Abstract
Asthma is one of the most common chronic diseases of childhood with significant morbidity. Management of asthma has evolved from inhaled corticosteroids to personalized therapy in recent years. This article summarizes the recent advances in the management of asthma. The severity of asthma is assessed retrospectively by the level of treatment required to control asthma symptoms.
Recently, there has been an emphasis on not to use short-acting beta-2 agonist (SABA) alone, even for intermittent or mild asthma. Single maintenance and reliever therapy (MART) is increasingly being recommended. Asthma therapy should be personalized based on phenotype (clinical presentations) and endotypes (distinct mechanistic pathways) of asthma and by a continuous cycle of assessment of asthma control and risk assessment, treatment, and reviewing the response in children on treatment. Various add-on therapy like tiotropium and biological monoclonal antibodies have been approved for use in a subset of children with severe asthma. Before considering biologicals, it is vital to have a proper diagnosis, good compliance and inhaler technique, and treatment of comorbidities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asthma is one of the most common chronic diseases of childhood, with a significant burden. Inhaled corticosteroids (ICS) have been the mainstay of treatment of asthma for more than 50 y. Over the years, with the understanding of the pathophysiology of asthma, there has been a trend towards personalized therapy. Here, the aim is to discuss recent advances in the long-term management of asthma in children above five years of age. In this article, ascertaining diagnosis, classification of severity of asthma, stepwise management of asthma, improving adherence, personalized asthma treatment, and management of severe asthma, including biologicals will be discussed.
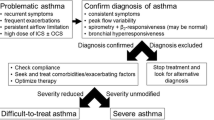
Ascertaining Diagnosis
Correct diagnosis of asthma is very crucial in the management of asthma. Before starting the therapy and later on, review the asthma diagnosis, and ensure that the diagnosis is correct before stepping up the therapy. Diagnosis of asthma is made with clinical features of episodic airway obstruction (cough, breathing difficulty, or wheezing) with documented variable (reversible) airflow obstruction using spirometry (preferred) or peak expiratory flow rate with or without a family history of asthma or atopy. It should be remembered that children with asthma may have normal spirometry parameters when they are in good health, and it may be due to differential involvement of small airways compared to large airways. But, asthma flare-up may produce airway obstruction with bronchodilator reversibility. Recently European Respiratory Society (ERS) has published evidence-based practice guidelines for diagnosing asthma in children [1].
Classification of Asthma Severity
Asthma severity is defined retrospectively after giving treatment for a few months [2]. Mild asthma is defined as asthma that is controlled with as-required SABA/ICS-formoterol or low- dose ICS. Moderate asthma is defined as asthma that is controlled with a low-to-moderate dose of ICS in a combination of LABA. Severe asthma is poorly controlled despite a high dose of ICS and a second controller drug with good compliance and technique and treatment of comorbidities.
Stepwise Management of Asthma
The treatment of a child with asthma is decided based on whether he or she is treatment naïve or already getting treatment. Initial therapy of a child with asthma, who is not receiving any treatment, is decided based on the frequency of daytime/nocturnal symptoms, activity limitation, and risk of a future asthma attack (Figs. 1 and 2). At follow-up, asthma control is assessed as controlled, partly controlled, and uncontrolled. They are evaluated for risk factors for asthma flare-ups, and treatment is optimized stepwise after ensuring correct asthma diagnosis, good compliance, and inhaler technique (Figs. 1 and 2) [2].
The updated stepwise management of asthma as per GINA 2021 [2] is summarized in Fig. 1. Briefly, now the preferred step 1 treatment is low-dose ICS taken whenever SABA is taken, and MART (single maintenance and reliever therapy) is suggested for step 3 and step 4 in children (Figs. 1 and 2). The change in step 1 management is based on a TREXA [3] trial, done in 5–18 y of age, and a pragmatic trial [4], done in 6–17 y of age. Further, there is a risk of severe exacerbations with SABA-alone therapy, and there is a concern of poor compliance with daily ICS for mild asthma. The recent expert panel working group guidelines also gave similar recommendations [5]. In step 5, add-on therapy may be used like Long-acting muscarinic antagonist (LAMA) or biological monoclonal antibody. The tiotropium, a LAMA, is recommended in children as add-on therapy. Tiotropium may be added if asthma is not controlled despite moderate-to-high doses of ICS along with LABA, once the basics of poorly controlled asthma (correct diagnosis, good compliance and inhaler technique, assessment and management of co-morbidities) have been taken care of [6]. Tiotropium may be more cost-effective than biologicals for poorly controlled asthma.
