Abstract
Carcinoma ex pleomorphic adenoma (CXPA) is a malignant tumor of the salivary gland that arises from pleomorphic adenoma (PA). Squamous cell carcinoma (SCC) is extremely rare in the salivary glands. We report two cases of acantholytic SCC (ASCC) ex PA. Case 1 involved a 72-year-old female, and case 2 involved a 67-year-old male. Histologically, both cases involved PA, and salivary duct carcinoma (SDC) components, which were positive for androgen receptor (AR) and gross cystic disease fluid protein (GCDFP)-15 but negative for HER2, were seen in the intracapsular regions. The invasive components consisted of ASCC, which were positive for cytokeratin 5/6 and p63 but negative for AR and GCDFP-15. The SDC and ASCC components were positive for the epidermal growth factor receptor. In both cases, the cytoplasmic localization or decreased expression of E-cadherin was observed in the ASCC. In the early phase, CXPA might emerge as SDC, and it might change into SCC as it invades beyond the capsule due to changes in microenvironment. Also, the aberrant expression of E-cadherin is related to acantholysis in SCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carcinoma ex pleomorphic adenoma (CXPA) is defined as a carcinoma arising from a primary (de novo) or recurrent benign pleomorphic adenoma (PA). Gnepp summarized approximately 60 series of CXPA lesions and noted that CXPA accounts for approximately 3.6% of all salivary gland neoplasms [1, 2]. In a previous study of CXPA, 56% of patients with high-grade carcinoma components died of their disease [1]. The malignant components of CXPA usually belong to a single histological subtype, and the most common histological subtype is salivary duct carcinoma (SDC), which was seen in approximately half of cases in a recent study [3]. The androgen receptor (AR) and gross cystic disease fluid protein (GCDFP)-15 are useful markers of SDC [4]. The large study indicated that immunopositivity for HER2 was detected in the cell membranes of 21% of high-grade SDC of CXPA [5].
Primary squamous cell carcinoma (SCC) of the salivary glands is extremely rare [6]. In cases in which SCC is found in the salivary glands, metastasis from tumors of the head and neck, skin, or other sites has to be excluded [6].
Acantholytic SCC (ASCC) is a rare subtype of SCC that arises in the skin or mucosa. Some authors have reported that ASCC is more aggressive than conventional SCC [7, 8], while others have reported that both subtypes exhibit similar outcomes [9].
Here, we report two cases of CXPA of the submandibular glands, which involved both ASCC and SDC components. To the best of our knowledge, these are the first such cases to be reported.
Methods
The tissue specimens were fixed in formalin, embedded in paraffin wax and cut into 3 µm-thick sections. We routinely performed the hematoxylin and eosin stain, Periodic acid-Schiff-alcian blue (PAS-AB) stain, and Elastica van Gieson (EvG) stain.
Immunohistochemical examinations were performed on formalin-fixed and paraffin-embedded tissue sections using Leica BOND III automatic immunostainer (Leica, Bannockburn, IL). The antibodies used in these examinations, and the results of the examinations are summarized in Table.
We used Image J (National Institutes of Health, Bethesda, MD) to estimate the percentage of Ki-67 positive tumor cell rates.
Case Presentation
Case 1: Clinico-pathological Findings
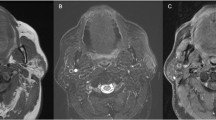
The patient was a 72-year-old Japanese female, who had experienced swelling of the left neck for 45 years. The swelling suddenly started to progress rapidly, and she was admitted to our hospital 2 months later. On an MRI scan, a 44 × 45 × 55-mm, irregularly shaped mass, which exhibited low signal intensity on T1- and T2-weighted imaging, was seen in the left submandibular gland (Fig. 1). Left submandibulectomy and left neck dissection were performed. After the operation, radiotherapy (20 Gy) was administered. She was alive without disease after 5 years.
