Abstract
Odontogenic tumors are rare entities, often derived from the epithelial remnants in the gnathic bones following odontogenesis. This brief manuscript will seek to address recent developments pertaining to odontogenic tumors as well as particularly uncommon odontogenic tumors and the difficulties in their diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Odontogenic tumors are rare entities, often derived from epithelial remnants in the gnathic bones following odontogenesis. Surgical excision is the mainstay of treatment for most tumors, though even some “benign” odontogenic tumors may require large oncologic-type resections, resulting in significant morbidity or with conservative enucleation resulting in incomplete excision and associated high recurrence rates [1]. Until recently, little was known about the molecular pathogenesis of odontogenic tumors. While this understanding still needs to be expanded tremendously, ultimately the hope is that this may allow for diagnostic and even therapeutic improvements. This brief manuscript will address recent developments pertaining to odontogenic tumors as the new found understanding of their molecular pathogenesis raises the exciting possibility of a future for targeted therapy. We will also discuss uncommon odontogenic tumors, their differential diagnosis, and diagnostic difficulties.
Recent Developments in Our Understanding of Odontogenic Tumor Pathogenesis
The decreasing cost and increased throughput capabilities in next generation sequencing (NGS) have led to rapid advances in the understanding of molecular pathogenesis of tumors including odontogenic tumors [2]. Various signaling pathways regulate the process of odontogenesis, of these, the three pathways with gene mutations most clearly implicated in the pathogenesis of odontogenic tumors and odontogenic cystic neoplasms include the mitogen activated protein kinase pathway (MAPK), the sonic hedgehog (SHH) pathway, and the Wnt signaling pathway (Fig. 1) [3]. The MAPK pathway is implicated in ameloblastoma and adenomatoid odontogenic tumor, the SHH pathway implicated in ameloblastoma and odontogenic keratocyst, formerly termed keratocystic odontogenic tumor (OKC/KCOT), and the Wnt signaling pathway in the family of odontogenic ghost cell tumors [4,5,6,7,8,9].
Ameloblastomas are locally aggressive neoplasms with a strong mandibular predilection and if incompletely excised a high recurrence rate is seen [10]. Several studies have characterized mutations in the MAPK pathway as well as the SHH pathway in the pathogenesis of ameloblastomas [4,5,6]. The MAPK pathway is important in odontogenesis and ameloblast development. The SHH participates in odontogenesis regulating tooth growth and shape, but it is also involved in tumorigenesis mediating epithelial mesenchymal interaction [11].
The most common mutation in ameloblastoma is BRAF V600E, present in 62.7% of mandibular ameloblastomas [12]. Clinically, the presence of BRAF V600E mutation is an independent predictor of recurrence free survival, is seen more frequently in younger patients, and is associated with the presence of cortical expansion [4, 13]. Other mutations in the RAS family (KRAS, HRAS, NRAS) as well as FGFR2 (a receptor that activates the MAPK pathway) have been seen in ameloblastoma less frequently and are usually mutually exclusive of BRAF V600E mutations. Interestingly, the molecular profile of ameloblastomas is strikingly different between the maxilla and mandible, with SMO mutations the most common mutation in maxillary ameloblastomas, often in conjunction with an additional RAS family or FGFR2 mutations. Notably, a higher recurrence rate is seen in ameloblastomas with a higher mutational burden [13]. Additionally, of the histologic subtypes seen in conventional ameloblastoma, a correlation between pattern and mutation is noted; that is the plexiform patterned ameloblastoma more frequently has a SMO mutation and follicular ameloblastomas more frequently have BRAF V600E mutations [5, 13].
One series reported a 100% concordance between IHC and NGS results of BRAF V600E on non-decalcified material (Fig. 2) [4]. Immunohistochemical testing for BRAF V600E on decalcified material may yield false negative results [14]. Other “ameloblastic” lesions (such as peripheral ameloblastoma, unicystic ameloblastoma, ameloblastic fibroma, and ameloblastic fibro-odontoma) have been shown to have BRAF V600E positivity [13, 15, 16].