Comorbid conditions such as allergic rhinitis, obesity, obstructive sleep apnea, dysfunctional breathing, gastroesophageal reflux, anxiety, and stress should also be assessed and treated. Addressing allergen exposure, indoor and outdoor pollution, and tobacco smoke exposure are essential steps in asthma management. The recent expert panel working group guidelines suggested multicomponent allergen-specific mitigation interventions (e.g., air purifiers/filters, pesticides, mattress covers, etc.) only for those asthma patients who had symptoms related to specific allergens (confirmed by history or allergy testing), not for all asthma patients [5].
Measures to Improve Adherence to Therapy
Adherence to treatment and technique of inhaler use is crucial for effective asthma management. Various methods/interventions have been adopted to improve adherence. Electronic devices are found to be helpful in improving adherence and asthma control in children [7]. But, these electronic devices are costly. Shields et al. tested feasibility (n = 12) and performed a pilot trial (n = 22) of mobile direct observation of therapy (MDOT) in poorly controlled asthma in children and reported that it was feasible and well-accepted, and it was associated with improvement in asthma control test score and normalization of FeNO [8].
Personalized Asthma Treatment
Asthma had various phenotypes (clinical presentations) and endotypes (distinct mechanistic pathways). The asthma clinical phenotypes include [2]: (i) Allergic asthma: Asthma starting in childhood, and it is associated with personal or family history of atopy. Sputum examination may reveal eosinophils, and they respond well to ICS. (ii) Nonallergic asthma: This asthma is not associated with allergy, and sputum profile may be neutrophilic, paucigranulocytic, or eosinophilic. These patients have a short-lasting response to ICS. (iii) Late-onset adult asthma: This asthma presents first time in adults, mainly in women, and has an inadequate response to ICS. (iv) Asthma with persistent airflow limitation: In some long-standing asthma, airway remodeling leads to fixed or incompletely reversible airway obstruction. (v) Asthma with obesity: This asthma is associated with obesity; patients may have less eosinophilic inflammation but more asthma symptoms.
Two types of asthma endotypes had been described: T-2 (type 2) high and T-2 low [9]. T-2 high inflammation is characterized by high eosinophils, high immunoglobin E (IgE), and increased fractional exhaled nitric oxide (FeNO). It results from increased activity of IL-4, IL-5, and IL-13. Therefore, biologicals active against IgE and these T-2 high interleukins may be helpful for severe asthma.
In personalized asthma treatment, asthma management is tailored to symptom control and treatment of modifiable risk factors for asthma attack or poor asthma control and considering asthma phenotype/endotype and patient/parents preferences [2]. It involves the continuous assessment cycle of symptoms and risk factors, pharmacological and nonpharmacological treatment, and reviewing response and lung function [2].
Sputum Eosinophilia-Tailored Treatment
A Cochrane review reported insufficient evidence for asthma management based on sputum eosinophilia (> 3%) in children [10].
Fractional Exhaled Nitric Oxide (FeNO)–Based Management
Fractional exhaled nitric oxide (FeNO) is a measurement of nitric oxide generated in airways, mainly lower airways, and it indicates the level of ongoing inflammation in airways.
FeNO is a good prediction of airway inflammation, and high FeNO levels may reflect asthma severity. FeNO levels should decrease with treatment so that it can be used as a surrogate marker for ICS compliance. The Cochrane review including 9 pediatric studies concluded that asthma management tailored by FeNO levels compared to guideline management reduced asthma exacerbations, but not the day-to-day symptoms and ICS doses, and recommended that FeNO-guided therapy should not be used universally, instead in a subset of children [11].
Uncontrolled, Difficult-to-Treat, and Severe Asthma
Uncontrolled asthma is defined as poorly controlled asthma in the form of frequent daytime symptoms or rescuer use, night awakening or exercise limitation or having ≥ 2/y exacerbations or ≥ 1/y severe exacerbation requiring hospital admission [2]. Difficult-to-treat asthma is defined as uncontrolled asthma despite a medium to high dose of ICS and another controller medication or maintenance oral steroids (GINA step-4 or step-5 treatment). It may be due to incorrect diagnosis, poor compliance, poor inhaler technique, or comorbidities in the majority. Severe asthma is defined as poorly controlled asthma despite a high dose of inhaled corticosteroids (ICS) along with a second controller drug with good compliance and technique, and treatment of comorbidities. Severe asthma is sometimes called severe refractory asthma, and it accounts for a subset of difficult-to-treat asthma. Severe asthma accounts for about 5%–10% of overall asthma.
