Abstract
Objectives
To compare the change in serum vitamin D levels and to compare the changes in serum levels of calcium, phosphate, alkaline phosphatase and parathyroid hormone in vitamin D supplemented and unsupplemented groups after 3 mo.
Methods
In this randomized, parallel group, nonblinded, controlled trial, 40 children, 2–12 y of age with newly diagnosed epilepsy, and vitamin D sufficient status, and started on valproate monotherapy, were randomized into the intervention group (n = 20), which was given daily oral 600 IU vitamin D supplementation, and the control group (n = 20), which was not given any supplementation. Changes in the biochemical parameters was measured in the two groups after 3 mo.
Results
There was a significant reduction in the median (IQR) vitamin D levels in the control group as compared to an increase seen in the intervention group [−6.64 (−8.4, −2.65) vs. 5.66 (1.81, 7.12); p < 0.001]. In the control group, 37.5% children developed vitamin D insufficiency and 12.5% developed deficiency whereas only 5% of the intervention group developed vitamin D insufficiency (p = 0.005). There was a significant decrease in ionized calcium (p = 0.02), increase in serum phosphate (p = 0.02), and alkaline phosphatase level (p = 0.003) in the unsupplemented group as compared to the supplemented group.
Conclusion
Vitamin D supplementation can reduce the valproate-associated decline in vitamin D levels and the negative impact on other markers of bone mineral metabolism.
Trial Registration
TCTR20200621002, 19.06.2020, retrospectively registered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antiseizure medications are the standard of care for epilepsy management in children. Many of these drugs have been demonstrated to negatively impact the bone mineral metabolism in children. According to the systematic review on antiseizure medication utilization in pediatrics by Egunsola et al. [1], most children (58%–94%) received monotherapy, and sodium valproate was the most frequently prescribed antiseizure medication accounting for 7%–66% of the prescriptions. Despite being a nonenzyme-inducing drug, multiple studies have shown that sodium valproate reduces vitamin D and calcium levels [2,3,4,5].
Population studies have reported a high prevalence of vitamin D insufficiency and deficiency in apparently healthy-looking children. Kapil et al. [6] reported that 93% of children 6–18 y of age in Himachal Pradesh, India, were deficient in vitamin D (25-hydroxy vitamin D < 20 ng/mL). In a study in children from lower and upper socioeconomic classes in New Delhi by Marwaha et al. [7], the prevalence of vitamin D deficiency (25-hydroxy vitamin D < 20 ng/mL) was found to be as high as 92.6% and 84.9% with a mean serum vitamin D level of 11.8 ± 7.2 ng/mL. Mandlik et al. [8] in a study in a semirural area of western India, reported that 24% children were vitamin D deficient (< 50 nmol/L), 71% were insufficient (50–74.9 nmol/L) and only 5% were sufficient (> 75 nmol/L). Long term use of sodium valproate in such populations may cause further deterioration of the vitamin D status, thereby affecting physical growth and bone mineral metabolism. The authors, therefore, compared the change in serum vitamin D levels and other serum markers of bone turnover among children with epilepsy receiving sodium valproate, with or without vitamin D supplementation.
Material and Methods
This study was conducted in the Department of Pediatrics of a public hospital attached to a medical college in New Delhi, India from February, 2019 to April, 2020 after clearance from the institutional ethics committee. The trial was registered at a public trial registry (TCTR20200621002).
Children 2–12 y of age presenting with new onset epilepsy and prescribed sodium valproate monotherapy were assessed for eligibility. Those with serum 25-hydroxy vitamin D levels > 20 ng/mL [9] were considered for enrollment. Children with chronic renal or liver disease, nonambulatory or tube-fed status and those who had received vitamin D supplementation in the last 3 mo were excluded. Informed written consent was taken from the parents and assent was taken from the children 7 y or older prior to enrollment. Enrollment was continued till the required sample size was met.
Detailed history, sun exposure (estimated by the UV score) [10, 11], and daily calcium intake (by 24-h dietary recall method) were calculated and noted in a structured form. Detailed physical examination and systemic examination was done. Anthropometry was interpreted, based on WHO growth charts [12] for children < 5 y and revised IAP growth charts [13] for children 5–12 y of age. A 5 mL blood sample was taken to assess baseline biochemical characteristics on the same day.
