Abstract
Pediatric point-of-care ultrasonography (POCUS) has grown in utilization and is now an integral part of pediatric acute care. Applications within the pediatric critical care, neonatology and pediatric emergency were once limited to evaluation of undifferentiated shock states, abdominal free fluid assessments in trauma resuscitation and procedural guidance. The body of pediatric POCUS literature is ever expanding and recently published international consensus guidelines are available to guide implementation into clinical practice. The authors present a review of emerging applications and controversies within thoracic, hemodynamic, neurologic, and ocular POCUS in pediatric acute care medicine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Point-of-care ultrasound (POCUS) is an integral part of pediatric acute care. It allows quick, focused, repeatable, and radiation-sparing imaging in real time at the bedside. Historically, POCUS utilization within pediatric critical care, neonatology and pediatric emergency medicine has centered on procedural guidance, hemodynamic assessment in undifferentiated shock, and free-fluid assessments in trauma. Growing evidence in both adult and pediatric populations, as well as international expert consensus and recommendations [1] have supported an expanding scope for POCUS use in diagnostic, procedural, and clinical decision-making applications within pediatric acute care [2]. The authors present a review of emerging applications and controversies within thoracic, hemodynamic, neurologic, and ocular POCUS in pediatric acute care medicine. Understanding the rationale and clinical implications of POCUS may improve patient outcomes and herald new ideas for further research and innovation.
Thoracic Point-of-Care Ultrasonography
Previously, pediatric lung ultrasonography was primarily used to distinguish parenchymal and pleural disease in an opacified hemithorax, as well as characterize focal mediastinal masses [3]. Many pediatric applications have been derived from adult experience, but when technical applications overlay pediatric-specific pathophysiologic processes, unique results relevant to pediatric clinical settings are revealed.
Metanalyses and evidence-based guidelines for critically ill adults have shown lung ultrasonography to be superior to chest radiography in the detection of pneumothorax [4]. In the neonatal population, lung POCUS is as reliable as radiography for pneumothorax detection, improves time to diagnosis, limits radiation exposure and may be more sensitive for small pneumothoraces [3, 4]. Lung POCUS is limited in its ability to assess pneumothorax size and may require more nuanced interpretation to delineate pneumothorax from other causes of absent lung sliding including pleural adhesions, pleurodesis, bullous emphysema, contusions and mainstem intubation [3, 4]. In traumatic injury scenarios, subcutaneous emphysema may obscure visualization of the pleura, limiting the diagnostic ability of lung POCUS.
Similarly, ultrasonography is superior to radiography and chest CT for assessment of pleural effusion presence, volume, and the presence of septations [3]. In children, early identification and treatment of pleural effusions of bacterial origin may prevent progression to empyema, and early drainage can reduce morbidity and mortality [3, 4]. Lung ultrasonography has shown improved sensitivity and specificity relative to radiography for the diagnosis of pneumonia and consolidation in multiple adult metanalyses and pediatric studies [3, 4]. A 2022 review encompassing 27 pediatric studies demonstrated that lung ultrasonography can diagnose pneumonia with high sensitivity and specificity [4]. Nonspecific findings of atelectasis and edema are found on lung ultrasonography in children with bronchiolitis and asthma, but ultrasound performs better than radiography to detect superimposed bacterial pneumonia in this patient population by identifying consolidation and dynamic air bronchograms [5]. Lung ultrasound is limited by an inability to see lung consolidations that are distant from the pleura and larger airways as well as in more central locations such as the paravertebral regions [3].
In neonates, lung ultrasonography can detect congenital pathology such as pulmonary sequestration, congenital pulmonary airway malformation (CPAM), and congenital diaphragmatic hernia [3, 4]. It is increasingly used in neonatal intensive care units to diagnose neonatal respiratory distress syndrome (RDS), transient tachypnea of the newborn (TTN), meconium aspiration syndrome, pneumonia, pneumothorax, atelectasis, bronchopulmonary dysplasia (BPD), and pulmonary hemorrhage. The predominant use is in distinguishing RDS and TTN, with each having characteristic sonographic findings [3, 4].