Ameloblastic carcinoma can arise de novo or alternatively, result from malignant transformation of a long standing ameloblastoma. Histologically, it may be impossible to make this distinction, which additionally, is not usually clinically relevant. Thus, in the fourth edition of the WHO Classification of Head and Neck Tumours, this distinction has been eliminated [17]. Ameloblastic carcinoma has shown a considerably lower rate of BRAF V600E mutation than conventional solid multicystic ameloblastoma, with combined results across four series demonstrating a rate of 26.3% (5/19) [4, 15, 16, 18]. Regarding MAPK pathway mutations in general as well as SHH pathway mutations, ameloblastic carcinoma also still shows a lower rate than conventional solid multicystic ameloblastoma (22.2% [2/9] and 11.1% [1/9], respectively). While data are limited, it appears that there is a third group of tumors in ameloblastic carcinoma that shows fairly non-specific alterations, including TP53, PIK3CA, CDK2NA mutations [18].
Some ameloblastic neoplasms fail to exhibit frank features of malignancy (marked cytonuclear atypia, perineural invasion, angiolymphatic invasion, necrosis, or distant metastases), but do exhibit increased mitotic activity or at most moderate pleomorphism. These can be termed ‘atypical ameloblastomas.’ Ameloblastic lesions can be considered to occur on a spectrum, with ameloblastomas at one end, ameloblastic carcinoma at the other, and atypical ameloblastomas would fall in the middle. Supporting this notion is preliminary data demonstrating the molecular profile of atypical ameloblastoma more is more in line with ameloblastic carcinoma than conventional solid multicystic ameloblastoma in terms of distribution of pathway alterations [18].
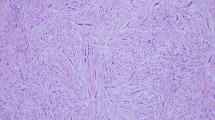
Adenomatoid odontogenic tumor (AOT) is a benign odontogenic tumor characterized by spindled shaped epithelial cells arranged in whorls or rosettes, duct-like structures, amyloid, and mineralization may be present (Fig. 3). Similar to ameloblastoma, AOT also harbors mutations in the MAPK pathway. Targeted NGS revealed KRASG12V mutations in 7 of 9 AOTs [8]. While both AOT and ameloblastoma have mutations in the MAPK, the clinical behavior is markedly different. AOTs are characterized by indolent clinical behavior; as well encapsulated lesions they are amenable to simple curettage, with recurrence unlikely.
Hematoxylin and eosin of an adenomatoid odontogenic tumor presenting in an 18 year-old female around the crown of an impacted canine. Spindled epithelial cells are arranged in sheets with whorls and focal rosette formation. Amyloid deposition is present with displacement of calcified massed leading to sectioning artifact
The family of odontogenic ghost cell tumors is comprised of calcifying cystic odontogenic tumor (re-classified in 2017 as calcifying odontogenic cyst) (CCOT/COC), dentinogenic ghost cell tumor (DGCT), and odontogenic ghost cell carcinoma (OGCC). While CCOT/COC are by far the most common odontogenic ghost cell tumors, representing the vast majority of odontogenic ghost cell tumors (93%), DGCT and GCOC represent 5 and 2%, respectively [19]. Tumors of this group (CCOT/COC, DGCT, and GCOC), are associated with CTNNB1 (β-catenin) mutations in the Wnt signaling pathway (Fig. 4) [20, 21]. β -catenin functions as a transcriptional activator of the Wnt signalling pathway. Key steps in the WNT pathway include, Wnt ligand activates transmembrane frizzled receptor, subsequently, β-catenin accumulates and translocates to the nucleus, complexing with LEF-1. In one series, 91% (10/11) cases of CCOT/COC demonstrated CTNNB1 point mutations, with one additional case having an APC mutation [22]. LEF-1 is reported to be positive in 64% (7/11) of CCOT/COC [23]. CCOT/COC is a benign cystic neoplasm of odontogenic origin that is usually cystic in nature, characterized by an ameloblastoma-like epithelium with pale eosinophilic anucleate ghost cells that may calcify. DGCT is characterized by a benign solid epithelial component, ghost cells, and sheets of dentinoid [22]. Abundant mitoses, perineural, or angiolymphatic invasion are features that may be present in GCOC; however, the distinction between DGCT and GCOC may be challenging when overt features of malignancy are lacking [9]. GCOC may arise de novo or from malignant transformation of DGCT or COC/CCOT. In a systematic review that included 35 cases of GCOC, 12/35 (34.3%) patients had a history of previously diagnosed COC/CCOT [21]. GCOC are associated with a higher recurrence rate (63.4%), and treatment often involves surgical resection with adjuvant chemoradiation [21].