The participants were randomized by block randomization with permuted blocks into two groups viz., intervention group, which was given daily 600 IU oral vitamin D supplementation and control group, which was not given any supplementation, using a computer-generated random number sequence. The randomization list and numbered packing of the intervention was prepared by the person who generated the randomization sequence.
Sequentially numbered, opaque, sealed envelopes were used for enclosing drug assignment. These envelopes were opened sequentially, only after the participants’ name and other details were written on the envelope. The intervention was provided to the parents using a unique serial number according to the randomization list. Each subject was assigned the next serial number (corresponding to the randomization code) of the intervention. The supplemented group received vitamin D 600 IU per day, orally. No masking of the study drugs was done.
The drug (vitamin D drops) was given from hospital pharmacy to the patients to be taken at home. All patients were followed up every 4 wk to ensure good compliance to valproate and vitamin D supplementation. Seizures and epilepsy were classified as per ILAE classification [14, 15] according to clinical history and results of further investigations (neuroimaging and electroencephalography). After 3 mo, blood sample for the measurement of biochemical markers was repeated.
Serum 25-hydroxy vitamin D and parathyroid hormone levels were assessed by electrochemiluminescence immunoassay (ECLIA). Total serum calcium and ionized calcium were assessed using spectrophotometry and ion-selective electrode method, respectively. Serum alkaline phosphatase was measured by calorimetric assay and serum phosphate level by photometry with molybdate UV method.
The sample size was calculated, based on the data from a pilot study, in which, mean (SD) vitamin D levels in children receiving antiseizure medications was 25.4 (9.3) ng/mL and 18.1 (9.4) ng/mL at baseline and after 90 d, respectively. To detect a minimum difference of 8 ng/mL between the intervention and control group on estimated pooled SD of 9 ng/mL, for a type I error of 5% and power of 80% the sample size required in each group was 20.
Categorical data were represented as numbers or proportions. Continuous data, including the various biochemical parameters, were expressed as median and interquartile range. Normality of the data was analyzed using Kolmogorov–Smirnov (K–S) test. As the data were non-normal in distribution, nonparametric statistical tests were used for comparisons. The baseline patient characteristics of the two groups were compared using Fischer exact test and Mann–Whitney U test. The baseline biochemical markers in the two groups were compared using Mann–Whitney U test. The change in biochemical markers over the study period between the two groups was analyzed by Wilcoxon signed-rank test. A p value less than 0.05 was considered significant. Statistical analysis was done as intention to treat.
Results
A total of 40 children (mean (SD) age 7.1 (2.8) y, 55% males) were enrolled in the study. In the control group, 2 patients were excluded from the study due to change/addition of antiseizure medication, and 2 children were lost to follow-up. Finally, data of 16 children in the control group and 20 in the intervention group were analyzed (Fig. 1).
There were no significant differences between the baseline characteristics, anthropometric variables, UV score, calcium intake, and socioeconomic status, and the biochemical parameters of the two groups (Table 1). Focal onset seizure was the commonest semiology (57.5%) and the commonest etiologies were structural (37.5%) and infectious (27.5%). Sodium valproate dose given ranged from 11 to 35 mg/kg/d.
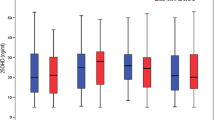
The median (IQR) vitamin D level of the control group reduced significantly (p = 0.002), whereas the intervention group showed a significant increase in serum vitamin D level (p = 0.009) over the 3 mo (Fig. 2). The median (IQR) difference for the control group was significantly different from the intervention group [−6.64 (−8.4, −2.65) vs. 5.66 (1.81, 7.12); p < 0.001] (Table 2).
In the control group, 14 (87.5%) children had a decline in vitamin D level, 12.5% became vitamin D deficient, and 37.5% became vitamin D insufficient. In the supplemented group, 16 (80%) showed increase in vitamin D levels with 5% developing insufficiency and no cases of vitamin D deficiency. This change in vitamin D status was statistically significant (p = 0.005).
