Abstract
Objective
To describe the prevalence and determinants of occurrence of dyslipidemia in children and adolescents with type 1 diabetes mellitus (T1DM).
Methods
A cross-sectional study was conducted in the diabetic clinic of a tertiary care referral hospital over two years. Subjects with T1DM aged 2–18 y had assessment of Lipid profile after 12 h of fasting. Glycemic control was assessed by glycosylated hemoglobin (HbA1C). Modifiable and nonmodifiable determining factors were assessed during clinic visit as per standard criteria. Body mass index (BMI) more than 23rd adult equivalent and 27th adult equivalent were considered as overweight and obesity, respectively. Lipid parameters were considered as abnormal if: Low density lipoprotein (LDL) > 100 mg/dL, high density lipoprotein (HDL) < 40 mg/dL, triglycerides > 100 mg/dL (< 10 y) and > 130 mg/dL (> 10 y).
Results
A total of 171 subjects (mean age: 11.8 ± 3.5 y, M:F = 75:96) were recruited during the study period. The mean fasting LDL level, HDL level, and triglyceride level observed in the study sample were 106.6 ± 26.9 mg/dL (62% abnormal), 52.6 ± 14.3 mg/dL (9.4% abnormal), and 85.3 ± 39.4 mg/dL (10.5% abnormal), respectively; 115 (67.3%) of the subjects had at least one abnormality in the serum lipid profile. On multivariate analysis, HbA1C was the most significant factor in determining the occurrence of dyslipidemia (p < 0.05). HbA1C was 9.9 ± 1.6 in subjects with abnormal LDL versus 9.1 ± 1.5 in those with normal LDL (p < 0.05).
Conclusion
Prevalence of dyslipidemia in the study children and adolescents with T1DM was 67.3%. HbA1C remains the most important modifiable determinant of the occurrence of dyslipidemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis resulting in cerebrovascular disease, coronary vascular disease, and peripheral vascular disease is an important cause for mortality in adults with T1DM [1]. Overt cardiovascular disease is not encountered in children, but subclinical disease is often present. Children with early cardiovascular changes are often known to track onto adulthood [2]. Globally, diabetes mellitus is emerging in an epidemic proportion. Type 1 diabetes mellitus (T1DM) accounts for 5%–10% of the total cases of diabetes worldwide [3]. With the available data, type 1 diabetes mellitus (T1DM) has been rising at an average rate of 3%–4% per year in children and adolescents [4]. The Diabetes Atlas 2017 estimates that there are 128,500 children and adolescents with diabetes in India [5]. Three sets of prevalence data show 17.93 cases/100,000 children in Karnataka, 3.2 cases/100,000 children in Chennai, and 10.2 cases/100,000 children in Karnal (Haryana) [6]. Children and adolescents with T1DM are prone to develop microvascular and macrovascular complications, and dyslipidemia is an important macrovascular complication of type 1 DM [7]. As the number of subjects with T1DM rise, the resulting increase in associated CVD has to be tackled by pediatricians and physicians dealing with T1DM. Limited data regarding pathology suggest that inflammation is more prominent in patients with DM when compared with the general population [8], and those with T1DM in particular are affected. Studies reveal that C-reactive protein is elevated within the first year of diagnosis of T1DM [9], and interleukin 6 and fibrinogen levels are high in individuals with an average disease duration of 2 y [10]. Elevated LDL-C is an established risk factor for cardiovascular disease (CVD) [11]. The American Diabetes Association (ADA) guidelines recommend screening for dyslipidemia in subjects beyond 10 y of age [12]. The International Society for Pediatric and Adolescent Diabetes (ISPAD) recommends screening lipid profile in children with T1DM after stabilization in children above 11 y or earlier in the presence of risk factors [7]. In the presence of risk factors like family history of atherosclerotic disease, screening can be commenced from 2 y of age [13]. Large population based studies from Europe and studies from Indian centers have demonstrated that there is a significant prevalence of cardiovascular risk factors including dyslipidemia in subjects ≤ 10 y with T1DM [14, 15]. Studies have looked into subclinical cardiovascular abnormalities in children and adolescents with various tools including: carotid intima media thickness, flow-mediated arterial dilatation, endothelial peripheral arterial tonometry, and arterial stiffness measured by pulse wave velocity [1]. Significant abnormalities have been observed in the first decade of onset of T1DM itself. Studies from various centers in developed and developing world have recognized the prevalence of dyslipidemia in this group of children [16,17,18]. However, there is paucity of data in this regard from South India. Hence, this study was conducted to identify the prevalence and determinants of dyslipidemia in children and adolescents with T1DM in an urban tertiary care hospital in South India.
