Abstract
Objective
To evaluate the efficacy and safety of 2% topical propranolol cream in the treatment of proliferating infantile strawberry hemangiomas.
Methods
A total of 40 infants were enrolled; 2% propranolol cream was applied three times daily. In the subsequent monthly visit, dynamic changes in tumor size, texture, and color were recorded. The adverse events (AEs) were observed. Treatment outcomes were scored on a four-point scale. All patients were followed up for 12 mo after treatment.
Results
The overall response was graded Scale 1 (poor response) in 2 patients, Scale 2 (moderate response) in 15 patients, Scale 3 (good response) in 17 patients, and Scale 4 (excellent response) in 6 patients. No significant differences were seen in treatment outcomes between female and male patients, among lesion locations/size, or in the age at the start of the treatment. No obvious AEs were reported.
Conclusions
2% topical propranolol cream is safe and effective for the treatment of proliferating infantile strawberry hemangiomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infantile hemangiomas (IHs) are common congenital benign tumors that arise from the abnormal proliferation of vascular endothelial cells (VECs) in the mesoderm. The estimated incidence of IHs is 1.1–2.6% in newborns [1]. Strawberry hemangiomas most commonly occur in the head and neck region, followed by the trunk and extremities. The natural course of hemangiomas is generally divided into the proliferating, plateau, and regression phases. During the proliferating phase, IH growth is rapid and complications such as ulceration, bleeding, and infections may occur. Their growth rate varies among individuals, but they commonly feature two periods of growth (after birth and 4–6 mo after birth) [2]. It is widely accepted that early therapeutic intervention should be considered during the proliferating phase [3, 4], which can slow the tumor growth, accelerate the IH regression, shorten the natural course, minimize the potential complications, and protect the patient’s physical function. Propranolol has been increasingly recommended as a new treatment for patients with IHs. However, the drug targeting and adverse effects of orally administered propranolol remain controversial [5]. The index report presents 40 cases with proliferating strawberry IHs that were treated with topical 2% propranolol cream. The aim of the current study was to assess the efficacy and safety profile of 2% propranolol cream.
Material and Methods
This was a case series study. From January 2012 through January 2013, a total of 40 outpatients (12 males, 28 females; age range 30 d to 12 mo) presenting with strawberry hemangiomas were enrolled in authors’ hospital. Based on their medical history, clinical data, and B-scan ultrasound, all patients had a confirmed diagnosis of strawberry hemangioma according to the 1996 International Society for the Study of Vascular Anomalies criteria. The enrolled patients did not receive any therapies for the hemangiomas prior to study entry. Some patients suffered two to five skin lesions, one of which was considered in the statistical analysis.
Patients who met the following criteria were excluded from the study: (1) condition complicated by any contraindications of propranolol including asthma, allergic rhinitis, tracheobronchitis, pneumonia, hypoglycemia, hypotension, platelet crisis, bradycardia, second- or third-degree atrioventricular block, acute heart failure, cardiogenic shock, liver or kidney dysfunction, glomerulonephritis, or thyroid dysfunction; (2) signs of acute inflammation; (3) incomplete data; (4) unwilling to comply with the study protocol; and (5) parents refused to provide written informed consent.
The study was approved by the Ethics Committee of Daping Hospital, Third Military Medical University (No. 2012006; January 10, 2012). The Ethics Committee is certificated by WHO/SCIDER, and consists of 14 members, including experts in clinics, nursing, pharmacy and management. For all patients, the past medical and family histories were recorded prior to treatment. Each patient underwent a thorough physical examination. Other indices included digital photographs of the hemangiomas, lesion size, ultrasound images, physical signs (body temperature, pulse, blood pressure, and respiratory frequency), cardiac color ultrasound images, blood routine examination, coagulation function, liver and renal function tests, and blood sugar analysis to avoid the contraindications of propranolol prior to the treatment. Changes in heart rate and blood pressure were measured before the first treatment of 2% topical propranolol cream and after 2 h of treatment. The patients’ parents were informed of the safety profile and risk of propranolol treatment of IHs and provided informed consent.