a Hematoxylin and eosin of a calcifying odontogenic cyst/calcifying cystic odontogenic tumor exhibiting abundant ghost cells as well as ameloblastic epithelium bβ-catenin exhibits nuclear positivity in tumoral cells c LEF-1 exhibits nuclear positivity in tumor cells as well as pre-B and T lymphocytes
While odontogenic ‘tumors’ are the focus of this manuscript, the pathogenesis of the cystic neoplasm OKC/KCOT should be mentioned for the sake of completeness, as the SHH pathway is implicated in their pathogenesis [7]. PTCH1 is a transmembrane receptor that inhibits SMO in the absence of sonic hedgehog protein or other activating mutations. PTCH1 has been found to be mutated in approximately 85% of syndromic OKC/KCOT (occurring in association with Nevoid Basal Cell Carcinoma syndrome) leading to constitutive active signaling [24, 25]. Other SHH pathway mutations such as PTCH2 and SUFU are seen in patients with Nevoid Basal Cell Carcinoma syndrome, at lower rates [26, 27]. PTCH1 mutations are also present in sporadic OKC/KCOT, although reported at lower rates (30 to 84%) [25].
Uncommon/Diagnostically Challenging Odontogenic Tumors
As odontogenic tumors are rare, some entities are infrequently encountered, making the diagnosis more difficult. Adenoid ameloblastoma was initially described by Charles Waldron in 1959, as “an essentially adenoid type growth which is not typical of either salivary gland tumors or as ameloblastomas as they are usually recognized” [28]. In 2015, a more recent article by Loyola et al. brought adenoid ameloblastoma to the forefront presenting 5 additional cases and, to date, less than 40 cases have been reported with numerous reports occurring in the last five years [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Given the small number of cases, it is difficult to draw conclusions, but these tumors appear to present in a wide age range (19–79, mean 43 years), as large tumors with a mean size of 4.4 cm, with a maxillary predilection (67%), and have a relatively high recurrence rate (10/13, 77%) [28, 32,33,34,35,36,37,38,39,40,41,42]. The histologic features include a cribriform architecture with duct-like structures, peripheral columnar cells with reverse polarity, clear cells, ghost cells (that may calcify), variable amounts of dentinoid/osteoid, increased mitotic activity, pseudopapillary areas, and distinctive whorls/morules (Fig. 5 a and b). Adenoid ameloblastoma is challenging diagnosis as it exhibits features of a dizzying array of odontogenic tumors as well as salivary tumors including: adenomatoid odontogenic tumor, adenoid cystic carcinoma, basal cell adenocarcinoma, adenomatoid odontogenic tumor, ameloblastoma, ameloblastic carcinoma, the ghost cell family of tumors, clear cell odontogenic carcinoma, and odontogenic carcinoma with dentinoid [43]. Adenoid ameloblastoma is positive for CK14, P63 and AE1/3, but negative for S-100, CK7, and P53 [29, 42]. Immunohistochemical studies eliminate biphasic salivary entities from the differential diagnosis (including adenoid cystic carcinoma and basal cell adenocarcinoma) as adenoid ameloblastoma is monophasic. Diagnosis of adenoid ameloblastoma can be made on the unique constellation of previously mentioned histologic features.