There was a significantly greater fall in the ionized calcium levels and increase in the phosphate levels and alkaline phosphatase levels of the unsupplemented group as compared to the supplemented group (Table 3). On imputing the missing values, the median (IQR) differences in these variables for the control group were −0.06 (−0.2, 0.0), 0.25 (−0.1, 0.5) and 30.25 (−4.87, 48.25), respectively. These differences retained their significance (p = 0.006, p = 0.01, and p = 0.002, respectively) on intention-to-treat analysis.
Discussion
This randomized, controlled trial of oral vitamin D supplementation in vitamin D sufficient children receiving sodium valproate monotherapy found significant benefits in the supplemented group. Half of the children not receiving vitamin D supplementation had a decline to vitamin D insufficient or deficient status, as compared to only 5% of the children receiving vitamin D supplementation. Other markers of bone mineral metabolism like calcium, alkaline phosphatase, and serum phosphate also improved in those being supplemented with vitamin D.
In the present study, 87.5% children in the unsupplemented group had a reduction in the serum vitamin D levels and the proportion of children found deficient or insufficient was comparable to the data from previous studies. A cross-sectional study in children on valproate or levetiracetam monotherapy for variable duration [16] has reported that 24% of children on the antiseizure monotherapy had vitamin D levels less than 20 ng/mL as compared to only 14% in the control population. In another study [4], it was reported that developmentally normal ambulatory children on valproate monotherapy for 6 mo or more having significantly lower median values of serum vitamin D levels as compared to controls with 37.5% of the children had vitamin D levels < 20 ng/mL. In another study [17], it was reported that 27.1% of children on antiseizure medications for minimum 6 mo had vitamin D levels < 12 mg/mL and 57.6% had levels < 20 ng/mL as compared to 11.2% and 46.1% in the same broad age range controls. The increase in vitamin D level and preservation of vitamin D sufficient status seen in the present study is similar to a study done in adults with new onset epilepsy started on phenytoin or valproate therapy [18], wherein the subjects given vitamin D supplementation showed a significant increase in vitamin D levels in 90 d. In a prospective interventional study, ambulatory children on long-term polytherapy with valproate and one or more other antiseizure medications, given daily vitamin D supplementation of 400 IU, orally, for 12 mo had a significantly higher serum vitamin D level with improvement in the biochemical markers of enhanced bone turnover [19]. In a study [20] conducted on ambulatory children and adolescents on long-term antiseizure therapy, vitamin D levels had increased by 30% and 54% in the study groups given daily vitamin D supplementation of 400 IU and 2000 IU, respectively, with an overall decrease in vitamin D deficient status from 14 to 6% over the one-year study period.
The present study did not show a significant change in serum parathyroid hormone levels, similar to the data from previous studies [16, 21, 22]. Offerman et al. [23] reported higher level of parathyroid hormone in children and adults on chronic antiseizure therapy which normalized on vitamin D supplementation for 9 mo, probably suggesting that changes in PTH levels with antiseizure medication use take longer to appear and require a longer duration of supplementation to improve. No significant change in serum calcium levels was found; although, many studies [16, 17, 22,23,24,25] have shown a decline in serum calcium levels in children on chronic antiseizure therapy, probably indicating that the decline in calcium levels takes longer to occur. In a study conducted on adults with new-onset epilepsy on valproate or phenytoin therapy, the group given only calcium supplementation showed a decline in calcium levels over 90 d, whereas the subjects given vitamin D and calcium supplementation showed an increase in serum calcium levels at 90 d [18], similar to the present study. Paradoxical deterioration of seizure control has been reported [26] due to antiseizure medication-induced hypocalcemia. In the present study, there was no difference in seizure control in the two groups. Many studies have reported higher alkaline phosphatase levels in children on chronic antiseizure medications [17, 21, 24]. A reduction in serum alkaline phosphate levels was found in those receiving supplementation similar to the data from a previous study [18].
