Abstract
A case of sclerosing polycystic adenosis without cysts is described. The lesion occurred as a painless slow growing superficial right parotid lump in a 47 years old woman. The tumor measured 14 × 10 mm and displayed extreme well circumscription, sclerotic stroma with scattered hyaline nodules, sprinkling of mononuclear inflammatory cells and islands of mature fat. The epithelial component was predominantly composed of tubules lined by low columnar to cuboidal epithelium with bland nuclear features. Only focal incomplete acinar differentiation with few cells containing small eosinophilic granules was present. The stroma contained a significant fibromyxoid component with increased cellularity. There was no recurrence on follow-up at 14 months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
History/Clinical Findings
The patient is a 47 years old woman with no significant past medical history. She had felt a lump in the right parotid gland for a couple of years. A superficial parotidectomy was performed and the specimen contained a well demarcated tumor measuring 10 × 14 mm. There has been no recurrence at 14 months follow-up.
Histopathology
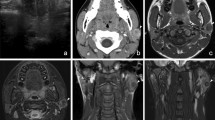
The tumor was unencapsulated with sharp separation from the adjacent salivary gland parenchyma (Fig. 1a, b). The stroma displayed three components in varying proportions. The most abundant stromal component was dense, sclerotic collagen which tended to be more prominent towards the periphery and focally formed hyalinized nodules with clefts in the sclerotic collagen (Fig. 1c). In addition, areas where the stroma had a more fibro-myxoid quality (especially in the center) as well as scattered foci of mature fat were present throughout the lesion (Fig. 1d). The stromal cellularity was significantly increased in these areas. Mononuclear inflammatory cells were focally distributed in a vaguely nodular distribution in the stroma. The epithelial component was chiefly made up of an adenosis-type of proliferation comprised of small tubules which were lined by a single layer of epithelial cells with bland nuclear features (Fig. 1e). A variable degree of stromal distortion of the tubules was present. In a limited area, the epithelial component exhibited a vague attempt to form incomplete tubulo-acinar structures where several of the epithelial cells had increased amount of pale, degenerative appearing cytoplasm (Fig. 1f). Very focally, epithelial cells with a few eosinophilic cytoplasmic granules were seen. (Fig. 1g). No mitotic activity was identified.
The tumor was non-encapsulated and sharply separated from the adjacent salivary gland parenchyma (a, b). The stroma contained hylinized nodules with clefts in the sclerotic collagen (c). Stroma with fibro-myxoid features and increased cellularity was seen, especially in the center (d). The epithelial component was predominantly comprised of small tubules which were lined by a single layer of epithelial cells with bland nuclear features (e). Incomplete acinar structures where the epithelial cells had increased amount of pale, degenerative appearing cytoplasm (f), and epithelial cells with a few eosinophilic cytoplasmic granules were focally present (g)
Diagnosis
Non-Cystic Sclerosing Polycystic Adenosis.
Discussion
Sclerosing polycystic adenosis (SPA) is a rare tumorous condition of salivary glands featuring a variable, albeit characteristic, set of histopathological features that was first described by Smith et al. [1]. SPA occurs most commonly in major salivary glands, with the parotid gland the most commonly affected site. Isolated cases of SPA have also been described in the oral cavity, lip, sinonasal tract and lacrimal gland. However, most of these studies contain poor microphotographs. In three reports, though, the figures are of good quality and these thus substantiate that SPA may arise outside the major salivary glands [2,3,4]. The biological nature of SPA is debated, but given that SPA is a clonal process, and may recur, a plausible perception of SPA is that it is a bona fide neoplasm. Given that the cystic component is an inconsistent finding, perhaps a better designation would be “sclerosing adenoma” [5]. The histopathological spectrum and other aspects of SPA have been previously comprehensively reviewed [6, 7]. The histopathological spectrum of SPA includes a characteristic set of epithelial and stromal components [7] (Table 1). The epithelial component includes acini, tubules and ducts with variable cytomorphological characteristics, including foamy, vacuolated (sebocyte-like), apocrine, mucous, clear/“ballooned”, squamous and oncocyte-like cells. Highly characteristic for SPA is the presence of acinar cells with eosinophilic cytoplasmic granules/globules of varying size. The focal presence of acinar cells with serous differentiation as an inherent component of SPA has received little attention in the literature, but is well documented [7]. In most cases, a prominent, cystic component is present (hence the name), however a paucicystic example has been published [8]. The cysts may be lined by flattened, columnar, apocrine, or vacuolated cells. Most commonly the epithelial components in SPA are embedded in a sharply delineated, dense, sclerotic collagenous stroma, as seen in the present case. The stroma may form hyalinized nodules with clefts in the sclerotic collagen, which were also present in the presented case. This case also contained a stromal adipocytic component which is commonly present. Uncommonly, the stroma may also display myxoid features with increased cellularity. This is a feature that has only infrequently been