In addition to diagnostic evaluation, POCUS reduces the risk of procedural complications during thoracentesis, thoracostomy tube placement and subclavian central venous catheter placement. Ultrasonography immediately prior to thoracentesis and thoracostomy tube placement enhances patient safety by localizing the diaphragm, pleura, surrounding visceral organs, and any aberrant intercostal vessels that may complicate needle insertion [6]. POCUS in subclavian central line placement has been shown to decrease time to placement, drug administration, the need for confirmatory chest radiograph, healthcare costs, and risk of associated complications including pneumothorax, hemothorax, hematoma, vascular injury, and nerve injury [7].
Lung and diaphragm POCUS are faster than radiography in endotracheal tube (ETT) confirmation in children and demonstrate > 90% accuracy [8]. Tracheal, rather than esophageal, placement of an ETT should result in bilateral pleural sliding and diaphragmatic excursion on POCUS, whereas mainstem intubation can result in absent or unilateral lung sliding, poor aeration, and reduced diaphragmatic excursion. A neonatal metanalysis found that tracheal ultrasonography was significantly faster to perform than radiography and was able to identify correct ETT position in 97% of patients [9].
Thoracic POCUS has also been explored as a tool for monitoring physiologic changes and guiding clinical decision making. POCUS can confirm resolution of a pneumothorax or pleural effusion to guide chest tube removal timing and minimize the need for radiography [10]. POCUS is a strong clinical adjunct to the physical exam in its ability to assess lung aeration. The degree of lung aeration is associated with characteristic sonographic findings leading to the creation of a lung ultrasound score (LUS) which has been validated against gold standard lung CT [11]. The LUS is a calculated sum of regional aeration scores from each of six thoracic regions per hemithorax consisting of anterior, lateral, and posterior zones divided into superior and inferior regions. Scores of 0 to 3 are assigned to each region based on the degree of aeration loss ranging from a normal aeration pattern to a consolidation pattern (Fig. 1) [11]. In children with bronchiolitis, a higher LUS is associated with increased oxygen requirement and increased need for mechanical ventilation (MV) [12]. In children with respiratory distress in the emergency department, a LUS > 12 is predictive of the need for escalated respiratory support to high flow nasal cannula (HFNC) or non-invasive or invasive MV [13].
B-mode ultrasound images illustrating lung ultrasound scoring ranging from well-aerated lung (score of 0) to consolidated lung (score of 3). Single headed arrows indicate A-lines, normal reverberation artifacts found in aerated lung. Asterisks indicate B-lines, reverberation artifacts seen in atelectatic or edematous lungs. B-lines increase in number to a point of coalescence as lung consolidation worsens as indicated by the double headed arrows. Complete lung consolidation results in the lung appearing similar to a solid organ in echotexture and ultimately separation of the parietal and visceral pleural layers due to the degree of lung consolidation. Scores are summed over 6 lung regions in each hemithorax for an overall lung score
Lung ultrasonography can also guide MV titration in acute respiratory failure. Lung POCUS provides real-time physiologic information in response to ventilator adjustments and helps differentiate focal from global aeration loss. Changes in LUS can guide titration of positive end-expiratory pressure (PEEP) in heterogenous lung pathology where balancing atelectasis and overdistension can be challenging [14]. Detecting overdistension has historically been a weakness of lung ultrasonography but more recently, regionally decreased lung sliding has been associated with segmental overdistension. Distinguishing overdistension-related reduced lung sliding from pneumothorax is important, particularly in the setting of high ventilator pressures. POCUS can determine that reduced lung sliding was due to overdistension if lung sliding improves in response to decreased ventilator pressures [15]. A randomized controlled trial of pediatric cardiac ICU patients found that perioperative lung ultrasound-guided recruitment maneuvers decreased post-operative desaturation events and shortened MV duration [3].
For ventilator-associated pneumonia, lung ultrasonography can assess post-antimicrobial lung re-aeration, which can re-direct therapies or interventions [16]. Lung ultrasound findings of dynamic air bronchograms are more specific for pneumonia and, with corresponding clinical and lab markers, may suggest the need for antibiotics. Absent or static air bronchograms suggest obstructed air flow and a potential to benefit from bronchoscopy to relieve the obstruction [17]. Excessive color Doppler signal in the setting of worsening hypoxemia and consolidation suggests intrapulmonary shunt as the physiologic etiology [17].