Macroscopically, case 1 involved an ill-defined grayish-white mass in the left submandibular gland (Fig. 2a, b). A yellowish firm nodule was seen in the mass. Histologically, the nodule contained a hyalinized stroma with a few spindle-shaped cells and benign ductal structures, which showed elastosis after EvG staining (Fig. 2c). The nodule was considered to be a residual PA. In the peripheral regions of the nodule, atypical ductal cells with large amounts of eosinophilic cytoplasm were observed, which were considered to be indicative of an SDC component (Fig. 2d). The SDC component showed cribriform, glandular, and solid growth patterns and comedo necrosis. The main tumor, which consisted of proliferating dysplastic squamous cells, was classified as a widely invasive carcinoma. The proliferation of single, scattered, round keratinocytes was seen in 60% of the main tumor, and the remaining 40% of the main tumor contained less cohesive acantholytic cells arranged in tumor nests combined with single-cell keratinization (Fig. 2e, f). The nuclei of these cells were round or polyhedral, centrally located, and had one or two large nucleoli. This component was found to be entirely negative for mucin (Fig. 2f). The main invasive tumor was considered to be an ASCC. One lymph node metastasis was also detected, and it consisted of conventional SCC.
Macroscopic and histological findings of the tumor in case 1. a Cut surface of the tumor; b Mapping of the three histological components PA, a hyalinized nodule, which was suggestive of a pre-existing PA (yellow area); SDC, the salivary duct carcinoma component (red area); ASCC, the acantholytic squamous cell carcinoma component (blue area). The hyalinized nodule showed moderate elastosis and hypocellularity (c hematoxylin–eosin staining, original magnification: ×200; inset, EvG staining, original magnification: ×400), which suggested that it was a pre-existing PA. In the peripheral portion of the PA, comedo necrosis and the proliferation of atypical ductal cells with relatively large amounts of eosinophilic cytoplasm, which were considered to be indicative of SDC, were observed (d hematoxylin–eosin staining, original magnification: ×200). The invasive carcinoma component displayed marked acantholysis; the loss of cell–cell adhesion (e hematoxylin–eosin staining, original magnification: ×200); and atypical round prickle cells, which did not produce mucin but exhibited a single-cell invasion pattern (f hematoxylin–eosin staining, original magnification: ×200; inset, mucicarmine staining, original magnification: ×400)
Case 2: Clinico-pathological Findings
The patient was a 67-year-old Japanese male, who had suffered from swelling of the right submandibular region 30 years earlier, when he was diagnosed with a benign lesion at a nearby clinic. Recently, this mass had swelled rapidly, and dysphagia had emerged. A fine-needle aspiration biopsy was performed, and he was diagnosed with a suspected malignant tumor. He was admitted to our hospital to undergo an operation within a month. MRI showed a 45 × 55 × 40-mm mass, which exhibited low signal intensity on T1-weighted imaging and a mixture of low and high signal intensity on T2-weighted imaging, in the right submandibular region. Right submandibulectomy and right neck dissection were carried out. Postoperative radiotherapy (60 Gy) was administered. No local recurrence or metastasis had been detected after 5 months.
Macroscopically, case 2 involved an ill-defined cystic tumor containing an intramural yellowish-white solid mass in the right submandibular gland. A firm nodule was present in the solid component (Fig. 3a–c). Histologically, the nodule, which exhibited marked elastosis during EvG staining, had a hyalinized stroma and contained spindle-shaped cells and double-layered ductal structures, and a myxoid stroma with spindle-shaped cells was seen in a part of the lesion (Fig. 3d). Dysplastic changes were also observed in the inner ductal cells. This nodule was considered to be a co-existing PA (which accounted for 10% of the tumor). Atypical ductal cells with a large amount of eosinophilic cytoplasm, which demonstrated cribriform, Roman-arch, and/or solid nest growth patterns, were observed at the periphery of the nodule (Fig. 3e). This lesion (which accounted for 35% of the tumor) was considered to be SDC. The SDC component did not display extracapsular invasion. The invasive component (which accounted for 40% of the tumor) consisted of proliferating dysplastic squamous cells, and acantholysis was observed in the central regions of the tumor nests, which demonstrated a pseudovascular growth pattern (Fig. 3f). This component was negative for mucin staining. The residual portion (which accounted for 15% of the tumor) was also composed of a keratinizing SCC (Fig. 3g). The invasive component was considered to be an ASCC. A single lymph node metastasis, which was diagnosed as an ASCC, was also detected during a histological examination.