highlighted in the literature [7]. Commonly the stroma harbors a variably intense, frequently nodular chronic inflammatory infiltrate which may contain bona fide lymphoid follicles. Also in the case presented here were there chronic inflammatory cells seen, although not conspicuously. The acinar component may or may not show a lobular architecture. There may be distortion of the epithelial components imparting an infiltrative appearance or formation of radial scar-like structures. SPA frequently harbors intraductal epithelial proliferations which may display atypia and morphological features of ductal carcinoma in situ (DCIS) [8,9,10]. Some degree of intraductal epithelial proliferations in SPA have been reported in at least 50% of cases in major salivary glands. However, the proportion of cases of SPA with epithelial proliferations that fulfill reasonably accepted criteria for high-grade DCIS does not exceed 10% of reported cases. One case with invasive carcinoma ex SPA has been documented [11]. If one is familiar with the histopathological features of SPA the diagnosis can be made on H&E-stained sections. The immunohistochemical features of SPA has been summarized previously [7]. Both ductal and acinar cells are positive for broad spectrum cytokeratins (AE1-3 and Cam5.2). Variable immunoreactivity for EMA, S-100 protein, antimitochondrial antibody has been reported whereas CEA, p53 and HER2 have been negative. GCDFP-15 is usually strongly expressed in the acinar component. Weak positivity for GCDFP-15 may also be seen in dysplastic or DCIS components. Consistent but variable expression of ER and PR is present in the acinar and ductal cells. ER and PR may or may not be present in the dysplastic/in situ intraductal proliferations. AR was expressed by 10% of the cells in the high-grade DCIS component in one case. The proliferative activity (Mib-1/Ki-67) is low (1–2%) in the benign (both ducular and adenosis-) components whereas significantly increased proliferation may be encountered in an associated high-grade DCIS. P63-, smooth muscle actin- (SMA) and calponin-positive myoepithelial cells are present in the periphery of the acini and ducts in SPA. An immunohistochemical study focusing of myoepithelial/basal cells may be necessary to reveal the pseudoinfiltrative nature in cases where the DCIS component has undergone prominent structural alterations due to stromal distortion [8].
In summary, a case of and adenosis-predominant non-cystic SPA with a fibro-myxoid stromal component is presented. This case expands the combination of histopathological changes that one may encounter when faced with SPA in clinical practice.
References
Smith BC, Ellis GL, Slater LJ, Foss RD. Sclerosing polycystic adenosis of major salivary glands. A clinicopathologic analysis of nine cases. Am J Surg Pathol. 1996;20(2):161–70.
Noonan VL, Kalmar JR, Allen CM, Gallagher GT, Kabani S. Sclerosing polycystic adenosis of minor salivary glands: report of three cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(4):516–20. https://doi.org/10.1016/j.tripleo.2006.08.033.
Park IH, Hong SM, Choi H, Chang H, Lee HM. Sclerosing polycystic adenosis of the nasal septum: the risk of misdiagnosis. Clin Exp Otorhinolaryngol. 2013;6(2):107–9. https://doi.org/10.3342/ceo.2013.6.2.107.
Su A, Bhuta SM, Berke GS, Lai CK. A unique case of sclerosing polycystic adenosis of the sinonasal tract. Hum Pathol. 2013;44(9):1937–40. https://doi.org/10.1016/j.humpath.2013.01.017.
Skalova A, Gnepp DR, Simpson RH, Lewis JE, Janssen D, Sima R, et al. Clonal nature of sclerosing polycystic adenosis of salivary glands demonstrated by using the polymorphism of the human androgen receptor (HUMARA) locus as a marker. Am J Surg Pathol. 2006;30(8):939–44.
Espinosa CA, Rua L, Torres HE, Fernandez Del Valle A, Fernandes RP, Devicente JC. Sclerosing polycystic adenosis of the parotid gland: a systematic review and report of 2 new cases. J Oral Maxillofac Surg. 2017;75(5):984–93. https://doi.org/10.1016/j.joms.2016.10.031.
Petersson F. Sclerosing polycystic adenosis of salivary glands: a review with some emphasis on intraductal epithelial proliferations. Head Neck Pathol. 2013;7(Suppl 1):97–106. https://doi.org/10.1007/s12105-013-0465-9.
Petersson F, Tan PH, Hwang JS. Sclerosing polycystic adenosis of the parotid gland: report of a bifocal, paucicystic variant with ductal carcinoma in situ and pronounced stromal distortion mimicking invasive carcinoma. Head Neck Pathol. 2011;5(2):188–92. https://doi.org/10.1007/s12105-011-0242-6.
Gnepp DR, Wang LJ, Brandwein-Gensler M, Slootweg P, Gill M, Hille J. Sclerosing polycystic adenosis of the salivary gland: a report of 16 cases. Am J Surg Pathol. 2006;30(2):154–64.
Skalova A, Michal M, Simpson RH, Starek I, Pradna J, Pfaltz M. Sclerosing polycystic adenosis of parotid gland with dysplasia and ductal carcinoma in situ. Report of three cases with immunohistochemical and ultrastructural examination. Virchows Arch. 2002;440(1):29–35.
Marques RC, Felix A. Invasive carcinoma arising from sclerosing polycystic adenosis of the salivary gland. Virchows Arch. 2014;464(5):621–5. https://doi.org/10.1007/s00428-014-1551-4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflict of interest.
Ethical Approval
It is author’s institution’s policy not to require formal ethical approval for reports on up to two patients.
Rights and permissions
About this article
Cite this article
Petersson, F. Non-cystic Sclerosing Polycystic Adenosis: Diagnosis of a Hitherto Undescribed Pattern. Head and Neck Pathol 13, 681–685 (2019). https://doi.org/10.1007/s12105-018-0979-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-018-0979-2