Adult literature has identified clinical applications for lung POCUS in directing ARDS therapies [18], intravenous fluid management and MV weaning [11, 19] but pediatric literature is limited. Increased heterogeneity of the pediatric population makes identifying corresponding lung ultrasonography parameters more complex. One study evaluating LUS during extubation readiness trials identified significant differences in measurements between children in whom extubation was successful vs. unsuccessful [20]. Another study of premature infants requiring MV for RDS showed that a LUS cut-off of 18, performed at a single time point prior to extubation, was predictive of extubation failure [21]. While early literature is promising in infants, significant gaps remain regarding the ability of lung POCUS to predict readiness for MV weaning and extubation readiness among other pediatric patients.
Finally, lung POCUS can differentiate neonatal RDS from TTN, which is essential for determining management since surfactant is administered in RDS, whereas TTN only requires respiratory supportive care [3]. Although radiography is standard for making this distinction, ultrasonography may allow for more timely differentiation, evidence-based treatment, and a radiation-sparing bedside alternative.
Diaphragm Point-of-Care Ultrasonography
Diaphragm POCUS can provide valuable information for clinicians in assessing for diaphragmatic dysfunction (DD) – a spectrum ranging from diaphragmatic weakness to paralysis. Diaphragm ultrasonography is the gold standard for diagnostic identification of DD, as it outperforms traditional techniques like fluoroscopy that are more time-consuming, unavailable at the bedside, and require radiation [22].
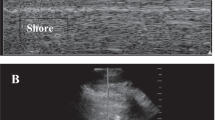
Diaphragm ultrasonography uses M-mode to measure diaphragm excursion (DE) and the difference in diaphragm thickness at end-inspiration and end-expiration to calculate the diaphragmatic thickening fraction (DTF) (Fig. 2). Both DE and DTF are indicators of diaphragm work and contractility. Often, the right hemidiaphragm is easier to visualize (due to the improved acoustic window through the liver), and adult and pediatric literature have shown no difference in measurements between the right and left hemidiaphragms [22, 23]. There are no established reference values for pediatric diaphragm ultrasound parameters, but a few studies have demonstrated age-based normative values that differ from preterm infants to adolescents. In DE assessment, an amplitude of ≤4 mm and a difference of >50% between hemidiaphragms are concerning for DD, with the more obvious absence of diaphragm motion or paradoxical motion being suspicious for paralysis [23]. There are no clear DTF values that define DD in pediatrics, although the lower limit of normal has been shown to be 20% in healthy adults [24].
M-mode image of diaphragm thickening fraction calculated as [(maximum diaphragm thickness - minimum diaphragm thickness)/minimum diaphragm thickness] x 100. The lower limit of normal diaphragm thickening fraction in adults is considered to be 20% [24]
DD exists in many diverse pediatric disease states. It is common in many congenital and acquired pediatric neuromuscular disorders and can occur after congenital cardiac surgery as a consequence of phrenic nerve injury [23]. DD is increasingly described in both children and adults during critical illness, especially in those requiring MV (referred to as ventilator-induced DD) [23, 25]. Diaphragmatic function can be affected by adjacent abdominal or lung pathology that increases intra-abdominal or intra-thoracic pressure [23]. It is unclear the degree to which sedation and MV affect diaphragmatic contractility. Mitigation of DD could help prevent its sequelae, including nosocomial infections, increased duration of MV, increased hospital and ICU length of stay, worse functional outcomes, and increased mortality [24].
There is growing interest in diaphragm POCUS as a tool to guide clinical decision-making. Existing literature suggests that critically ill children undergoing MV experience diaphragmatic atrophy with a median 3.4% decrease in thickness daily, which is accelerated by exposure to neuromuscular blockade [25]. POCUS, therefore, may be a useful adjunct to predict readiness for MV weaning and extubation. Recent prospective studies in children undergoing MV found significant differences in post-extubation readiness testing DE and DTF [20] between children with extubation failure and those with success. Post-extubation readiness testing cutoff values of approximately 6–8 mm for DE and 23–26% for DTF were suggested as being predictive for MV weaning or extubation failure [20, 26]. Despite promising early data, heterogeneous study populations and methods necessitate further studies to better determine the utility of diaphragm POCUS in guiding MV weaning and extubation readiness.