Material and Methods
A cross-sectional study was conducted in the diabetic clinic of a tertiary care referral hospital in South India over a period of two years from 2019 to 2021. Children aged 2 y to 18 y who were diagnosed as type 1 DM were recruited. Children with type 1 DM with 1 y of diabetes duration were recruited. Subjects with celiac disease, untreated or uncontrolled hypothyroidism and eating disorder were excluded. Data on age of onset, chronological age, duration of diabetes, sex, insulin regimen, and dose of insulin were collected from the parents by standard interview in the clinic by primary investigator (MS). Pattern of unhealthy food intake, frequency of physical activity and screen time were interviewed in the clinic visit.
Anthropometric measures were recorded as per standard techniques [19,20,21]. A calibrated stadiometer was used to record the height. Without shoes or socks, the child would be standing with parallel feet on an even platform with the arms hanging by the sides. The head was held in the Frankfurt plane (lower border of eye orbit and the external canal of ear on the same horizontal plane), with the buttocks, heels and scapulae touching the rod. The head piece was lowered to the top of the head and height was measured. With light clothing, the weight of the children was recorded using a calibrated electronic weighing scale. The weight was recorded to the nearest 50 g and height to the nearest 0.1 cm. Using a nonstretchable tape, waist circumference was recorded at the midpoint of the iliac crest and lower rib cage to the nearest 0.1 cm. During measurement, the children would be in the standing position in the end tidal expiration. A Harpenden skin-fold calliper was used to record the triceps skin-fold thickness (TSFT). A point was marked on the back of the arm between the acromion process of the scapula and the olecranon process of the ulna.
Body mass index (BMI) was calculated from the formula, BMI = Weight (kg)/Height (m2). BMI more than 23rd adult equivalent and 27th adult equivalent were considered as overweight and obesity, respectively. Anthropometric measures were interpreted using appropriate references [19,20,21,22,23].
SMR assessments were accurately performed by MS and HKP after obtaining consent. The assessments were performed on the girls with minimal clothing in complete privacy in the presence of a female staff nurse and the mother. Breast stage, pubic hair, testicular size, and penile size were classified into one of the five stages described by Tanner based on verbal and pictorial description. The assessments were performed on the boys with minimal clothing in complete privacy in the presence of the father.
Blood pressure was recorded using an appropriate-sized cuff which covers two-third size of arm with the bladder encircling 40% of the arm circumference. Using the palpatory method, systolic blood pressure (SBP) was recorded. After inflating to 20 mm Hg above the SBP measured, the stethoscope was placed medial to the biceps brachii tendon. Appearance of first Korotkoff sound and point of disappearance of Korotkoff sound were taken as SBP and diastolic blood pressure (DBP), respectively [24]. Blood pressure more than the 95th percentile was considered as hypertension. The study subjects were assessed for microvascular complications: indirect ophthalmoscopy for fundus screening by trained pediatric ophthalmologist, and urinary albumin-to-creatinine ratio in first voided urine and nervous system examination by a single pediatrician (HKP) for peripheral neuropathy. Blood sample was collected after 12 h of fasting for glycosylated hemoglobin (to assess glycemic control) and lipid profile. HbA1C was estimated by immunoturbidimetry method (Roche diagnostics, COBAS Integra 400 Plus). In lipid profile, total cholesterol and triglyceride were measured by cholesterol oxidase peroxidase method (CHOD-POD) and glycerol-3-phosphate oxidase peroxidase (GPO-POD) method, respectively, and HDL and LDL were done by direct enzymatic method (Roche diagnostics, COBAS Integra 400 Plus). Lipid parameters were considered as abnormal if: LDL was > 100 mg/dL, HDL < 40 mg/dL, and triglycerides > 100 mg/dL and > 130 mg/dL in less than and more than 10 y, respectively [7, 13].
The study was approved by the institutional ethics committee. All eligible children were enrolled in the study after informed consent or assent obtained from the parents/study subjects as appropriate. Sample size was estimated using previous study [16] with a prevalence of dyslipidemia in type 1 diabetes mellitus of 72.5%, with a 95% confidence level and 7% absolute precision arrived was 171 children.