Severe asthma affects the child and their entire family, and its course is variable. Children with severe asthma may have frequent exacerbations, including life-threatening exacerbations, and lung function may decline with each exacerbation. Such children may need frequent courses of oral steroids resulting in steroid-induced side effects like hypertension, weight gain, diabetes, psychological effects, and more infections. Children with asthma, from developing countries may have significant morbidity and even mortality due to uncontrolled asthma because of poor access to essential medications like ICS. Thus, socioeconomic status may affect asthma control.
Management of Severe Asthma
Severe asthma in children should be managed by a multidisciplinary team at a specialized centre by experts in Pediatric pulmonology using pediatric-specific guidelines. The children with severe asthma should be evaluated for the clinical or inflammatory phenotype to decide on add-on therapy. The assessment includes atopy (eczema, allergic rhinitis), blood eosinophil levels, FeNO, sputum eosinophils, total IgE, aspergillus-specific, or other allergens–specific IgE, and chest radiograph or HRCT chest.
A few studies suggested that lung clearance index (LCI) may be abnormal (high) in severe therapy-resistant asthma compared to difficult asthma [12]; however, routine LCI monitoring in clinical management of children with severe asthma is limited to very few specialized centers only.
An algorithm for the management of severe asthma is summarized in Fig. 3. Type 2 airway inflammation is characterized by blood eosinophils ≥ 150/μL, FeNO ≥ 20 ppb, sputum eosinophils ≥ 2%, allergic asthma, or requiring oral maintenance steroids [2]. If Type 2 response is there, consider biologicals as per eligibility and give a trial for at least 4–6 mo. If there is a good response to biologicals, titrate the other therapy and decide the duration of it. Before considering biologicals, it should be ensured that the diagnosis is asthma-only, and there is no possibility of asthma mimickers like airway malacias, bronchopulmonary dysplasia, aspiration, vocal cord dysfunction, etc. [13].
Biological Agents
Before using biologicals, oral corticosteroids (OCS) were used as a last resort treatment of severe asthma as step-5 therapy, but OCS had unacceptable side effects. Therefore, there is a need to use nonsteroid agents in severe asthma, and biological agents are such therapy.
Biologicals therapies are monoclonal antibodies targeted against IgE or Type 2 interleukins or their receptors, and are useful in T-2 high asthma [14]. While choosing a particular biological agent, the following things should be kept in mind: Severity of asthma, endotype/phenotype of the patient, weight and age of child, affordability and acceptability, drug licence, side effects, and dosing schedule. It is difficult to assess the superiority of one agent over another because of the lack of studies for head-to-head comparisons of various biological agents. The doses and side effects of multiple biologicals approved for use in children and adolescents are mentioned in Table 1. The high cost with poor affordability by patients are the limitations in using the biologicals therapies in developing countries.
Omalizumab
Omalizumab is a biological monoclonal antibody against IgE. In clinical trials and real-life studies, omalizumab had shown lesser asthma exacerbations, reduced seasonal symptoms, and more symptom-free days [15].
It should be used for at least 16 wk. It should be administered in a hospital setting with facilities to manage anaphylaxis, as rarely it may happen.
Anti-Interleukin-5/5R
Mepolizumab (monoclonal antibody against interleukin -5) and benralizumab (monoclonal antibody against IL-5 receptor alpha): Mepolizumab had been studied in children 6–11 y of age with a marked decrease in eosinophil counts similar to adults [16]. Gupta et al. [17] reported the safety and efficacy of mepolizumab in children 6–11 y of age with severe eosinophilic asthma.
Dupilumab
Dupilumab is a monoclonal antibody against the alpha subunit of the IL-4 receptor and it blocks the production of cytokine IL-4 and IL-13. Use of dupilumab in patients ≥ 12 y of age with uncontrolled asthma was associated with a reduced rate of severe asthma exacerbations and reduced steroid use. The benefit was more in patients with high baseline eosinophil levels [18]. Recently, results of the VOYAGE phase 3 trial were reported where dupilumab was used in children aged 6–11 y, and it significantly reduced asthma exacerbations [19].
High-Dose Inhaled Steroids
Step Up Yellow Zone Inhaled Corticosteroids to Prevent Exacerbations (STICS) trial reported that in children 5–11 y of age with mild-to-moderate persistent asthma there was at least one exacerbation in the last one year, and the use of a fivefold increased dose of ICS with early signs of poor asthma control did not decrease the rate of asthma exacerbations. It was associated with a decrease in height gain [21]. Therefore, intermittent use of a high dose of ICS is not recommended for poorly controlled asthma in children 5–11 y.