Macroscopic and histological findings of the tumor in case 2. a Cut surface of the tumor; b Mapping of the three histological components PA, a hyalinized nodule, which was suggestive of a co-existing PA (yellow area); SDC, the salivary duct carcinoma component (red area); ASCC, the acantholytic squamous cell carcinoma component (blue area). SDC area and ASCC area clearly separated without the transitional zone (c hematoxylin–eosin staining, original magnification: ×20). The elastic-hard nodule contained proliferating plump myoepithelial cells within a myxoid stroma (d hematoxylin–eosin staining, original magnification: ×200), which was suggestive of a co-existing PA. Roman-arch structures, which consisted of large eosinophilic atypical ductal cells displaying comedo necrosis were observed at the periphery of the PA, and this component was diagnosed as an SDC (e hematoxylin–eosin staining, original magnification: ×200). The invasive portion mainly showed a vascular-like growth pattern, which was derived from the degeneration of the central sections of the tumor nests, and this component was considered to be an ASCC (f hematoxylin–eosin staining, original magnification: ×200; inset, PAS-AB staining, original magnification: ×400). However, a small part of the invasive portion was composed of SCC cells and exhibited keratin pearl formation and so was considered to be a conventional SCC (g hematoxylin–eosin staining, original magnification: ×200)
Immunohistochemical Analysis of Cases 1 and 2 (Table)
In both cases, the atypical ductal cells of the SDC component exhibited diffuse positivity for cytokeratin (CK) 7, epithelial membrane antigen (EMA), GCDFP-15, AR, and p53 (Fig. 4a, b). Both cases were positive for the epidermal growth factor receptor (EGFR) (Fig. 4c). The lesion in case 1 was entirely negative for HER2 (Allred score: 0), whereas that in case 2 was weakly positive for HER2 (Allred score: 1 +). Both tumors were positive for mammaglobin (MMG) and S-100P, whereas they were negative for prostatic specific antigen (PSA) and SCC markers.
Immunohistochemical results for the carcinoma cells in each case. The atypical ductal cells were positive for GCDFP-15 (a immunostaining, original magnification: ×200, case 1), the AR (b immunostaining, original magnification: ×200, case 2), and the EGFR (c immunostaining, original magnification: ×200, case 2). The dysplastic squamous cells were positive for CK5/6 (d: immunostaining, original magnification: ×200, case 1) i.e., they displayed the SCC phenotype. The ASCC cells in case 1 were entirely negative for p53 (e immunostaining, original magnification: ×200; inset, the SDC cells were strongly positive for p53; immunostaining, original magnification: ×400, case 1), whereas the ASCC cells in case 2 were strongly positive for p53 (f immunostaining, original magnification: ×200; inset, the SDC cells were strongly positive for p53; immunostaining, original magnification: ×400, case 2)
In both cases, the dysplastic squamous cells of the ASCC component were diffusely positive for CK5/6, p63, p40, and EGFR (Fig. 4d). ASCC components in both cases were partially positive for EMA. Only case 1 was partially positive for CK7. The ASCC component in case 1 was entirely negative for p53, whereas case 2 was strongly positive for p53 (Fig. 4e, f). Both ASCC components were negative for MMG, PSA, and SDC markers, such as AR and GCDFP-15, whereas case 1 was focally positive for S-100P.