In this study, only ambulatory children with vitamin D sufficient status were enrolled eliminating many confounding factors like nonambulatory status causing altered bone mineral metabolism and pre-existing vitamin D deficiency resulting from chronic dietary deficiency of vitamin D intake with reduced exposure to sunlight. All the subjects enrolled were drug naïve to eliminate the impact of intake of antiseizure medications taken previously for variable duration. Only children started on valproate monotherapy were included to ensure a relatively homogenous participant group for the study. Dietary calcium intake was calculated for all children enrolled in the study and all the children were counseled for adequate intake of calcium in diet. Sun exposure, an important determinant of serum vitamin D level, was calculated for all subjects at the baseline. The patients were followed up for 90 d, which in a previous study [16] has been shown as the time required to have significant changes in bone mineral metabolism caused by initiation of antiseizure medication.
The major limitation of the present study is that it was a sample size of convenience. It was a nonblinded trial with no placebo given to the unsupplemented group. Exposure to sunlight was measured using UV score rather than a solarimeter/UV dosimeter, which has a better reliability. Compliance to medications was ensured verbally at regular follow-up; however, the authors did not check physically for remaining syrup with the participant to confirm compliance. Impact of anti-seizure medication and supplementation on bone mineral density of children could not be studied. Also, the authors could not measure bone-specific alkaline phosphatase due to nonavailability of test, and measured total alkaline phosphate level, which is relatively nonspecific.
The study indicates that a significant proportion of children with vitamin D sufficient status become vitamin D insufficient or deficient on receiving valproate monotherapy over a short duration of 3 mo. This was prevented in majority of children in the study by daily oral supplementation of vitamin D in recommended doses as per RDA (600 IU). In the backdrop of high prevalence of vitamin D deficiency or insufficiency in healthy-looking Indian children, this points towards the need for screening children for vitamin D deficiency when being started on valproate (and possibly other antiseizure medications), and treatment with vitamin D of those who are found deficient. In resource-deficient areas, supplementation of daily oral maintenance dose of vitamin D can be considered in those started on valproate monotherapy. Further studies are required to elucidate the requirement of supplementation of vitamin D with all antiseizure medications and the adequate dose and duration required to prevent decline in vitamin D status and bone mineral density in these children.
References
Egunsola O, Choonara I, Sammons HM. Antiepileptic drug utilisation in paediatrics: a systematic review. BMJ Paediatr Open. 2017;1:e000088.
Vestergaard P. Effects of antiepileptic drugs on bone health and growth potential in children with epilepsy. Paediatr Drugs. 2015;17:141–50.
Zhang Y, Zheng YX, Zhu JM, Zhang JM, Zheng Z. Effects of antiepileptic drugs on bone mineral density and bone metabolism in children: a meta-analysis. J Zhejiang Univ Sci B. 2015;16:611–21.
Lee Y-J, Park KM, Kim YM, Yeon GM, Nam SO. Longitudinal change in vitamin status in children with epilepsy on anti-epileptic drugs: prevalence and risk factors. Pediatr Neurol. 2015;52:153–9.
Oner N, Kaya M, Karasalihoğlu S, Karaca H, Celtik C, Tütüncüler F. Bone mineral metabolism changes in epileptic children receiving valproic acid. J Paediatr Child Health. 2004;40:470–3.
Kapil U, Pandey RM, Goswami R, et al. Prevalence of vitamin D deficiency and associated risk factors among children residing at high altitude in Shimla district, Himachal Pradesh. India Indian J Endocr Metab. 2017;21:178–83.
Marwaha RK, Tandon N, Reddy DR, et al. Vitamin D and bone mineral density status of healthy school children in Northern India. Am J Clin Nutr. 2005;82:477–82.
Mandlik R, Kajale N, Ekbote V, et al. Determinants of vitamin D status in Indian school children. Indian J Endocr Metab. 2018;22:244–8.
Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101:394–415.
Seth A, Aneja S, Singh R, Majumdar R, Sharma N, Gopinath M. Effect of impaired ambulation and anti-epileptic drug intake in vitamin D status of children with cerebral palsy. Pediatr Int Child Health. 2017;37:193–8.
Aggarwal V, Seth A, Aneja S, et al. Role of calcium deficiency in development of nutritional rickets in Indian children: a case control study. J Clin Endocrinol Metab. 2012;97:3461–6.
WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta paediatr Suppl. 2006;450:76–85.