The basic characteristics were presented by descriptive analysis as mean (standard deviation) or frequencies (%). Comparison of the qualitative variables of the groups were done using chi square test. Quantitative variables among the groups were compared using student independent t-test. The z scores were calculated using the formula: z score = X – mean/SD, where X is the measured value, SD is the standard deviation. A p value of ≤ 0.05 was considered statistically significant, and two-tailed tests were used for testing significance. Statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS, version 22) software. A multiple regression analysis was performed to determine the likely determinants of occurrence of dyslipidemia (abnormal LDL).
Results
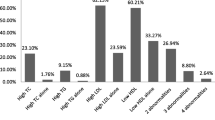
During the study period, 175 children and adolescents with T1DM presented to the endocrine unit and among them 171 satisfied the inclusion criteria and were recruited in to the study. The baseline data of the study population are described in Table 1. The mean fasting LDL level, HDL level and triglyceride level observed in the study sample were 106.6 ± 26.9 mg/dL, 52.6 ± 14.3 mg/dL and 85.3 ± 39.4 mg/dL, respectively. In the present study population, 106 (62%), 16 (9.4%) and 18 (10.5)% had high LDL, low HDL and high triglycerides, respectively. In the present study population, 115 (67.3%) subjects had at least one abnormality in the serum lipid profile.
It was observed that 46 out of 75 males (61.3%) had one abnormal lipid parameters versus 69 of the 96 females (71.9%), (p > 0.05). It was also observed that 35 (66%), 3 (5.7%), 4 (7.5%) and 36 (67.9%) subjects aged less than 10 y had abnormal LDL, abnormal triglycerides, low HDL and at least one abnormal lipid value, respectively. Among the subjects with elevated BMI (n = 49), 34 (69.4%), 5 (10.2%) and 1 (2%) of the subjects had an abnormality in serum LDL, Triglyceride and HDL value. Among the children with high LDL, high triglycerides and low HDL, 34 (32.1%), 5 (27.8%) and 1 (6.2%) of the children had elevated BMI.
On univariate analysis, diabetic age and HbA1C were found to be factors that determine the occurrence of abnormal LDL in subjects with type 1 diabetes mellitus. A multivariate regression analysis was performed to identify the independent factor determining the occurrence of abnormal LDL in the present study subjects. It was observed that HbA1C was the most independent factor in determining the onset of dyslipidemia (p < 0.05) (Table 2). A weak correlation was observed between HbA1C and LDL (r = 0.22, p < 0.05) (Fig. 1). To further understand the relationship between dyslipidemia and HbA1C, the children were divided into two groups (children with normal LDL and children with abnormal LDL). It was observed that mean HbA1C was 9.1 ± 1.5 in the former versus 9.9 ± 1.6 in the latter group (p < 0.05) (Fig. 2).
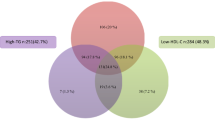
To further elucidate the relationship between HbA1C and dyslipidemia, tertiles of HbA1C were constructed and the percentage of children with abnormal LDL was computed. It was observed that the percentage of children with abnormal LDL increased across tertiles of HbA1C: 51.6%, 63%, and 72.7% in tertile 1, 2, and 3, respectively.
On physical examination, none of the subjects had any obvious external markers of dyslipidemia like xanthoma or arcus juvenilis. The children with abnormal lipid profile were referred to a dietician, advised diet, restricting junk-food intake and reducing screen time with increase in physical activity. None of the present study subjects were initiated on pharmacological therapy.
Discussion
As far as the authors know, this is the first South Indian study to describe the prevalence of dyslipidemia in children with T1DM. The prevalence of dyslipidemia, observed in the present study, of 67.3% is an eye-opener to pediatricians caring for subjects with T1DM to screen for these subjects early and initiate on lifestyle measures. This observation is in agreement with high prevalence reported from an Indian study of 47.2% and 65% reported from another South Asian center [15, 18]. It is noteworthy that the Indian study catered to a low socioeconomic population, whereas the authors cater to the middle income group.