Both ASCC components had high Ki-67 labeling indices (82% and 89% in cases 1 and 2, respectively). In both cases, the spindle-shaped cells in the nodules were positive for α-smooth muscle actin (ASMA), p63, p40, and CK5/6; therefore, these cells were considered to be neoplastic myoepithelial cells, and the nodules were considered to be pre-/co-existing PA.
In the ASCC component in case 1, E-cadherin and β-catenin were mainly localized in the cytoplasm although membranous expression of these molecules was focally seen, whereas the ASCC component in case 2 demonstrated decreased expression of E-cadherin and β-catenin (Fig. 5). In both cases, E-cadherin and β-catenin were expressed on the membranes of the SDC components as well as on normal salivary ducts and acinar cells. Strong cytoplasmic expression of syndecan-1 was seen in the ASCC and SDC cells of both cases. Although the ASCC component in case 1 was focally and weakly positive for vimentin, that in case 2 was negative for vimentin.
Immunohistochemical staining of cell–cell adhesion molecules in the ASCC components in each case. In the invasive ASCC cells seen in case 1, E-cadherin mainly localized in the cytoplasm (a immunostaining, original magnification: ×200, case 1; inset, E-cadherin immunostaining of the SDC component, original magnification: ×400), as did β-catenin (b immunostaining, original magnification: ×200, case 1; inset, β-catenin immunostaining of the SDC component, original magnification: ×400). In case 2, the invasive ASCC cells displayed decreased expression of E-cadherin (c immunostaining, original magnification: ×200, case 2; inset, E-cadherin immunostaining of the SDC component, original magnification: ×400) and β-catenin (d immunostaining, original magnification: ×200, case 2; inset, β-catenin immunostaining of the SDC component, original magnification: ×400)
We finally diagnosed both cases as ASCC and SDC ex-PA, widely invasive, of the submandibular glands.
Discussion
In CXPA, the most common subtypes of the malignant component are SDC, adenocarcinoma not otherwise specified, undifferentiated carcinoma, myoepithelial carcinoma, and epithelial–myoepithelial carcinoma [10]. It is extremely rare for SCC to arise as a carcinomatous component of CXPA [1]. Only a few cases of such lesions have been reported [11,12,13,14]. Recently, a case of combined SCC and SDC, which was suspected to be a CXPA, was reported [15]. In recent studies, no CXPA patients (0%) harbored SCC components [10]. To diagnose SCC ex-PA, the identification of pre-/co-existing PA is essential [12]. Another differential diagnosis is mucoepidermoid carcinoma; however, this type of tumor typically does not exhibit prominent keratinization and is characteristically composed of epidermoid, intermediate, and mucous cells, which are positive for intracellular mucin. Thus, it is important to perform mucin staining, which produced negative results in our cases.
SDC is one of the most common carcinoma subtypes seen in CXPA, with approximately 25–50% arising from a pre-existing PA [3]. Hyalinized nodules in SDC, which display elastosis with EvG staining, are considered to be pre-existing PA, and a careful search for residual PA components should be performed when a SDC is encountered, as the PA component can be overgrown by the SDC component [3]. SDC is characterized by positivity for the AR and GCDFP-15 [4]. Skalova et al. detected HER2 protein overexpression in SDC as well as in malignant cells of CXPA, but not in benign PA, and suggested that HER2 is useful for detecting the malignant transformation of PA [16]. Based on the immunoprofiles of their large series of SDC, Takase et al. recently proposed that SDC, including CXPA cases, should be divided into “apocrine A”, “apocrine B”, “apocrine HER2”, “HER2-enriched”, and “double negative” subtypes. Moreover, the “double negative” subtype was further subclassified into “basal-like” and “unclassified” types [17]. According to their classification, our cases belonged to the “apocrine B” subtype. However, as the SDC components of both cases were also positive for the EGFR, EGFR overexpression might be related to the transformation of a basal-like type SDC to an ASCC.
