateral pulmonary nodules. No other metastatic lesion was identified in the abdomen or pelvis. CT brain with and without contrast showed no metastasis. In March 2016, a repeat CT chest, abdomen and pelvis showed disease progression in the form of increased mediastinal and perihilar lymphadenopathy (paratracheal nodes increased from 1.2 to 2.9 cm and 1.0 to 1.6 cm; subcarinal node increased from 3.7 to 4.4 cm) and new lytic bone lesions at T8 and L3 vertebrae, radiologically consistent with metastatic disease. Clinically, his symptoms of Cushing’s syndrome became severe with more weight gain, ecchymosis, muscle weakness, progressive bilateral symmetrical peripheral edema and occasional low blood pressure, with an increased morning serum cortisol 360.2 µg/dL (range 6.2–19.4) levels. The patient was then referred to our institution to seek consultation regarding treatment options. Due to significant bone pain secondary to vertebral lytic bone lesions, the patient received a course of palliative chemo-radiotherapy and his Cushing’s syndrome was managed with lisinopril, pravastatin, metformin, mifepristone, and ranitidine.
Pathologic Findings
In May 2016, per request by the clinical team, review of his pathology material was performed at our institution. Histologic sections of the right parotidectomy showed that majority of the tumor (90–95%) was composed of infiltrating poorly differentiated carcinoma with diffuse non-descript solid/nested and glandular patterns with desmoplasia and areas of necrosis (Fig. 1a, b). The tumor cell in these areas showed nuclear pleomorphism with high nuclear to cytoplasmic ratio, vesicular nuclei with prominent nucleoli, frequent mitosis and apoptosis with scattered areas of necrosis (Fig. 1c, e). Lymphovascular invasion was identified; however, no perineural invasion was noted. Focally, a low-grade conventional/differentiated acinic cell carcinoma component (approximately 5–10% of tumor) composed of polyhedral acinar/epithelial cells arranged in a microcystic and lobular nested pattern was noted (Fig. 1a, b). The neoplastic cells in these areas have relative low nuclear to cytoplasmic ratio; abundant basophilic granular cytoplasm admixed with scattered vacuolated cells, and with no mitosis, apoptosis or necrosis (Fig. 1d, f). The resection margins were negative for tumor, albeit focally tumor was very close to inked resection edges (less than 0.5 mm). Four out of forty-two lymph nodes were positive for metastatic carcinoma (two periparotid and two right neck level II lymph nodes), comprised entirely of high-grade component with largest metastatic focus measured 3.0 cm in greatest dimension with extranodal extension (stage pN3b according to the current AJCC 8th edn) (Fig. 2a).
A composite photomicrograph of histologic and ACTH immunostain in acinic cell carcinoma with high-grade transformation. Histologic sections from two different areas show infiltrating poorly differentiated carcinoma (high-grade transformation) in a non-descript nested/solid and glandular patterns with accompanying desmoplasia and fibrosis (left side of panel a, b) [hematoxylin and eosin stain (H&E), ×40]. Juxtaposed and focally admixed were residual focal areas of differentiated/conventional acinic cell carcinoma in microcystic, solid and follicular patterns with scattered tumor-associated lymphoid infiltrate (right side of panel a, b). High-grade transformed areas lack differentiation and show pleomorphic cells with high nuclear to cytoplasmic ratio, vesicular nuclei with coarse chromatin, distinct nucleoli and frequent mitotic figures (arrows). Scattered areas of necrosis were also noted [panel c (H&E, ×100) and E (H&E, ×200)]. The neoplastic cells in the differentiated component composed of round to oval cells with abundant basophilic granular cytoplasm and indistinct cell borders with admixed vacuolated cells [panel d (H&E, ×100) and f (H&E, ×200)]. ACTH immunostaining shows heterogeneous cytoplasmic positivity in the high-grade transformed component (panel g) and was absent in the differentiated component of acinic cell carcinoma (panel h)
Panel a show right neck level II lymph node metastasis with extranodal extension (arrows) (H&E, ×40). High power magnification shows metastatic carcinoma is composed of high-grade transformed component of acinic cell carcinoma (inset) (H&E, ×200). Mirror image of panel a shows positive ACTH immunostaining of the tumor cells in the metastatic foci (panel b, ×40) (inset, higher magnification, ×200). Panel c shows focus of infiltrating papillary thyroid carcinoma, follicular variant involving the isthmus (H&E, ×40). The neoplastic cells show enlarged round to elongated optically clear nuclei with overlapping, irregular nuclear contours and fine dispersed chromatin (panel d, H&E, ×100). Panels e, f shows focus of medullary thyroid microcarcinoma in the background of C-cell hyperplasia involving right thyroid lobe. Incidentally, rare admixed solid cell nests (arrows) were also noted (H&E, ×40 and ×200 respectively). Immunostains show the neoplastic cells in the focus of medullary thyroid microcarcinoma and background C-cell hyperplasia were positive for calcitonin (panel g) and negative for ACTH (panel h, inset, higher magnification, ×200). Foci of solid cell nests (arrows) were negative for calcitonin and ACTH
Histologic sections of the thyroid showed a 2.0 cm papillary thyroid carcinoma, follicular variant (Fig. 2c, d) involving isthmus as well as multifocal (2×) medullary thyroid microcarcinomas (3.0 mm and 2.0 mm) in the background of C-cell hyperplasia (Fig. 2e, f) involving the right thyroid lobe. The foci of medullary thyroid microcarcinomas showed small infiltrating solid nests and single cells with fibrosis that were positive for calcitonin (Fig. 2g) and TTF-1 immunostains (not shown). As the patient presented with features of Cushing’s syndrome, immunohistochemical stain for ACTH was performed on both parotid and thyroid malignant tumors which showed heterogeneous cytoplasmic expression in the high-grade transformed component of acinic cell carcinoma, both in the primary parotid tumor (Fig. 1g) and in lymph node metastasis (Fig. 2b); and was absent in the conventional/differentiated acinic cell carcinoma component (Fig. 1h) as well as medullary (Fig. 2h) and papillary thyroid carcinoma (not shown).