Azithromycin
GINA 2021 recommends azithromycin (three times a week) as add-on therapy in adults receiving high dose ICS-LABA. An ECG to look for long QTc and assessment for atypical mycobacteria should be done before starting azithromycin therapy [2]. It should be used for 6 mo when used. There is a lack of efficacy studies for azithromycin in children with severe asthma.
Breathing Exercises
Sometimes, dysfunctional breathing may be associated with asthma morbidity. The proposed mechanisms include abnormal breathing patterns due to anxiety, increased perception of forthcoming symptoms, hyperventilation leading to drying and cooling of the airways resulting in hyperresponsiveness, and emotional stimulus causing constriction of airways through cholinergic pathways. Dysfunctional breathing may not respond by stepping up the treatment and management include various breathing exercises [22].
Allergen Immunotherapy
It is usually used for three allergens—pollen, animal dander, house dust mites. The role of allergen immunotherapy (AIT) is limited. It may improve symptoms in mild-to-moderate asthma in patients who are allergic to one or two allergens only, and it may reduce medication burden [5]. The expert panel suggested subcutaneous immunotherapy in such cases rather than sublingual immunotherapy [5]. AIT is contraindicated in severe asthma, as it may precipitate an exacerbation.
A recent Cochrane review including 66 studies (31 in children) concluded that there is limited evidence for main outcomes like exacerbations and quality-of-life to draw a firm conclusion. Most studies focused on nonvalidated symptom scores. The authors suggested a need for further evaluation of immunotherapy in uncontrolled asthma [23].
Vitamin D Supplementation
Vitamin D had been implicated in the pathogenesis of asthma, and observational studies showed a relation between vitamin D deficiency and poor asthma control [24]. Still, the results of interventional studies are not consistent. A systematic review including individual participant data from seven studies (five in children) concluded that vitamin D supplementation was associated with decreased asthma exacerbations requiring systemic steroids, more so in participants with 20 (OH) vitamin D levels < 25 nmol/L (10 ng/ml) [25]. But two recent RCTs reported that vitamin D supplementation in asthmatic children with vitamin D deficiency was not associated with reduced asthma exacerbation [26] or better asthma control [27].
Temperature-Controlled Laminar Airflow (TLA) Devices
TLA devices reduce exposure to allergens/particulate material when installed in the bedroom over the bed. Boyle et al. [28] studied TLA devices in 312 patients of 7–70 y of age with poorly controlled atopic asthma for one year and found that TLA improved quality of life, reduced FeNO, and was associated with less increment in cat-specific IgE.
Bronchial thermoplasty is not recommended in children less than 18 y old.
Asthma Remission
The aim should be to achieve asthma remission as the final treatment goal while managing asthma. There was a lack of well-defined definitions for asthma remission. Recently, Menzies-Gow et al. [29] defined the remission as clinical and complete as per on and off treatment. Clinical remission on treatment is defined as the continued absence of significant asthma symptoms based on validated criteria for ≥ 12 mo along with optimization and stabilization of lung function, agreement of disease remission by both patient and physician, and no use of OCS therapy for exacerbation and long-term control for ≥ 12 mo. The clinical remission criteria off treatment are the same but without asthma medications for ≥ 12 mo [28]. Complete remission on treatment is defined as clinical remission plus objectively documented resolution of previously observed asthma inflammation, e.g., reduced FeNO, sputum or blood eosinophils, and/or other measures with current negative bronchial hyper-responsiveness, performed in appropriate settings. The complete remission criteria off treatment are the same but without asthma medications for ≥ 12 mo [28]. But, it should be noted that asthma remission is not the same as cure and patients with asthma remission may have asthma exacerbations later in life.
Conclusion
Asthma is not a single disease; it is an umbrella term for a group of respiratory diseases with similar symptoms but with several etiologies/phenotypes and endotypes. There is a trend towards personalized therapy as one size doesn’t fit all. Assessment of different phenotypes/endotypes of asthma will help in personalized asthma therapy. Exposure to repeated courses of oral steroids has a significant burden of side effects. Recently available biological agents may be a breakthrough in the management of severe asthma in children.
References
Gaillard EA, Kuehni CE, Turner S, et al. European Respiratory Society clinical practice guidelines for the diagnosis of asthma in children aged 5–16 years. Eur Respir J. 2021;58:2004173.
2021 GINA Main Report. Global Initiative for Asthma - GINA. 2021. Available at: https://ginasthma.org/gina–reports/. Accessed on 7 May 2021.
Martinez FD, Chinchilli VM, Morgan WJ, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:650–7.