Indian Academy of Pediatrics Growth Charts Committee, Khadilkar V, Yadav S, Agrawal KK, et al. Revised IAP growth charts for height, weight and body mass index for 5-to 18-year-old Indian children. Indian Pediatr. 2015;52:47–55.
Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:512–21.
Fisher RS, Cross JH, D'souza C, et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58:531–42.
Durá-Travé T, Gallinas-Victoriano F, Malumbres-Chacón M, Moreno-Gónzalez P, Aguilera-Albesa S, Yoldi-Petri ME. Vitamin D deficiency in children with epilepsy taking valproate and levetiracetam as monotherapy. Epilepsy Res. 2018;139:80–4.
Vijaykumar M, BK A, George B, Bhatia V. Vitamin D status in children on anticonvulsant therapy. Indian J Pediatr. 2021. https://doi.org/10.1007/s12098-021-03853-y.
Krishnamoorthy G, Nair R, Sundar U, Kini P, Shrivastava M. Early predisposition to osteomalacia in Indian adults on phenytoin or valproate monotherapy and effective prophylaxis by simultaneous supplementation with calcium and 25-hydroxy vitamin D at recommended daily allowance dosage: a prospective study. Neurol India. 2010;58:213–9.
Papassava M, Siomou E, Nakou I, Cholevas V, Challa A, Tzoufi M. Effects of long-term antiepileptic polytherapy on bone biochemical markers in ambulatory children and adolescents and possible benefits of vitamin D supplementation: a prospective interventional study. Epilepsy Behav. 2021;115:107708.
Mikati MA, Dib L, Yamout B, Sawaya R, Rahi AC, Fuleihan Gel-H. Two randomized vitamin D trials in ambulatory patients on anticonvulsants: impact on bone. Neurology. 2006;67:2005–14.
Babayigit A, Dirik E, Bober E, Cakmakci H. Adverse effects of anti-epileptic drugs on bone mineral density. Pediatr Neurol. 2006;35:177–81.
Albaghdadi O, Alhalabi MS, Alourfi Z, Youssef LA. Bone health and vitamin D status in young epilepsy patients on valproate monotherapy. Clin Neurol Neurosurg. 2016;146:52–6.
Offermann G, Pinto V, Kruse R. Antiepileptic drugs and vitamin D supplementation. Epilepsia. 1979;20:3–15.
Chaudhuri JR, Mridula KR, Rathnakishore C, Balaraju B, Bandaru VS. Association of 25-hydroxyvitamin D deficiency in pediatric epileptic patients. Iran J Child Neurol. 2017;11:48–56.
Christiansen C, Rodbro P, Lund M. Effect of vitamin D on bone mineral mass in normal subjects and in epileptic patients on anticonvulsants: a controlled therapeutic trail. Br Med J. 1973;2:208–9.
Gauci Z, Rizzo C, Mifsud S, Cachia MJ. Paradoxical deterioration in seizure control due to anticonvulsant-induced hypocalcemia. BMJ Case Rep. 2019;12:e232429
Funding
None.
Author information
Authors and Affiliations
Contributions
DM conceptualized and planned the study, supervised the conduct of the study, and assisted in the statistical analysis and finalization of the manuscript; SM assisted in planning the study and statistical analysis, evaluated and enrolled the patients and carried out sample collection, carried out follow-up and assessed the outcomes, and prepared the initial draft of the manuscript; MM provided important intellectual inputs in planning and conduct of the study, and assisted in manuscript preparation; BM provided important intellectual inputs in planning and conduct of the study, and supervised the sample storage and analysis; AMK provided important intellectual inputs in planning and conduct of the study, and the data analysis. All authors approved the final manuscript. DM will act as the guarantor for this paper.
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Maulana Azad Medical College, University of Delhi (F.No./17/IEC/MAMC/2018/09).
Consent to Participate
Informed consent was taken from parents and assent was taken from all participants 7 to 12 y of age.
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mishra, S., Mishra, D., Mahajan, B. et al. Effect of Daily Vitamin D Supplementation on Serum Vitamin D Levels in Children with Epilepsy Receiving Sodium Valproate Monotherapy: A Randomized, Controlled Trial. Indian J Pediatr 90, 450–456 (2023). https://doi.org/10.1007/s12098-022-04225-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-022-04225-w