Dynamic Point-of-Care Ultrasound to Assess Fluid Responsiveness
Intravenous fluid administration is integral in the resuscitation of critically ill patients. Intravascular volume increases venous return and augments cardiac contractility via optimization of ventricular myocyte stretch mechanics. Fluid responsiveness is defined by an increase in stroke volume and cardiac output following the administration of fluids. Unfortunately, it is frequently unclear where a given patient lies on the Frank-Starling curve prior to fluid administration and difficult to predict who will benefit and who may be harmed by volume expansion. Only half of hemodynamically unstable patients have a positive response to fluid [27] and adverse effects of fluid overload are well-documented [28]. No static variable (e.g., heart rate, mean arterial pressure, central venous pressure) consistently predicts fluid responsiveness accurately. Fluid responsiveness research has since shifted to dynamic measures, which utilize preload fluctuations that can be mimicked by normal heart-lung interactions during the respiratory cycle, applied liver pressure, or a passive leg raise maneuver to predict how stroke volume might respond to exogenous fluid. Some traditionally dynamic measures require invasive monitors, such as central venous catheters or arterial lines. Fortunately, POCUS can non-invasively assess a patient’s response to preload changes at the bedside in real time.
Fluid responsiveness has no standard research definition, but generally refers to an increase in cardiac index, cardiac output, or stroke volume of >10–15% in response to a 10-20 cc/kg fluid challenge. A frequently used ultrasound-based method to assess fluid responsiveness is inferior vena cava (IVC) respiratory variability. From a subcostal window, the IVC is longitudinally imaged near its confluence with the hepatic vein to capture the dynamic change in IVC diameter caused by fluctuations in intrathoracic pressure during the respiratory cycle. When negative intrathoracic pressure is generated, recruitable blood is returned to the heart and the IVC collapses. During exhalation, the relatively positive intrathoracic pressure decreases venous return and the IVC diameter increases. An IVC collapsibility index (ICI) can be calculated from these measurements using the equation 100 x [(IVCmax – IVCmin) / IVCmax]. The only published prospective study measuring IVC collapsibility in spontaneously breathing pediatric patients with sepsis showed that, the ICI was a poor predictor of fluid responsiveness, with an area under receiver operator characteristic (AUROC) of 0.38 [29].
This relationship between IVC diameter and respiratory phase is the opposite in intubated and paralyzed patients receiving positive pressure ventilation. In positive pressure ventilation, air is delivered by positive force resulting in an increase in intrathoracic pressure, decreased venous return and a corresponding increase in IVC diameter. The IVC distensibility index (IDI) is the measure of IVC respiratory variability in mechanically ventilated patients and is a more promising measure of IVC-based fluid responsiveness prediction in pediatrics. Measurements are taken in the same location as the ICI and the IDI is calculated as 100 x [(IVCmax – IVCmin) / IVCmin]. Using cutoff values ranging from 15.9-23.5%, multiple pediatric studies of mechanically ventilated patients in varied clinical settings have demonstrated good predictive ability of IDI for fluid responsiveness with AUROCs >0.85 [27]. However, other pediatric studies have not demonstrated similarly promising predictive ability [30]. Among other reasons why IVC POCUS may not accurately predict fluid responsiveness, inter- and intra-rater variability can be high [29].
The POCUS technique to assess fluid responsiveness in children, most supported by the evidence, is measurement of peak aortic outflow velocity (Vpeak_Ao) variation. Vpeak_Ao is measured at the aortic annulus or left ventricular outflow tract by pulsed wave Doppler ultrasound. In a paralyzed and mechanically ventilated patient, aortic outflow velocity is maximal during inspiration due to a relative increase in preload to the left side of the heart and at a minimum during exhalation. Percent change in peak aortic outflow velocity (%Δ Vpeak_Ao) is defined as 100 × (Vpeak_AoMax − (Vpeak_AoMin) / [(Vpeak_AoMax + Vpeak_AoMin) / 2] (Fig. 3). Using cutoff values ranging from 7-20%, multiple pediatric studies have demonstrated that %Δ Vpeak_Ao can predict fluid responsiveness in intubated patients; two metanalyses found AUROC ranges from 0.91-0.94 [31, 32]. Despite %Δ Vpeak_Ao’s potential predictive ability, it may be less reliable in smaller children and limited by operator dependence [31].