The oral propranolol preparation was previously ground and mixed with glycerin at a concentration of 20 mg/g. All of the procedures were conducted in the pharmacy department of Daping Hospital. The workshop of pharmacy department was GMC C-class and the production approval was authorized by Ministry of Public Health, Chengdu Military Region. After the skin was cleaned, 2% topical propranolol cream was applied evenly on the lesion surface three times daily, followed by gentle massage to facilitate complete absorption. Patients with ulceration were given propranolol until the wound healed. Parents were warned about the potential treatment complications including hypotension, bradycardia, hypoglycemia, and tracheospasm.
All of the adverse events (AEs) were recorded. Patients were sent to the hospital if symptoms such as low spirits, drowsiness, or loss of appetite occurred. All patients were followed up via telephone every 2 wk. If redness, itching, or other propranolol-related AEs were reported during the follow-up period, the interruption of propranolol was permitted and symptomatic treatment was adopted. A subsequent visit was required every month after treatment, and the dynamic changes in tumor size, texture, and color were observed and captured. A regularly scheduled re-examination including routine blood screening, liver and renal function tests, and blood sugar level checking was conducted every 2 mo. According to the standards for the withdrawal of oral propranolol, the discontinuation of propranolol treatment was indicated by the successful disappearance of hemangiomas or the 8-mo treatment duration [6].
The recordings obtained from patients and the clinical, visual and manual examinations were considered the subjective assessment. Meanwhile, the objective assessment involved the pre- and post-treatment differences in the digital photographs and ultrasound images and the regression degree. Tumor size was recorded using the hemisphere measurements [7] and digital photographs. Treatment outcomes were scored according to a four-point scale [8], which was defined as follows: scale 1 (poor response), 0–25% shrinkage; scale 2 (moderate response), 26–50% shrinkage and color fading; scale 3 (good response), 51–75% shrinkage and color fading; and scale 4 (excellent response), 76–100% shrinkage as well as total tumor disappearance and normal or nearly normal skin color restoration.
The observed and recorded AEs after propranolol treatment included edema, erythema, blistering, erosion, ulceration, hyperpigmentation, hypopigmentation, depigmentation, scarring, low spirits, drowsiness, hypotension, bradycardia, hypoglycemia, and tracheospasm. The temporary interruption of propranolol due to AEs was permitted until all symptoms disappeared completely.
Descriptive statistics were used to summarize the demography (age and sex) and the background characteristics (tumor size and location) of the patients. According to the age at the start of the treatment, the patients were divided as follows: 0–3 mo, >3–6 mo, and >6–12 mo. Patients were also divided according to the size and the locations of the treated lesions (0–5 cm2, > 5–10 cm2, and >10 cm2, on the head and neck, trunk and extremities, respectively). Among the responded groups, the Kruskal-Wallis test was used to compare the age, tumor size and location. The Mann-Whitney U test was used to compare the gender group. The results were considered significant if P < 0.05.
Results
A total of 40 infants (12 male, 28 female; median age, 4.5 mo; range, 30 d to 12 mo) who fulfilled the inclusion and exclusion criteria were ultimately enrolled. The tumor areas were 1.0 cm × 1.5 cm to 3.0 cm × 5.0 cm. Of the 40 lesions, 23 were located on the head and neck, nine were on the trunk, and eight were on the extremities.
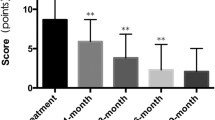
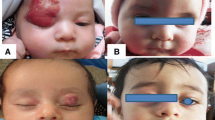
After 1 wk of treatment, most of the tumor color began to fade and the tumor was softening with a decreased skin surface temperature. The tumor shrank over time and looked darker, had a soft texture, and showed a plaque-like regression. After 3 mo of treatment, most of the hemangiomas were completely regressed, with mild telangiectasias on the surface. All patients were followed for 12 mo after treatment. Among these patients, three had complete regression after 2 mo of treatment; however, the hemangiomas recurred after 5 mo of propranolol withdrawal but were managed with a second course of propranolol therapy. The overall response was graded scale 1 in two patients, scale 2 in 15 patients, scale 3 in 17 patients, and scale 4 in six patients (Figs. 1, 2 and 3). There were no significant differences in the treatment outcomes between female and male patients or in the age at the start of treatment. The differences in outcomes related to lesion location and size were also not statistically significant (Table 1). Two patients reported gastrointestinal reactions such as mild diarrhea and loss of appetite, but the symptoms were manageable with propranolol discontinuation and the therapy was resumed once until their symptoms were alleviated and had disappeared.