Primary SCC of the salivary glands is typically composed of moderately to well differentiated tissue and exhibits infiltrative growth, desmoplasia, and invasion into the periglandular soft tissue of, or near, a major salivary gland [6]. The presence of squamous dysplasia in a dilated salivary duct is indicative of a primary tumor that originates from a salivary gland. The differential diagnoses include metastasis from cutaneous SCC of the head and neck or other sites [6]. Moreover, squamous metaplasia might be also seen in SDC and/or salivary gland carcinomas undergoing high-grade transformation. Squamous metaplasia never showed the keratinization and/or keratin pearl formation unlike SCC, and moreover, in the even case of SDC without squamous metaplasia, some invasive areas partially indicate the immuno-positivity for SDC markers, such as AR or GCDFP-15. Therefore, salivary gland SCC have to be diagnosed very carefully. Our cases focally or partially indicated the single cell keratinization or keratin pearl formation, In the present cases, the invasive portions were entirely negative for SDC markers, and SDC and ASCC components were clearly separated without the transitional zone, so that we considered that the widely invasive components were not squamous metaplasia but true SCC.
ASCC is an uncommon, histologically distinctive variant of SCC. In addition to arising in sun-exposed skin in the head and neck region, it can also occur in mucosal membranes [9, 18]. Histologically, ASCC shows a gland-like or pseudovascular/angiosarcoma-like pattern; SCC with rhabdoid features is rarely seen [19]. Among our cases, case 1 involved acantholysis of the central areas of the tumor nests, some of which displayed rhabdoid features, whereas case 2 demonstrated a pseudovascular histology. Dysregulation and altered expression of intercellular adhesion molecules have been reported in SCC, but the literature offers limited insight into these abnormalities in ASCC. ASCC was reported to exhibit decreased expression of desmoglein 3 and E-cadherin compared with conventional SCC, whereas similar syndecan-1 staining patterns were noted in ASCC and SCC [20]. On the other hand, Bayer-Garner and Smoller demonstrated that in ASCC reduced syndecan-1 and E-cadherin expression were associated with decreased cellular differentiation and were also seen in the acantholytic areas, where both molecules displayed greater cytoplasmic than membranous expression [21]. Such aberrant immunolocalization of E-cadherin is a characteristic of lobular carcinoma of the breast, which is associated with mutations, deletions, and/or methylation of the CDH1 gene [22]. Recently, we reported that in SDC with rhabdoid features the loss of cell–cell adhesion is also related to the absence or aberrant expression of E-cadherin and β-catenin [23]. Both of the present cases displayed decreased or cytoplasmic expression of E-cadherin and β-catenin; therefore, these adhesion molecules were associated with acantholytic changes in our cases. On the other hand, the syndecan-1 expression seen in the ASCC component was similar to those observed in the SDC component and normal salivary gland tissue. Therefore, syndecan-1 expression was not related to morphological changes in our cases. Gu et al. reported that in ASCC acantholysis is related to epithelial–mesenchymal transition (EMT) according to co-expression of CK and vimentin in ASCC cells [24]. A case of SCC with rhabdoid features that we reported previously [19] co-expressed pan-CK and vimentin, but in the current ASCC cases the upregulation of vimentin expression was not observed. Thus, EMT might not occur in salivary ASCC.
In conclusion, we reported two extremely rare cases of CXPA, in which the carcinomatous components were composed of ASCC and SDC. To the best of our knowledge, this is the first case report about ASCC ex-PA. In such cases, changes in the microenvironment beyond the PA capsule are related to histological transformation from SDC to ASCC. Moreover, in salivary ASCC, acantholytic changes might be caused by the aberrant expression of E-cadherin and β-catenin rather than syndecan-1.
Availability of Data and Materials
The dataset supporting the conclusions of this article in included within the article.