Pulse wave doppler image of blood flow out of the aortic valve from an apical probe position. The peak velocity of blood flow through the aortic valve varies throughout the respiratory cycle with aortic peak velocity variability calculated as (aortic peak velocity max – aortic peak velocity min) / mean aortic peak velocity x 100%. Values greater than 18–20% are considered indicative that a patient will have an improvement in cardiac output with bolus fluid administration [31, 32]
Point-of-Care Neuroimaging and Ocular Ultrasound
Head ultrasound (HUS) is ubiquitous in the neonatal intensive care unit to evaluate intracerebral pathology. Standard images are acquired by insonating through the anterior fontanelle. The posterior and mastoid fontanelles can be used to evaluate the lateral ventricles [33] and posterior fossa structures [34], respectively. Neonatology providers use point-of-care HUS to evaluate infants with acute clinical deterioration, and adult neurocritical care providers are increasingly following suit. While performance is dependent upon practice environment and resource availability, the value of HUS is its rapid bedside assessment of the need for emergent intervention. This is particularly helpful for patients who are unsafe to transport for a CT scan. In acutely symptomatic infants, HUS is the first-line imaging modality to evaluate for hemorrhage, stroke, and venous thrombosis. It can also diagnose a variety of asymptomatic, subacute, and chronic conditions, including intraventricular hemorrhage, hypoxic-ischemic injury, periventricular leukomalacia, hydrocephalus, infectious complications, and congenital malformations [33, 35]. While HUS is less sensitive than CT or magnetic resonance imaging (MRI) for many of the above diagnoses, it may be preferable due to cost, portability, accessibility, repeatability, and speed.
Transcranial doppler (TCD) measures blood flow velocity in the cerebral vasculature with an ultrasound probe positioned over the temporal, suboccipital, or orbital region of the skull or over the fontanelle. The most common indication in children is to screen for and prevent stroke in children with sickle cell anemia [36]. In critically ill adults, TCD is used to screen for vasospasm following subarachnoid hemorrhage and other intracranial processes [37]; TCD has shown promise as a diagnostic tool in children with acute neurocritical illness and normative values for critically ill children are established [38]. Research in defining the optimal role of TCD in managing children with neurocritical illness is growing, and practice recommendations guiding use in the PICU have been published [39]. Five broad categories for TCD use have been proposed: assessment of cerebral hemodynamics, cerebral autoregulation and vasoreactivity, evaluation of intracranial pressure (ICP), screening for cerebral vasospasm (Fig. 4), and emboli detection [40].
Point-of-care ocular ultrasound is increasingly being used in acute care environments as a diagnostic modality and potential alternative to CT in resource-poor environments. For ocular ultrasound, the ultrasound probe is placed on a closed eyelid and directed posteriorly. It can diagnose a variety of ocular injuries [41]. Ocular ultrasound may also evaluate for elevated ICP (Fig. 5). In predominantly adult metanalyses, optic nerve sheath diameter by ocular US (US-ONSD) had an AUROC of 0.93 for detection of elevated ICP [42] and performs well compared to CT [43]. In pediatrics, CT-ONSD and US-ONSD have been shown to be correlated [44]. Many, but not all, pediatric studies have demonstrated the ability for US-ONSD to predict elevated ICP, though cutoff ranges vary [45].
Conclusions
Pediatric acute care providers are uniquely positioned to follow a patient’s clinical trajectory and perform serial POCUS assessments to guide ongoing management in continuously evolving scenarios. Recent international guidelines have laid the groundwork for expanded POCUS utilization in the care of critically ill children and neonates. This review highlights recent advances and controversies in POCUS utilization as non-imaging specialist exposure to ultrasound technology broadens. When operated within a clinician’s scope of practice, focused POCUS assessments can be invaluable in narrowing differential diagnoses, answering discrete clinical questions and monitoring a patient’s response to interventions. Future POCUS implementation should continue to capitalize on the clinician’s continuous bedside presence and knowledge of the patient’s ongoing physiologic state to optimize its complementary role alongside diagnostic ultrasound.
References
Singh Y, Tissot C, Fraga MV, et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care. 2020;24:65.
Conlon TW, Nishisaki A, Singh Y, et al. Moving beyond the stethoscope: diagnostic point-of-care ultrasound in pediatric practice. Pediatrics. 2019;144:e20191402.
Musolino AM, Tomà P, De Rose C, et al. Ten years of pediatric lung ultrasound: a narrative review. Front Physiol. 2022;12:721951.
Rizvi MB, Rabiner JE. Pediatric point-of-care lung ultrasonography: a narrative review. West J Emerg Med. 2022;23:497–504.