Follow Up
Due to progressive metastatic disease, the patient was ineligible for bilateral adrenalectomy and was advised to pursue palliative systemic therapy with doxorubicin and cyclophosphamide, and to follow up with his oncologist at his parent institution. However, patient declined further therapy was lost to follow up.
Discussion
Acinic cell carcinoma is a malignant salivary gland neoplasm characterized by proliferation of neoplastic cells with acinar differentiation showing abundant cytoplasmic zymogen secretory granules. In majority of cases (90–95%), parotid gland is the most commonly implicated site. Acinic cell carcinoma accounts for 1–7% of all tumors and 7–15% of malignant tumors originating in the major salivary glands [21,22,23,24,25]. It is considered a low-grade neoplasm as majority of the cases have an indolent course. However, minor proportion of cases, almost exclusive to the parotid gland, can undergo high-grade transformation (also described previously as “dedifferentiation”) and show aggressive behavior with regional and distant metastasis [21,22,23,24,25]. Compared to conventional acinic cell carcinoma, cases with high-grade transformation have been reported to have a shorter mean overall survival (40 months vs 125 months) [24, 26].
In this study, we report a rare interesting case of ectopic ACTH production associated with Cushing’s syndrome in a patient with concurrent parotid and thyroid gland carcinomas. Similar to previously reported instances, the diagnosis was belatedly made post-resection which coincided with progressive widely metastatic disease [9,10,11,12,13,14,15,16,17]. In contrast to previous reports of salivary gland tumors, our patient presented with multiple primary tumors including medullary and papillary thyroid carcinomas and parotid carcinoma leading to initial delay in identifying the source of ectopic ACTH production [7,8,9,10,11,12,13,14,15,16,17]. Initially, medullary thyroid carcinoma was suspected to be the source of ACTH, but the lack of its expression led to the analysis of the parotid primary. Interestingly, cytoplasmic ACTH expression was exclusively limited to the high-grade transformed component of the primary acinic cell carcinoma and in metastatic lymph nodes. Similar findings have previously been reported in acinic cell carcinoma with high-grade transformation, thereby showing the propensity of ectopic ACTH activation with tumor progression [12, 13, 21]. However, ectopic ACTH expression has also been reported in differentiated acinic cell carcinoma and adenoid cystic carcinomas with progressive disease. Together these findings suggest the ectopic ACTH in both adenoid cystic and acinic cell carcinomas is associated with disease progression irrespective of cellular differentiation [9,10,11,12,13,14,15].
Recurrent genomic rearrangement of t(4;9)(q13;q31) as a genetic driver event has been recently described in acinic cell carcinoma [27]. This rearrangement causes constitutive upregulation of oncogenic transcription factor nuclear receptor subfamily 4 member 3 (NR4A3) which is also known as NOR1 receptor. Nuclear expression by immunohistochemical staining for NR4A3/NOR1 has been suggested as a highly sensitive and specific novel marker for acinic cell carcinoma [27, 28]. Interestingly, it has previously been shown that NR4A3/NOR1 (one of the three members of NR4A superfamily of orphan nuclear receptors) expression has been described in adrenal cortex and pituitary gland [29]. According of this study, ACTH induces transcription activation and expression of NOR1 mRNA in mice cultures of adrenal gland fasiculata cells and is involved in regulation of hypothalamic–pituitary–adrenal (HPA) axis [29]. The selective expression of NR4A3/NOR1 in acinic cell carcinoma and their previously demonstrated role in HPA axis regulation might play a role in development of Cushing syndrome in some of these patients.