No obvious propranolol-related AEs were reported, including bradycardia, hypotension, altered respiratory rate, or abnormalities in blood routine and lung function tests. No cases of allergic reaction, tissue infections, or necrosis occurred.
Discussion
Strawberry hemangiomas, also known as isolated hemangiomas, are benign tumors identified by the rapid proliferation of VECs and the formation of new capillaries. The pathogenesis of strawberry hemangiomas has not yet been fully clarified [9]. Some hemangiomas leave recognizable marks after spontaneous regression, such as mild telangiectasias and skin shrinkage, tissue ischemia and necrosis, tissue injuries, and scarring, all of which would seriously impact an individual’s physical function and appearance as well as cause economic and psychological burdens [10]. Hemangioma growth rates are unpredictable and their regression rates are highly variable. Thus, it is widely accepted that prompt diagnosis and therapeutic intervention of these lesions should be considered during their proliferating phase, which can inhibit the tumor growth, shorten the natural course, reduce complications, protect the patient’s physical function, and not alter their appearance.
In 2008, French researchers found that oral propranolol effectively reduced IH volume and color [11]. A variety of clinical studies have demonstrated that propranolol treatment can shorten the natural course of IH and suppress its proliferation [4, 12–14]. Moreover, propranolol can exert its therapeutic effects on the proliferating and regression phases of IHs. Propranolol is characterized by its rapid onset of action, better patient tolerance, and few AEs. Thus, propranolol is increasingly considered a first-line therapy for hemangiomas in the clinical setting. Nevertheless, the mechanisms of biological action of propranolol in the treatment of IHs remain unclear. Propranolol can inhibit nitric oxide production and release, reduce the expression of angiogenic factors including vascular endothelial growth factor, basic fibroblast growth factor, and matrix metalloproteinase (MMP) as well as down-regulate the mitogen-activated protein kinase pathway, but it can also inhibit MMP-9 and human brain microvascular endothelial cell expression, activate mesenchymal stem cell differentiation, and induce G-protein–coupled receptor polymorphism [15, 16]. It was previously discovered that a dose-dependent effect of propranolol on IHs and the drug-related AEs were associated with administration route [6]. Orally administered drugs with first-pass metabolism have low bioavailability, and only approximately 25% of drugs can enter the blood circulation and exert their therapeutic effects. However, higher doses are always associated with an increased risk of AEs. The potential propranolol-related AEs of nonselective β-blocker mainly include bradycardia, hypotension, hypoglycemia, and tracheospasm. In addition, insomnia, drowsiness, night terrors, fatigue, and gastrointestinal reactions such as nausea, vomiting, acute abdominal pain, and mild diarrhea have also been reported in patients treated with propranolol. Intravenous injection can increase the absorption over that of oral administration but is both inconvenient and has poor compliance. Based on these findings, propranolol was made with glycerin for external use on the skin to ensure good efficacy while minimizing the AEs [17]. After using topical propranolol, the cutaneous parts of the hemangiomas had changed in color from bright red to purple with areas of gray, and subcutaneous tumor had changed in texture from hard to soft. There was visible shrinkage of the hemangioma and/or skin color shallowed [18, 19]. Topical propranolol can make a reduction in volume, color, and elevation of IHs, which can control the growth of hemangioma, or promote tumor regression. The concentration of propranolol was higher in index study than those in previous studies (2% vs. 1%) [20–22], which can greatly increase the transdermal absorption ratio, improve targeting, and achieve better efficacy. The first-pass metabolism and systemic effects due to oral administration can be prevented, as can the laser therapy–related AEs including ulceration, bleeding, and infections. Furthermore, the external administration route is safe and convenient, able to decrease local skin inflammation, and can reduce treatment costs and the need for hospital admission.