Biagi C, Pierantoni L, Baldazzi M, et al. Lung ultrasound for the diagnosis of pneumonia in children with acute bronchiolitis. BMC Pulm Med. 2018;18:191.
Millington SJ, Koenig S. Better with ultrasound: pleural procedures in critically ill patients. Chest. 2018;153:224–32.
Raman D, Sharma M, Moghekar A, Wang X, Hatipoğlu U. Utilization of thoracic ultrasound for confirmation of central venous catheter placement and exclusion of pneumothorax: a novel technique in real-time application. J Intensive Care Med. 2019;34:594–8.
Kerrey BT, Geis GL, Quinn AM, Hornung RW, Ruddy RM. A prospective comparison of diaphragmatic ultrasound and chest radiography to determine endotracheal tube position in a pediatric emergency department. Pediatrics. 2009;123:e1039–44.
Congedi S, Savio F, Auciello M, Salvadori S, Nardo D, Bonadies L. Sonographic evaluation of the endotracheal tube position in the neonatal population: a comprehensive review and meta-analysis. Front Pediatr. 2022;10:886450.
Soult MC, Collins JN, Novosel TJ, Weireter LJ, Britt LD. Thoracic ultrasound can predict safe removal of thoracostomy tubes. J Trauma Acute Care Surg. 2014;77:256–61.
Mongodi S, De Luca D, Colombo A, et al. Quantitative lung ultrasound: technical aspects and clinical applications. Anesthesiology. 2021;134:949–65.
Supino MC, Buonsenso D, Scateni S, et al. Point-of-care lung ultrasound in infants with bronchiolitis in the pediatric emergency department: a prospective study. Eur J Pediatr. 2019;178:623–32.
Giorno EPC, Foronda FK, De Paulis M, et al. Point-of-care lung ultrasound score for predicting escalated care in children with respiratory distress. Am J Emerg Med. 2023;68:112–8.
Bouhemad B, Brisson H, Le-Guen M, Arbelot C, Lu Q, Rouby JJ. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med. 2011;183:341–7.
Markota A, Golub J, Stožer A, et al. Absence of lung sliding is not a reliable sign of pneumothorax in patients with high positive end-expiratory pressure. Am J Emerg Med. 2016;34:2034–6.
Bouhemad B, Liu ZH, Arbelot C, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med. 2010;38:84–92.
Mongodi S, Bouhemad B, Iotti GA, Mojoli F. An ultrasonographic sign of intrapulmonary shunt. Intensive Care Med. 2016;42:912–3.
Haddam M, Zieleskiewicz L, Perbet S, et al. Lung ultrasonography for assessment of oxygenation response to prone position ventilation in ARDS. Intensive Care Med. 2016;42:1546–56.
Soummer A, Perbet S, Brisson H, et al; Lung Ultrasound Study Group. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress*. Crit Care Med. 2012;40:2064–72.
Abdel Rahman DA, Saber S, El-Maghraby A. Diaphragm and lung ultrasound indices in prediction of outcome of weaning from mechanical ventilation in pediatric intensive care unit. Indian J Pediatr. 2020;87:413–20.
Liang Z, Meng Q, You C, Wu B, Li X, Wu Q. Roles of lung ultrasound score in the extubation failure from mechanical ventilation among premature infants with neonatal respiratory distress syndrome. Front Pediatr. 2021;9:709160.
Turton P, ALAidarous S, Welters I. A narrative review of diaphragm ultrasound to predict weaning from mechanical ventilation: where are we and where are we heading? Ultrasound J. 2019;11:2.
Weber MD, Lim JKB, Glau C, Conlon T, James R, Lee JH. A narrative review of diaphragmatic ultrasound in pediatric critical care. Pediatr Pulmonol. 2021;56:2471–83.
Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5:113.
Glau CL, Conlon TW, Himebauch AS, et al. Progressive diaphragm atrophy in pediatric acute respiratory failure. Pediatr Crit Care Med. 2018;19:406–11.
Yao Y, He L, Chen W, et al. Predictive value of diaphragmatic ultrasonography for the weaning outcome in mechanically ventilated children aged 1–3 years. Front Pediatr. 2022;10:840444.
Carioca FL, de Souza FM, de Souza TB, et al. Point-of-care ultrasonography to predict fluid responsiveness in children: a systematic review and meta-analysis. Paediatr Anaesth. 2023;33:24–37.