The restricted occurrence of ectopic ACTH to acinic cell and adenoid cystic carcinoma phenotypes, exclusive of other aggressive salivary carcinomas is currently uncertain but empirically suggests tumor context specificity [9,10,11,12,13,14,15,16,17]. We, therefore, posit that ectopic ACTH may selectively be induced in certain biologically aggressive acinic cell and adenoid cystic carcinomas patients as a result of alterations of the POMC gene during progression. Transcript of the POMC, a highly conserved gene located on chromosome 2p23 region, has been detected at low levels in multiple normal tissues but its expression in salivary glands is unknown. It is likely that activation of this gene during progression in a subset of acinic cell salivary carcinoma may underlie the aberrant ACTH expression and Cushing’s syndrome [21, 30, 31]. Further studies of the genetic and epigenetic modification, and/or changes disrupting the regulation of this gene may shed more light on the aberrant activation and specificity of this gene in acinic cell carcinoma patients. Future retrospective studies of ACTH expression in aggressive subset of these tumors may shed more light on the incidence of this feature in aggressive salivary carcinomas for early detection and intervention.
Our case and those previously reported cases underscore the insidious nature and associated comorbidities that preclude early detection and timely management in these patients. Thus, high level of clinical suspicion and awareness of the association of ectopic ACTH production in certain aggressive tumors and timely testing and interpretation of metabolic imbalances is necessary for early detection of ectopic ACTH secretion [14,15,16,17]. This contention is supported by evidence that early diagnosis leads to improved 5-year overall survival of these patients [18, 20] and that early detection of the ectopic source of aberrant ACTH and treatment with adrenal enzyme inhibitors, and/or bilateral adrenalectomy can lead to improvement in patients outcome [18,19,20]. We, therefore recommend advance judicial use of immunohistochemistry for ACTH detection in selected cases of aggressive adenoid cystic and acinic cell carcinomas patients in coordination with treating clinicians.
In conclusion, our case represents a unique example of ectopic ACTH secretion by acinic cell carcinoma with high-grade transformation causing paraneoplastic Cushing’s syndrome. The selective propensities for ectopic ACTH production to acinic cell and adenoid cystic carcinomas exclusive of other salivary carcinoma subtypes suggest that a tumorigenic event acquired during progression underlie the activation of a constitutionally silent gene in certain tumors.
References
DeLellis RA, Xia L. Paraneoplastic endocrine syndromes: a review. Endocr Pathol. 2003;14(4):303–17.
Alexandraki KI, Grossman AB. The ectopic ACTH syndrome. Rev Endocr Metab Disord. 2010;11(2):117–26.
Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90(8):4955–62.
Kamp K, Alwani RA, Korpershoek E, Franssen GJ, de Herder WW, Feelders RA. Prevalence and clinical features of the ectopic ACTH syndrome in patients with gastroenteropancreatic and thoracic neuroendocrine tumors. EurJ Endocrinol. 2016;174(3):271–80.
Ballav C, Naziat A, Mihai R, Karavitaki N, Ansorge O, Grossman AB. Mini-review: pheochromocytomas causing the ectopic ACTH syndrome. Endocrine. 2012;42(1):69–73.
Thomas T, Zender S, Terkamp C, Jaeckel E, Manns MP. Hypercortisolaemia due to ectopic adrenocorticotropic hormone secretion by a nasal paraganglioma: a case report and review of the literature. BMC Res Notes. 2013;6:331.
Marques P, Vieira Mda S, Bugalho MJ. Ectopic cushing in a patient with medullary thyroid carcinoma: hypercortisolism control and tumor reduction with Sunitinib. Endocrine. 2015;49(1):290–2.
Singer K, Heiniger N, Thomas I, Worden FP, Menon RK, Chen M. Ectopic Cushing syndrome secondary to metastatic medullary thyroid cancer in a child with multiple endocrine neoplasia syndrome type 2B: clues to early diagnosis of the paraneoplastic syndromes. J Pediatr Endocrinol Metab. 2014;27(9–10):993–6.
Dacruz T, Kalhan A, Rashid M, Obuobie K. An Ectopic ACTH Secreting Metastatic Parotid Tumour. Case Rep Endocrinol. 2016;2016:4852907.
Jamieson L, Taylor SM, Smith A, Bullock MJ, Davis M. Metastatic acinic cell carcinoma of the parotid gland with ectopic ACTH syndrome. Otolaryngol Head Neck Surg. 2007;136(1):149–50.