a Hematoxylin and eosin of adenoid ameloblastoma demonstrating at low power a cribriform architecture, with subtle palisading of the cells at the periphery of the tumor nests. Amorphous, anucleate areas representing ghost cell keratinization (solid arrow) are present, as well as more basophilic areas of cellular condensation or morules (open arrow). b In other more solid areas, cellar clearing is noted with masses of dentinoid
Calcifying epithelial odontogenic tumor (CEOT) is one of the least frequently encountered odontogenic tumors [44]. CEOTs are characterized by sheets of polygonal, pleomorphic epithelium with eosinophilic cytoplasm, prominent intercellular bridging, and variable amounts of amyloid and concentric calcifications (Fig. 6a). While not a difficult diagnosis per se, CEOT-like features can be found within other odontogenic lesions and over interpretation of focal features should be discouraged. Small odontogenic rests have been reported in the wall of dental follicles in 79% of cases with calcification present in 37% [45]. Thus, CEOT-like change in the fibrous connective tissue wall of a dental follicle is a common, incidental, well recognized focal finding (Fig. 6b) [46]. While a diagnostic pitfall, these areas are small and occurring in the context of a developmental odontogenic cyst. The clear cell variant of CEOT (CCCEOT) is an uncommon variant of CEOT, representing only 10.7% of CEOTs, it is debated in the literature as to whether CCCEOT behaves more aggressively that conventional CEOT [47,48,49]. On small biopsy material CCCEOT can be mistaken for clear cell odontogenic carcinoma (CCOC). A lack of EWSR1 rearrangement, a feature of nearly 90% of CCOC, may be especially useful in this context [50]. CEOT-like areas may be seen in conjunction with AOT (Fig. 6c) [51]. Clinically, these combined CEOT/AOT lesions will have the same indolent behavior as AOTs, and surgeons should be advised they are to be managed as such.
a Calcifying epithelial odontogenic tumor exhibiting sheets of polygonal epithelial cells with interspersed amyloid and concentric calcifications. b A focal area of calcifying epithelial odontogenic tumor-like change in the wall of a hyperplastic dental follicle surrounding an impacted mandibular molar in a 14 year-old male. c A focal calcifying epithelial odontogenic tumor-like area occurring in an adenomatoid odontogenic tumor in a 21 year-old female in the anterior maxilla. d Hematoxylin and eosin of amyloid rich variant of central odontogenic fibroma. Abundant amyloid is present with small islands of odontogenic epithelium
The amyloid rich variant of central odontogenic fibroma (COF) is perhaps the most difficult differential diagnosis for CEOT and this distinction remains controversial (Fig. 6d) [52]. COF have a predilection for females, presenting most commonly as a well-defined radiolucency in the maxillary anterior region in a peri- or inter- radicular region, depression of the palatal bone may be present; histopathologically, COF are characterized by moderately cellular connective tissue with islands or strands of epithelium [53, 54]. The amyloid in the amyloid-rich variant of COF is the same odontogenic ameloblast-associated protein as found in CEOT. The amyloid rich variant of COF exhibits a network of dendritic cells that may be highlighted by CD1a or S100. A non-calcifying Langerhans cell-rich variant of CEOT has been described [55,56,57], with morphologic features that overlap with amyloid rich variant of COF. The distinction between amyloid rich variant of COF from the non-calcifying Langerhans cell-rich variant of CEOT is clinically relevant, as COFs are generally expected to behave non-aggressively with low rates of recurrence after treatment with curettage. Lack of calcifications radiographically as well as histologically, anterior maxillary location, inter-radicular location, and female gender are features that favor an amyloid rich variant of COF over the non-calcifying Langerhans cell-rich variant of CEOT. While controversy remains, several authors argue that these tumors are better classified as a variant of COF rather than a variant of CEOT classification, to avoid overtreatment [52,53,54].
The pathogenesis of CEOT is not clearly characterized, with proposed mechanisms conflicting. It has been theorized the SHH pathway is implicated in the pathogenesis of CEOT [58, 59] ; however, initial NGS data does not support this [18, 60]. Five CEOTs analyze with NGS revealed mutations in tumor suppressor genes PTEN and CDKN2A as well as oncogenes MET and JAK3 and mutations [60]. We have reported NGS results on two CEOTs, one with mutation of the MET oncogene and another CEOT that was multiply recurrent, and clinically aggressive with mutations of multiple tumor suppressor genes (ATRX, CDK2NA, RB1) [18]. Additional studies are required to more fully characterize the molecular pathogenesis of CEOT.