Abbreviations
- CXPA:
-
Carcinoma ex pleomorphic adenoma
- PA:
-
Pleomorphic adenoma
- SDC:
-
Salivary duct carcinoma
- AR:
-
Androgen receptor
- GCDFP-15:
-
Gross cystic disease fluid protein-15
- HER2:
-
Human epidermal growth factor receptor 2
- SCC:
-
Squamous cell carcinoma
- ASCC:
-
Acantholytic squamous cell carcinoma
- EvG:
-
Elastica van Gieson
- CK:
-
Cytokeratin
- EGFR:
-
Epidermal growth factor receptor
- MMG:
-
Mammaglobin
- PSA:
-
Prostate specific antigen
- ASMA:
-
α-smooth muscle actin
- EMT:
-
Epithelial–mesenchymal transition
- P/M:
-
Polyclonal/monoclonal
- AMACR:
-
Alpha-methylacyl-CoA racemase
- Cy:
-
Cytoplasmic positivity
- Myo:
-
Myoepithelial cells
- Duct:
-
Ductal cells
References
Gnepp DR. Malignant mixed tumours of the salivary glands: a review. Pathol Annu. 1993;28:279–328.
Nagao T, Licitra L, Loening T, et al. Salivary duct carcinoma. In: El-Nagger AK, Chan JKC, Grandis JR et al, editors. WHO classification of head and neck tumours. Lyon: IARC; 2017. pp. 173–5.
Lim CM, Hobson C, Kim S, et al. Clinical outcome of patients with carcinoma ex pleomorphic adenoma of the parotid gland: a comparative study from a single tertiary center. Head Neck. 2015;37:543–7.
Udager AM, Chiosea SI. Salivary duct carcinoma: an update on morphologic mimics and diagnostic use of androgen receptor immunohistochemistry. Head Neck Pathol. 2017;11:288–94.
Rosa JC, Fonseca I, Felix A, et al. Immunohistochemical study of c-cerbB-2 expression in carcinoma ex-pleomorphic adenoma. Histopathology. 1996;28:247–52.
Taxy JB. Squamous cell carcinoma in a major salivary gland: a review of the diagnostic considerations. Arch Pathol Lab Med. 2001;125:740–5.
Weedon D. Strutton G, Rubin A. Adenoid squamous cell carcinoma. Weedon’s skin pathology, 3th edn. London: Churchill Livingstone; Elsevier; 2010. p. 694–695.
Cassarino DS, Derienzo DP, Barr RJ. Cutaneous squamous cell carcinoma (adenoacanthoma): a comprehensive clinicopathologic classification. Part one. J Cutan Pathol. 2006; 33: 191–206.
Garcia C, Crowson AN. A catholytic squamous cell carcinoma: is it really a more-aggressive tumor? Dermatol Surg. 2011;37:353–6.
Lewis JE, Olsen KD, Sebo TJ. Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol. 2001;32:596–604.
Nakamori K. Ohuchi T, Hasegawa T, et al. Carcinoma ex pleomorphic adenoma of the buccal region is composed of salivary duct carcinoma and squamous cell carcinoma components. Int J Oral Maxillofac Surg. 2009;38:1116–8.
Iino M, Yamada H, Ishikawa H, et al. Carcinoma ex pleomorphic adenoma of the submandibular gland: report of a case with an unusual malignant component of clear cell squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:e30–4.
Garth RJ. Squamous liver metastases from a carcinoma arising within a pleomorphic adenoma of the parotid gland. J Laryngol Otol. 1990;104:152–3.
Magaki SD, Bhuta S, Abemayer E, et al. Carcinoma ex-pleomorphic adenoma of the parotid gland consisting of high-grade salivary duct carcinoma and keratinizing squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:e169–73.
Enokida T, Fujii S, Kuno H, et al. Combined salivary duct carcinoma and squamous cell carcinoma suspected of carcinoma ex pleomorphic adenoma. Pathol Int. 2016;66:460–5.