Alobaidi R, Morgan C, Basu RK, et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:257–68.
Si X, Xu H, Liu Z, et al. Does respiratory variation in inferior vena cava diameter predict fluid responsiveness in mechanically ventilated patients? A systematic review and meta-analysis. Anesth Anal. 2018;127:1157–64.
Lee JH, Kim EH, Jang YE, Kim HS, Kim JT. Fluid responsiveness in the pediatric population. Korean J Anesthesiol. 2019;72:429–40.
Wang X, Jiang L, Liu S, Ge Y, Gao J. Value of respiratory variation of aortic peak velocity in predicting children receiving mechanical ventilation: a systematic review and meta-analysis. Crit Care. 2019;23:372.
Desgranges FP, Desebbe O, Pereira de Souza Neto E, Raphael D, Chassard D. Respiratory variation in aortic blood flow peak velocity to predict fluid responsiveness in mechanically ventilated children: a systematic review and meta-analysis. Pediatr Anesth. 2016;26:37–47.
Maller VV, Cohen HL. Neonatal head ultrasound: a review and update—part 1: techniques and evaluation of the premature neonate. Ultrasound Q. 2019;35:202–11.
Lowe LH, Bailey Z. State-of-the-art cranial sonography: Part 1, modern techniques and image interpretation. Am J Roentgenol. 2011;196:1028–33.
Maller VV, Choudhri AF, Cohen HL. Neonatal head ultrasound: a review and update-Part 2: the term neonate and analysis of brain anomalies. Ultrasound Q. 2019;35:212–23.
Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial doppler ultrasonography. N Engl J Med. 1998;339:5–11.
Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. Stroke. 2012;43:1711–37.
O’Brien NF. Reference values for cerebral blood flow velocities in critically ill, sedated children. Childs Nerv Syst. 2015;31:2269–76.
O’Brien NF, Reuter-Rice K, Wainwright MS, et al. Practice recommendations for transcranial doppler ultrasonography in critically ill children in the pediatric intensive care unit: a multidisciplinary expert consensus statement. J Pediatr Intensive Care. 2021;10:133–42.
Lovett ME, O’Brien NF. Transcranial doppler ultrasound, a review for the pediatric intensivist. Children. 2022;9:727.
Seguin J, Le CK, Fischer JW, Tessaro MO, Berant R. Ocular point-of-care ultrasound in the pediatric emergency department. Pediatr Emerg Care. 2019;35:e53–8.
Aletreby W, Alharthy A, Brindley PG, et al. Optic nerve sheath diameter ultrasound for raised intracranial pressure: a literature review and meta-analysis of its diagnostic accuracy. J Ultrasound Med. 2022;41:585–95.
Ohle R, McIsaac SM, Woo MY, Perry JJ. Sonography of the optic nerve sheath diameter for detection of raised intracranial pressure compared to computed tomography. J Ultrasound Med. 2015;34:1285–94.
Bhandari D, Udupi Bidkar P, Adinarayanan S, Narmadhalakshmi K, Srinivasan S. Measurement of changes in optic nerve sheath diameter using ultrasound and computed tomography scan before and after the ventriculoperitoneal shunt surgery in patients with hydrocephalus - A prospective observational trial. Br J Neurosurg. 2019;33:125–30.
Abbinante G, Vitiello L, Coppola A, Salerno G, Gagliardi V, Pellegrino A. Optic nerve ultrasound evaluation in children: a review. Diagnostics (Basel). 2023;13:535.
Author information
Authors and Affiliations
Contributions
TWC and CLG conceived of the paper; AEB and KLD wrote the first draft. All authors were involved in editing further versions of this paper. CLG will act as guarantor for this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Andrew E. Becker and Kristopher L. Dixon declare that they have no competing interests to declare that are relevant to the content of this article. Matthew P. Kirschen is supported by a grant from NIH NINDS (K23NS116120). Thomas W. Conlon and Christie L. Glau disclose that they have received travel reimbursement and honoraria for serving as course faculty in point-of care ultrasound courses for the Society of Critical Care Medicine.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Becker, A.E., Dixon, K.L., Kirschen, M.P. et al. Advances in Point-of-Care Ultrasound in Pediatric Acute Care Medicine. Indian J Pediatr (2024). https://doi.org/10.1007/s12098-024-05180-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12098-024-05180-4