Mean BMI z score in the present study of −0.05 ± 1.0 is higher than −0.4 ± 0.9 observed in the Indian study [15] and a parallel higher prevalence of dyslipidemia; 28.7% of the present study subjects had elevated BMI. Improved glycemic control from intensive insulin therapy is associated with weight gain and worsening of cardiovascular risk factor profile. Data from DCCT have demonstrated that subjects in the top quartile of weight gain had highest waist-to-hip ratio, proatherogenic lipid profile, and blood pressure [25]. The present study group is predominantly on intensive insulin therapy. A high prevalence of overweight or obesity has been observed previously of 15.1% and 15.6% in subjects with type 1 diabetes mellitus [16, 26]. Obesity and metabolic syndrome in subjects with T1DM is said to be associated with increased visceral adiposity, blood pressure, adverse lipoprotein changes, and insulin resistance [1]. Insulin resistance appears to be an independent predictor of microangiopathy, left ventricular hypertrophy and diastolic dysfunction [25]. Thus, it is mandatory that pediatricians caring for subjects with T1DM should monitor BMI on the new IAP growth charts and identify abnormal BMI, early.
HbA1C has emerged as the single independent determinant of occurrence of dyslipidemia in the present study. This is in agreement with various previous studies [16, 17]. Long-term glycemic control has been observed to be a determinant of occurrence of atherosclerotic plaques on ultrasound without symptoms of coronary vascular disease. Intensive insulin therapy has observed to be beneficial in improvement of cardiovascular events in subjects with childhood onset T1DM [27]. Few mechanisms have been proposed suggesting a direct effect of hyperglycemia on gene transcription of coagulation factors due to hyperglycemia-induced oxidative stress, direct glycation of coagulation factors, altering their activity and loss of the endothelial glycocalyx layer. Insulin is a natural antagonist of platelet hyperactivity. It enhances endothelial generation of PGI2 and NO and sensitizes the platelet to PGI [28]. Thus, the deficiency of insulin in T1DM on a long term, with the combination of poor glycemic control, dyslipidemia and dysfunctional coagulation cascade together may form a significant risk for dyslipidemia. The extent of atherosclerosis by intravascular ultrasound also correlated with HbA1C over 18 y of follow-up in the Oslo Study; a 1% increase in mean HbA1C was associated with a 6.4% increase in coronary vessel stenosis [29]. Thus, optimization of glycemic control and maintenance of HbA1C in target range as recommended by ISPAD is the most important strategy for prevention of dyslipidemia. Limitations of the present study include: single-center data, lack of follow-up and lack of control group.
Conclusion
There is a significant prevalence of dyslipidemia in the present sample of children and adolescents with type 1 DM (67.3%). Maintenance of good glycemic control remains the most important strategy to be adopted by pediatricians to prevent the occurrence of dyslipidemia.
Data Availability
On request.
References
de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American heart association and American diabetes association. Circulation. 2014;130:1110–30.
Truong UT, Maahs DM, Daniels SR. Cardiovascular disease in children and adolescents with diabetes: where are we, and where are we going? Diabetes Technol Ther. 2012;14(Suppl 1):S11-21.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–7.
Bell RA,Mayer-Davis EJ, Beyer JW, et al. SEARCH for Diabetes in Youth Study Group. Diabetes in non-hispanic white youth: prevalence, incidence, and clinical characteristics:the SEARCH for diabetes in youth study. Diabet Care. 2009;32 Suppl 2:S102–11.
International Diabetes Federation. IDF Diabetes Atlas, 8th edn. Brussels, Belgium. In: International Diabetes Federation. 2017. Available at: http://www.diabetesatlas.org. Accessed on 17 Feb 2019.
Virmani A. Type 1 diabetes in India: the numbers show the way ahead. Indian Pediatr. 2019;56:189–90.
Donaghue KC, Marcovecchio ML, Wadwa RP, et al. ISPAD clinical practice consensus guidelines 2018: microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes. 2018;19(Suppl 27):262–74.
Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102:2180–4.
Hayaishi-Okano R, Yamasaki Y, KatakamiN, et al. Elevated C-reactive protein associates with early-stage carotid atherosclerosis in young subjects with type 1 diabetes. Diabetes Care. 2002;25:1432–8
Schölin A, Siegbahn A, Lind L, et al. Diabetes Incidence Study in Sweden Group. CRP and IL–6 concentrations are associated with poor glycemic control despite preserved beta-cell function during the first year after diagnosis of type 1 diabetes. Diabetes Metab Res Rev. 2004;20:205–10.