Cox ML, Gourley RD, Kitabchi AE. Acinic cell adenocarcinoma of the parotid with ectopic production of adrenocorticotropic hormone. Am J Med. 1970;49(4):529–33.
Butt MI, Rose SC, Robinson AM. Cushing syndrome secondary to ectopic ACTH secretion by dedifferentiated acinic cell carcinoma of the parotid gland. Endocrinologist. 2008;18:161–2.
Shenoy VV, Lwin Z, Morton A, Hardy J. Ectopic adrenocorticotrophic hormone syndrome associated with poor prognosis in metastatic parotid acinic cell carcinoma. Otolaryngol Head Neck Surg. 2011;145(5):878–9.
Alcantara V, Urgell E, Sancho JF, Chico A. Severe ectopic cushing syndrome caused by adenoid cystic carcinoma of a salivary gland. Endoc Pract. 2013;19(5):e118–21.
Sugawara M, Hagen GA. Ectopic ACTH syndrome due to salivary gland adenoid cystic carcinoma. Arch Intern Med. 1977;137(1):102–5.
Marks AD, Kim YN, Kroop HS. Letter: ectopic production of ACTH by a minor salivary gland tumor. Ann Intern Med. 1975;83(4):521–2.
Southgate HJ, Archbold GP, el-Sayed ME, Wright J, Marks V. Ectopic release of GHRH and ACTH from an adenoid cystic carcinoma resulting in acromegaly and complicated by pituitary infarction. Postgrad Med J. 1988;64(748):145–8.
Davi MV, Cosaro E, Piacentini S, et al. Prognostic factors in ectopic Cushing’s syndrome due to neuroendocrine tumors: a multicenter study. Eur J Endocrinol. 2017;176(4):453–9.
Isidori AM, Kaltsas GA, Pozza C, et al. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab. 2006;91(2):371–7.
Ejaz S, Vassilopoulou-Sellin R, Busaidy NL, et al. Cushing syndrome secondary to ectopic adrenocorticotropic hormone secretion: the University of Texas MD Anderson Cancer Center Experience. Cancer. 2011;117(19):4381–9.
Nagao T. “Dedifferentiation” and high-grade transformation in salivary gland carcinomas. Head Neck Pathol. 2013;7:S37–47.
Stanley RJ, Weiland LH, Olsen KD, Pearson BW. Dedifferentiated acinic cell (acinous) carcinoma of the parotid gland. Otolaryngol Head Neck Surg. 1988;98:155–61.
Perzin KH, LiVolsi VA. Acinic cell carcinomas arising in salivary glands: a clinicopathologic study. Cancer. 1979;44(4):1434–57.
Simpson RHW, Chiosea S, Katabi N, Leivo I, Vielh P, Williams MD. Acinic cell carcinoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. 4th ed. Lyon: IARC/WHO Press; 2017. p. 166–7.
Thompson LD, Aslam MN, Stall JN, Udager AM, Chiosea S, McHugh JB. Clinicopathologic and immunophenotypic characterization of 25 cases of acinic cell carcinoma with high-grade transformation. Head Neck Pathol. 2016;10(2):152–60.
Chiosea SI, Griffith C, Assaad A, Seethala RR. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol. 2012;36:343–50.
Haller F, Bieg M, Will R, et al. Enhancer hijacking activates onocogenic transcription factor NR4A3 in acinic cell carcinomas of the salivary glands. Nat Commun. 2019;10:368.
Haller F, Skalova A, Ihrler S, et al. Nulcear NR4A3 immunostainig is a specific and sensitive novel marker for acinic cell carcinoma of the salivary glands. Am J Surg Pathol. 2019. https://doi.org/10.1097/PAS.0000000000001279.
Fernandez PM, Brunel F, Jiminez MA, et al. Nuclear receptors NOR1 and NGF1-B/Nur77 play similar, albeit distinct, roles in the hypothalmo-pituitary-adrenal axis. Endocrinology. 2000;141:2392–400.
Oliveira JH, Vieira JG, Abucham J, Lengyel AM. GHRP-6 is able to stimulate cortisol and ACTH release in patients with Cushing’s disease: comparison with DDAVP. J Endocrinol Invest. 2003;26(3):230–5.
Landon J, Ratcliffe JG, Rees LH, Scott AP. Tumour-associated hormonal products. J Clin Pathol Suppl (R Coll Pathol). 1974;7:127–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No financial support was needed for the preparation of this manuscript. There was no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saluja, K., Ravishankar, S., Ferrarotto, R. et al. Ectopic ACTH Production and Cushing’s Syndrome in a Patient with Parotid Acinic Cell Carcinoma with High-Grade Transformation: Tumor Context and Clinical Implications. Head and Neck Pathol 14, 562–569 (2020). https://doi.org/10.1007/s12105-019-01054-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-019-01054-w