Conclusions
The advances in understanding of the molecular pathogenesis of odontogenic tumors have many potential ramifications including: diagnostic value, affording greater precision in the diagnosis of challenging cases, prognostication of tumor behavior based on characteristics of the mutational burden, and prediction of the response to potential therapeutic targets. There is exciting potential for the use of targeted therapies in the treatment of odontogenic tumors. This may lead to reduced surgical morbidity and decreased recurrence rates; given the accessibility of the anatomic region, local delivery systems may be considered. Odontogenic tumors, as a whole, are rare, and the combination of their infrequency with their morphologic overlap makes diagnosis challenging.
References
Slootweg PJ, Odell EW, Baumhoer D, Carlos R, Hunter KD, Taylor AM, et al. Data set for the reporting of malignant odontogenic tumors: explanations and recommendations of the guidelines from the international collaboration on cancer reporting (ICCR). Arch Pathol Lab Med. 2018. https://doi.org/10.5858/arpa.2018-0417-SA.
Koboldt DC, Steinberg KM, Larson DE, Wilson RK, Mardis ER. The next-generation sequencing revolution and its impact on genomics. Cell. 2013;155(1):27–38. https://doi.org/10.1016/j.cell.2013.09.006.
Diniz MG, Gomes CC, de Sousa SF, Xavier GM, Gomez RS. Oncogenic signalling pathways in benign odontogenic cysts and tumours. Oral Oncol. 2017;72:165–73. https://doi.org/10.1016/j.oraloncology.2017.07.021.
Brown NA, Rolland D, McHugh JB, Weigelin HC, Zhao L, Lim MS, et al. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res. 2014;20(21):5517–26. https://doi.org/10.1158/1078-0432.CCR-14-1069.
Sweeney RT, McClary AC, Myers BR, Biscocho J, Neahring L, Kwei KA, et al. Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat Genet. 2014;46(7):722–5. https://doi.org/10.1038/ng.2986.
Kurppa KJ, Caton J, Morgan PR, Ristimaki A, Ruhin B, Kellokoski J, et al. High frequency of BRAF V600E mutations in ameloblastoma. J Pathol. 2014;232(5):492–8. https://doi.org/10.1002/path.4317.
Li TJ. The odontogenic keratocyst: a cyst, or a cystic neoplasm? J Dent Res. 2011;90(2):133–42. https://doi.org/10.1177/0022034510379016.
Gomes CC, de Sousa SF, de Menezes GH, Duarte AP, Pereira Tdos S, Moreira RG, et al. Recurrent KRAS G12V pathogenic mutation in adenomatoid odontogenic tumours. Oral Oncol. 2016;56:e3–5. https://doi.org/10.1016/j.oraloncology.2016.03.001.
Ohata Y, Kayamori K, Yukimori A, Sumikura K, Ohsako T, Harada H, et al. A lesion categorized between ghost cell odontogenic carcinoma and dentinogenic ghost cell tumor with CTNNB1 mutation. Pathol Int. 2018;68(5):307–12. https://doi.org/10.1111/pin.12659.
Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer Part B. 1995;31B(2):86–99.
Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127(22):4775–85.
Brown NA, Betz BL. Ameloblastoma: a review of recent molecular pathogenetic discoveries. Biomark Cancer. 2015;7(Suppl 2):19–24. https://doi.org/10.4137/BIC.S29329.
Gultekin SE, Aziz R, Heydt C, Senguven B, Zoller J, Safi AF, et al. The landscape of genetic alterations in ameloblastomas relates to clinical features. Virchows Arch. 2018;472(5):807–14. https://doi.org/10.1007/s00428-018-2305-5.
Heikinheimo K, Huhtala JM, Thiel A, Kurppa KJ, Heikinheimo H, Kovac M, et al. The mutational profile of unicystic ameloblastoma. J Dent Res. 2018:22034518798810. https://doi.org/10.1177/0022034518798810.