Schwab KO, Doerfer J, Marg W, Schober E, Holl RW. DPV Science Initiative and the Competence Network Diabetes mellitus. Characterization of 33 488 children and adolescents with type 1 diabetes based on the gender-specific increase of cardiovascular risk factors. Pediatr Diabetes. 2010;11:357–63
Chiang JL, Maahs DM, Garvey KC, et al. Type 1 diabetes in children and adolescents: a position statement by the American diabetes association. Diabetes Care. 2018;41:2026–44.
Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 Suppl 5:S213–56.
Margeirsdottir HD, Larsen JR, Brunborg C, Overby NC, Dahl-Jørgensen K. Norwegian Study Group for Childhood Diabetes. High prevalence of cardiovascular risk factors in children and adolescents with type 1 diabetes: a population-based study. Diabetologia. 2008;51:554–61.
Shah N, Khadilkar A, Gondhalekar K, Khadilkar V. Prevalence of dyslipidemia in Indian children with poorly controlled type 1 diabetes mellitus. Pediatr Diabetes. 2020;21:987–94.
Homma TK, Endo CM, Saruhashi T, et al. Dyslipidemia in young patients with type 1 diabetes mellitus. Arch Endocrinol Metab. 2015;59:215–9.
Kim G, DeSalvo D, Guffey D, et al. Dyslipidemia in adolescents and young adults with type 1 and type 2 diabetes: a retrospective analysis. Int J Pediatr Endocrinol. 2020;2020:11.
Zabeen B, Balsa AM, Islam N, Parveen M, Nahar J, Azad K. Lipid profile in relation to glycemic control in type 1 diabetes children and adolescents in Bangladesh. Indian J Endocrinol Metab. 2018;22:89–92.
WHO Child growth standards. Acta Pediatr Supplement. 2006;450:5–101.
Indian Academy of Pediatrics Growth Charts Committee, Khadilkar V, Yadav S, Agrawal KK, et al. Revised IAP growth charts for height, weight and body mass index for 5- to 18-year-old Indian children. Indian Pediatr. 2015;52:47–55.
Chalil VK, Prasad HK, Nassir SAMA, Arulalan KV, Sangaralingam T, Krishnamoorthy N. A study on new IAP 2015 growth references in rural South Indian children. Indian J Pediatr. 2021;88:645–9.
Khadilkar A, Ekbote V, Chiplonkar S, et al.Waist circumference percentiles in 2–18 year old Indian children. J Pediatr. 2014;164:1358–62.
Khadilkar A, Mandlik R, Chiplonkar S, Khadilkar V, Ekbote V, Patwardhan V. Reference centile curves for triceps skinfold thickness for Indian children aged 5 to 17 years and cut offs for predicting risk of childhood hypertension: a multi-centric study. Indian Pediatr. 2015;52:675–80.
Flynn JT, Kaelber DC, Baker-Smith CM, et al. Subcommittee On Screening And Management Of High Blood Pressure In Children. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904.
Nadeau KJ, Regensteiner JG, Bauer TA, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95:513–21.
Purnel JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes control and complications trial. JAMA. 1998;280:140–6.
Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53.
Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24:1476–85.
Larsen J, Brekke M, Sandvik L, Arnesen H, Hanssen KF, Dahl-Jorgensen K. Silent coronary atheromatosis in type 1 diabetic patients and its relation to long-term glycemic control. Diabetes. 2002;51:2637–41.
Acknowledgements
The authors would like to thank Prof Nedunchelian K. for reading and reviewing this manuscript. They also thank all the children and their families for participating in this study.
Author information
Authors and Affiliations
Contributions
MS was involved in data collection and tabulation; HKP, TS, BP, and SW were involved in the clinical management of cases and study conceptualization; BP and TS were involved in data analysis; SW, HKP, BP, TS contributed to the manuscript; All authors have read the final manuscript and approve the final version of the manuscript. HKP will act as the guarantor for this paper.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by Mehta Mutispeciality Hospitals IEC, Chennai. Approval number/IRB number: IRB-MCH/011/2019.
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Selvaraj, M., Prasad, H.K., White, S. et al. Prevalence and Determinants of Occurrence of Dyslipidemia in Subjects with Type 1 Diabetes Mellitus. Indian J Pediatr 90, 118–123 (2023). https://doi.org/10.1007/s12098-022-04130-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-022-04130-2