Skalova A, Starek I, Vanecek T, et al. Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by fluorescence in situ hybridization and immunohistochemistry. Histopathology. 2003;42:348–56.
Takase S, Kano S, Taka Y, et al. Biomarker immunoprofile in salivary duct carcinomas; clinicopathological and prognostic implications with evaluation of the revised classification. Oncotarget. 2017;8:59023–35.
Kusafuka K, Ebihara M, Ishiki H, et al. Primary adenoid squamous cell carcinoma of the oral cavity. Pathol Int. 2006;56:78–83.
Kusafuka K, Onitsuka T, Miki T, et al. Squamous cell carcinoma with rhabdoid features of the gingiva: a case report with unusual histology. Med Mol Morphol. 2014;47:240–5.
Griffin JR, Wriston CC, Peters MS, et al. Decreased expression of intercellular adhesion molecules in acantholytic squamous cell carcinoma compared with invasive well-differentiated squamous cell carcinoma of the skin. Am J Clin Pathol. 2013;139:442–7.
Bayer-Garner IB, Smoller BR. The expression of syndecan-1 is preferentially reduced compared with that of E-cadherin in acantholytic squamous cell carcinoma. J Cutan Pathol. 2001;28:83–9.
Lakhani SR, Rakha E, Simpson PT. Invasive lobular carcinoma. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver M, editors. World Health Organization Classification of Tumours of the Breast. Lyon: IARC; 2012. 40–42.
Kusafuka K, Kawasaki T, Maeda M, et al. Salivary duct carcinoma with rhabdoid features: a salivary counterpart of pleomorphic lobular carcinoma of the breast. Histopathology. 2017;70:164–73.
Gu X, Jiang R, Fowler MR. Acantholytic squamous cell carcinoma in upper aerodigestive tract: Histopathology, Immunohistochemical profile and epithelial mesenchymal transition phenotype change. Head Neck Pathol. 2012;6:438–44.
Acknowledgements
The authors thank Mr. Isamu Hayashi, Mr. Kiyoshi Tone, Ms. Sachiyo Ono, Mr. Koji Muramatsu, Mr. Masato Abe, Mr. Hiroshi Tashiro, Mr. Masatake Honda, Ms. Chiho Tashiro, Ms. Shiori Masujima, Mr. Hiroyuki Shiiya, Mr. Shogo Fujii, Ms. Yoko Kosaka, and Ms. Miho Naka (the staff at the Pathology Division, Shizuoka Cancer Center, Shizuoka, Japan) for their excellent technical assistance. We are also grateful to Ms. Minako Ishii, (the secretary of the Pathology Division, Shizuoka Cancer Center, Shizuoka, Japan) for her valuable help during the preparation of this manuscript.
Funding
The authors declare that they received no funding support for this study.
Author information
Authors and Affiliations
Contributions
KK designed and drafted the manuscript, and KK, TNa and TS made the histopathological diagnosis. TO, NH, KM, TM, TNi, TK, and YI belong to Division of Head and Neck Surgery, Shizuoka Cancer Center, Shizuoka, Japan.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in association with this study.
Ethics Approval and Consent to Participate
This study was approved by the institutional review board of Shizuoka Cancer Center (No. 19–49). All subjects signed informed consent forms to participate.
Consent for Publication
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient. A copy of the consent form is available for review by the Editor of this journal.
Rights and permissions
About this article
Cite this article
Kusafuka, K., Kawasaki, T., Onitsuka, T. et al. Acantholytic Squamous Cell Carcinoma and Salivary Duct Carcinoma Ex-pleomorphic Adenoma of the Submandibular Gland: A Report of Two Extremely Rare Cases with an Immunohistochemical Analysis. Head and Neck Pathol 14, 230–238 (2020). https://doi.org/10.1007/s12105-018-0987-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-018-0987-2