Diniz MG, Gomes CC, Guimaraes BV, Castro WH, Lacerda JC, Cardoso SV, et al. Assessment of BRAFV600E and SMOF412E mutations in epithelial odontogenic tumours. Tumour Biol. 2015;36(7):5649–53. https://doi.org/10.1007/s13277-015-3238-0.
Brunner P, Bihl M, Jundt G, Baumhoer D, Hoeller S. BRAF p.V600E mutations are not unique to ameloblastoma and are shared by other odontogenic tumors with ameloblastic morphology. Oral Oncol. 2015;51(10):e77-8. https://doi.org/10.1016/j.oraloncology.2015.07.010.
El-Naggar A, Chan J, Grandis J, Takata T, Slootweg P, editors WHO classification of head and neck tumours. 4th ed., 2017.
Bilodeau E, Chiosea S, Berg A, Muller S, Purgina B, Seethala R. Molecular profiling of rare odontogenic tumors. Mod Pathol. 2018;31:473. https://doi.org/10.1038/modpathol.2018.12.
Reichart PA, Philipsen H. Calcifying ghost cell odontogenic cysts/ tumors. odontogenic tumors and allied lesions. London: Quintessence; 2004. pp. 155–78.
Sekine S, Sato S, Takata T, Fukuda Y, Ishida T, Kishino M, et al. Beta-catenin mutations are frequent in calcifying odontogenic cysts, but rare in ameloblastomas. Am J Pathol. 2003;163(5):1707–12.
de Arruda JAA, Monteiro J, Abreu LG, de Oliveira Silva LV, Schuch LF, de Noronha MS, et al. Calcifying odontogenic cyst, dentinogenic ghost cell tumor, and ghost cell odontogenic carcinoma: a systematic review. J Oral Pathol Med. 2018;47(8):721–30. https://doi.org/10.1111/jop.12727.
Yukimori A, Oikawa Y, Morita KI, Nguyen CTK, Harada H, Yamaguchi S, et al. Genetic basis of calcifying cystic odontogenic tumors. PLoS ONE. 2017;12(6):e0180224. https://doi.org/10.1371/journal.pone.0180224.
Bilodeau EA, Acquafondata M, Barnes EL, Seethala RR. A comparative analysis of LEF-1 in odontogenic and salivary tumors. Hum Pathol. 2015;46(2):255–9. https://doi.org/10.1016/j.humpath.2014.10.018.
Bresler SC, Padwa BL, Granter SR. Nevoid basal cell carcinoma syndrome (Gorlin Syndrome). Head Neck Pathol. 2016;10(2):119–24. https://doi.org/10.1007/s12105-016-0706-9.
Qu J, Yu F, Hong Y, Guo Y, Sun L, Li X, et al. Underestimated PTCH1 mutation rate in sporadic keratocystic odontogenic tumors. Oral Oncol. 2015;51(1):40–5. https://doi.org/10.1016/j.oraloncology.2014.09.016.
Smith MJ, Beetz C, Williams SG, Bhaskar SS, O’Sullivan J, Anderson B, et al. Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol. 2014;32(36):4155–61. https://doi.org/10.1200/JCO.2014.58.2569.
Xu LL, Li TJ. PTCH2 gene alterations in keratocystic odontogenic tumors associated with nevoid basal cell carcinoma syndrome. Beijing Da Xue Xue Bao Yi Xue Ban. 2008;40(1):15–8.
Waldron CA. The importance of histologic study of the various radiolucent areas of the jaws. Oral Surg Oral Med Oral Pathol. 1959;12(1):19–30.
Adorno-Farias D, Muniz V, Soares AP, Cury PR, Rabelo RG, Fernandez-Ramires R, et al. Ameloblastoma with adenoid features: a series of eight cases. Acta Histochem. 2018;120(5):468–76. https://doi.org/10.1016/j.acthis.2018.05.006.
Rai HK, Pai SM, Dayakar A, Supriya H. Adenoid ameloblastoma with dentinoid: a rare hybrid variant. J Oral Maxillofac Pathol. 2017;21(2):319. https://doi.org/10.4103/jomfp.JOMFP_53_15.