Sumino K, Bacharier LB, Taylor J, et al. A pragmatic trial of symptom-based inhaled corticosteroid use in African-American children with mild asthma. J Allergy Clin Immunol Pract. 2020;8:176–85.
Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146:1217–70
Sunther M, Marchon K, Gupta A. Tiotropium in the management of paediatric and adolescent asthma: systematic review. Paediatr Respir Rev. 2021;38:58–62.
Gupta RS, Fierstein JL, Boon KL, et al. Sensor-based electronic monitoring for asthma: a randomized controlled trial. Pediatrics. 2021;147:e20201330.
Shields MD, ALQahtani F, Rivey MP, McElnay JC. Mobile direct observation of therapy (MDOT) – A rapid systematic review and pilot study in children with asthma. PloS One. 2018;13:e0190031.
Kuruvilla ME, Lee FE-H, Lee GB. Understanding asthma Ppenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56:219–33.
Petsky HL, Li A, Chang AB. Tailored interventions based on sputum eosinophils versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev. 2017;8:CD005603.
Petsky HL, Kew KM, Chang AB. Exhaled nitric oxide levels to guide treatment for children with asthma. Cochrane Database Syst Rev. 2016;11:CD011439.
Irving S, Fleming L, Ahmad F, et al. Lung clearance index and steroid response in pediatric severe asthma. Pediatr Pulmonol. 2020;55:890–8.
Russo D, Di Filippo P, Attanasi M, Lizzi M, Di Pillo S, Chiarelli F. Biologic therapy and severe asthma in children. Biomedicines. 2021;9:760.
Cevhertas L, Ogulur I, Maurer DJ, et al. Advances and recent developments in asthma in 2020. Allergy. 2020;75:3124–46.
Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136:1476–85.
Gupta A, Pouliquen I, Austin D, et al. Subcutaneous mepolizumab in children aged 6 to 11 years with severe eosinophilic asthma. Pediatr Pulmonol. 2019;54:1957–67.
Gupta A, Ikeda M, Geng B, et al. Long-term safety and pharmacodynamics of mepolizumab in children with severe asthma with an eosinophilic phenotype. J Allergy Clin Immunol. 2019;144:1336–42.
Castro M, Corren J, Pavord ID, et al. Dupilumab Efficacy and Safety in Moderate–to–Severe Uncontrolled Asthma. N Engl J Med. 2018;378:2486–96.
Bacharier LB, Maspero JF, Katelaris CH, et al. Dupilumab efficacy and safety in children with uncontrolled, moderate-to-severe asthma: the phase 3 VOYAGE study. Am J Respir Crit Care Med. 2021;203:A1204.
Abrams EM, Becker AB, Szefler SJ. Current state and future of biologic therapies in the treatment of asthma in children. Pediatr Allergy Immunol Pulmonol. 2018;31:119–31.
Jackson DJ, Bacharier LB, Mauger DT, et al. Quintupling inhaled Ggucocorticoids to prevent childhood asthma exacerbations. N Engl J Med. 2018;378:891–901.
Thomas M, Bruton A. Breathing exercises for asthma. Breathe. 2014;10:312–22.
Fortescue R, Kew KM, Leung MST. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2020;9:CD011293.
Gupta A, Sjoukes A, Richards D, et al. Relationship serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med. 2011;184:1342–9.
Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881–90.
Forno E, Bacharier LB, Phipatanakul W, et al. Effect of vitamin D3 supplementation on severe asthma exacerbations in children with asthma and low vitamin D levels: the VDKA randomized clinical trial. JAMA. 2020;324:752–60.
Jat KR, Goel N, Gupta N, et al. Efficacy of vitamin D supplementation in asthmatic children with vitamin D deficiency: A randomized controlled trial (ESDAC trial). Pediatr Allergy Immunol. 2021;32:479–88.
Boyle RJ, Pedroletti C, Wickman M, et al; 4A Study Group. Nocturnal temperature controlled laminar airflow for treating atopic asthma: a randomised controlled trial. Thorax. 2012;67:215–21.
Menzies-Gow A, Bafadhel M, Busse WW, et al. An expert consensus framework for asthma remission as a treatment goal. J Allergy Clin Immunol. 2020;145:757–65.
Author information
Authors and Affiliations
Contributions
KRJ: Literature search, manuscript writing, and final approval of manuscript; AG: Critical intellectual input, review of manuscript, and final approval of manuscript. KRJ will act as the guarantor for this paper.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jat, K.R., Gupta, A. Recent Advances in Long-Term Management of Asthma. Indian J Pediatr 89, 378–386 (2022). https://doi.org/10.1007/s12098-021-04060-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-021-04060-5