Khalele BAO. Adenoid ameloblastoma with dentinoid and cellular atypia: a case report and literature review. Pathologica. 2017;109(4):379–81.
Loyola AM, Cardoso SV, de Faria PR, Servato JP, Eisenberg AL, Dias FL, et al. Adenoid ameloblastoma: clinicopathologic description of five cases and systematic review of the current knowledge. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(3):368–77. https://doi.org/10.1016/j.oooo.2015.05.011.
Saxena K, Jose M, Chatra LK, Sequiera J. Adenoid ameloblastoma with dentinoid. J Oral Maxillofac Pathol. 2012;16(2):272–6. https://doi.org/10.4103/0973-029X.99088.
Ghasemi-Moridani S, Yazdi I. Adenoid ameloblastoma with dentinoid: a case report. Arch Iran Med. 2008;11(1):110–2. doi:08111/AIM.0021.
Evans BL, Carr RF, Phillipe LJ. Adenoid ameloblastoma with dentinoid: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98(5):583–8. https://doi.org/10.1016/S1079210404001866.
de Andrade Sobrinho J, de Carvalho MB, Rapoport A, Saba LM. Odontogenic adenomatoid tumor of the mandible (adenoameloblastoma). Int Surg. 1978;63(1):39–42.
Tajima Y, Yokose S, Sakamoto E, Yamamoto Y, Utsumi N. Ameloblastoma arising in calcifying odontogenic cyst. Report of a case. Oral Surg Oral Med Oral Pathol. 1992;74(6):776–9.
Matsumoto Y, Mizoue K, Seto K. Atypical plexiform ameloblastoma with dentinoid: adenoid ameloblastoma with dentinoid. J Oral Pathol Med. 2001;30(4):251–4.
Ide F, Mishima K, Saito I, Kusama K. Diagnostically challenging epithelial odontogenic tumors: a selective review of 7 jawbone lesions. Head Neck Pathol. 2009;3(1):18–26. https://doi.org/10.1007/s12105-009-0107-4.
Sonone A, Hande A, Chaudhary M, Sheorain A, Agni N. Adenoid ameloblastoma with dentinoid and ghost cells. A composite odontogenic tumour: a rare case report and review of the literature. Oral Surg. 2011;4:77–81.
Kumar K, Shetty DC, Wadhwan V, Dhanapal R, Singh HP. Dentinoameloblastoma with ghost cells: A rare case report with emphasis on its biological behavior. Dent Res J (Isfahan). 2013;10(1):103–7. https://doi.org/10.4103/1735-3327.111809.
Bilodeau E, Seethala R. Adenoid ameloblastoma: A series of 5 tumors. Paper presented at the 72nd Annual Meeting of the American Academy Oral Maxillofacial Pathology; June 26, 2018; Vancouver.
Mosqueda-Taylor A, Neville BW, Tatemoto Y, Ogawa I, Takata T. Odontogenic carcinoma with dentinoid: a new odontogenic carcinoma. Head Neck Pathol. 2014;8(4):421–31. https://doi.org/10.1007/s12105-014-0586-9.
Philipsen HP, Reichart PA. Calcifying epithelial odontogenic tumour: biological profile based on 181 cases from the literature. Oral Oncol. 2000;36(1):17–26.
Kim J, Ellis GL. Dental follicular tissue: misinterpretation as odontogenic tumors. J Oral Maxillofac Surg. 1993;51(7):762–7.
Azevedo RS, Mosqueda-Taylor A, Carlos R, Cabral MG, Romanach MJ, de Almeida OP, et al. Calcifying epithelial odontogenic tumor (CEOT): a clinicopathologic and immunohistochemical study and comparison with dental follicles containing CEOT-like areas. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(6):759–68. https://doi.org/10.1016/j.oooo.2013.08.023.
Anavi Y, Kaplan I, Citir M, Calderon S. Clear-cell variant of calcifying epithelial odontogenic tumor: clinical and radiographic characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(3):332–9. https://doi.org/10.1067/moe.2003.8.
Sabir H, Kumbhare S, Redij S, Gajbhiye N. Clear Cell Variant of Calcifying epithelial odontogenic tumor: a rare clinical entity. Gulf J Oncolog. 2017;1(24):55–60.
Rangel AL, da Silva AA, Ito FA, Lopes MA, de Almeida OP, Vargas PA. Clear cell variant of calcifying epithelial odontogenic tumor: is it locally aggressive? J Oral Maxillofac Surg. 2009;67(1):207–11. https://doi.org/10.1016/j.joms.2007.11.029.
Bilodeau EA, Weinreb I, Antonescu CR, Zhang L, Dacic S, Muller S, et al. Clear cell odontogenic carcinomas show EWSR1 rearrangements: a novel finding and a biological link to salivary clear cell carcinomas. Am J Surg Pathol. 2013;37(7):1001–5. https://doi.org/10.1097/PAS.0b013e31828a6727.
Damm DD, White DK, Drummond JF, Poindexter JB, Henry BB. Combined epithelial odontogenic tumor: adenomatoid odontogenic tumor and calcifying epithelial odontogenic tumor. Oral Surg Oral Med Oral Pathol. 1983;55(5):487–96.
Eversole LR. Odontogenic fibroma, including amyloid and ossifying variants. Head Neck Pathol. 2011;5(4):335–43. https://doi.org/10.1007/s12105-011-0279-6.
Ide F, Matsumoto N, Miyazaki Y, Kikuchi K, Kusama K. What is the non-calcifying langerhans cell-rich variant of calcifying epithelial odontogenic tumor? Head Neck Pathol. 2018. https://doi.org/10.1007/s12105-018-0968-5.
Zhou CX, Li TJ. A clinicopathologic study on central odontogenic fibroma: with special reference to amyloid variant. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126(6):513–20. https://doi.org/10.1016/j.oooo.2018.08.019.
Santosh N, McNamara KK, Kalmar JR, Iwenofu OH. Non-calcifying langerhans cell-rich variant of calcifying epithelial odontogenic tumor: a distinct entity with predilection for anterior maxilla. Head Neck Pathol. 2018. https://doi.org/10.1007/s12105-018-0958-7.
Asano M, Takahashi T, Kusama K, Iwase T, Hori M, Yamanoi H, et al. A variant of calcifying epithelial odontogenic tumor with Langerhans cells. J Oral Pathol Med. 1990;19(9):430–4.
Wang YP, Lee JJ, Wang JT, Liu BY, Yu CH, Kuo RC, et al. Non-calcifying variant of calcifying epithelial odontogenic tumor with Langerhans cells. J Oral Pathol Med. 2007;36(7):436–9.
Junior BC, Muniz V, Vidal MTA, Gurgel CA, Leon JE, De Azevedo RA, et al. Clear cell variant of calcifying epithelial odontogenic tumor: a case report and preliminary immunohistochemical study of the SHH pathway. Appl Immunohistochem Mol Morphol. 2017;25(10):e95-e9. https://doi.org/10.1097/PAI.0000000000000467.
Peacock ZS, Cox D, Schmidt BL. Involvement of PTCH1 mutations in the calcifying epithelial odontogenic tumor. Oral Oncol. 2010;46(5):387–92. https://doi.org/10.1016/j.oraloncology.2010.02.023.
de Sousa SF, Diniz MG, Franca JA, Fontes Pereira TDS, Moreira RG, Santos JND, et al. Cancer genes mutation profiling in calcifying epithelial odontogenic tumour. J Clin Pathol. 2018;71(3):279–83. https://doi.org/10.1136/jclinpath-2017-204813.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report nor funding sources to disclose. This work is exempt from IRB review.
Rights and permissions
About this article
Cite this article
Bilodeau, E.A., Seethala, R.R. Update on Odontogenic Tumors: Proceedings of the North American Head and Neck Pathology Society. Head and Neck Pathol 13, 457–465 (2019). https://doi.org/10.1007/s12105-019-01013-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-019-01013-5