Prior to the treatment, all patients underwent clinical assessments including electrocardiography, cardiac color ultrasonography evaluation, blood glucose, blood pressure, and heart rate measurements. Patients were hospitalized for at least 3 d before the propranolol treatment. A stepped care program was adopted during the treatment. The present study revealed that 2% topical propranolol cream is an effective treatment for proliferating infantile strawberry hemangiomas. The response of hemangiomas was graded scale 1 in two patients, scale 2 in 15 patients, scale 3 in 17 patients, and scale 4 in six patients. No obvious differences were found in the treatment outcomes between female and male patients with hemangiomas or in the age at the start of the treatment. The authors also found no significant differences in location/size-related outcomes. No obvious AEs were reported in index study.
Therefore, it is concluded that early therapeutic intervention with propranolol cream is a necessity for infants with red patches or plaques of skin present at birth or when a condition is definitely diagnosed as hemangiomas, to prevent progression to the rapid proliferation phase. Moreover, propranolol combined with laser therapy can be recommended for patients with hemangiomas who have dismal outcomes after treatment with propranolol alone. Based on these findings, 2% topical propranolol cream has proven effective and safe in the treatment of proliferating infantile strawberry hemangiomas.
It is generally accepted that the external preparation of propranolol has a better therapeutic effect on superficial hemangioma than on deep hemangioma. Thus, further studies are required to verify the feasibility of this drug delivery system for treating deep hemangiomas by increasing the percutaneous absorption and infiltration of propranolol into the dermis and center of the lesion under the skin.
In summary, the current study demonstrated that the administration of 2% topical propranolol cream is a safe and effective approach for the treatment of proliferating infantile strawberry hemangiomas and may become an optimal treatment in clinical practice.
References
Schwartz RA, Sidor MI, Musumeci ML, Lin RL, Micali G. Infantile haemangiomas: a challenge in paediatric dermatology. J Eur Acad Dermatol Venereol. 2010;24:631–8.
Smith Jr SP, Buckingham ED, Williams EF 3rd. Management of cutaneous juvenile hemangiomas. Facial Plast Surg. 2008;24:50–64.
Oksiuta M, Matuszczak E, Debek W, Dzienis-Koronkiewicz E, Hermanowicz A. Treatment of rapidly proliferating haemangiomas in newborns with propranolol and review of the literature. J Matern Fetal Neonatal Med. 2016;29:64–8.
Tan CE, Itinteang T, Leadbitter P, Marsh R, Tan ST. Low-dose propranolol regimen for infantile haemangioma. J Paediatr Child Health. 2015;51:419–24.
Breur JM, de Graaf M, Breugem CC, Pasmans SG. Hypoglycemia as a result of propranolol during treatment of infantile hemangioma: a case report. Pediatr Dermatol. 2011;28:169–71.
Holmes WJ, Mishra A, Gorst C, Liew SH. Propranolol as first-line treatment for rapidly proliferating infantile haemangiomas. J Plast Reconstr Aesthet Surg. 2011;64:445–51.
Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118:882–7.
Achauer BM, Chang CJ, Vander Kam VM. Management of hemangioma of infancy: review of 245 patients. Plast Reconstr Surg. 1997;99:1301–8.
Greenberger S, Bischoff J. Pathogenesis of infantile haemangioma. Br J Dermatol. 2013;169:12–9.
Manunza F, Syed S, Laguda B, et al. Propranolol for complicated infantile haemangiomas: a case series of 30 infants. Br J Dermatol. 2010;162:466–8.
Leaute-Labreze C. Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taieb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–51.
Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99–108.
Mazereeuw-Hautier J, Hoeger PH, Benlahrech S, et al. Efficacy of propranolol in hepatic infantile hemangiomas with diffuse neonatal hemangiomatosis. J Pediatr. 2010;157:340–2.
Schiestl C, Neuhaus K, Zoller S, et al. Efficacy and safety of propranolol as first-line treatment for infantile hemangiomas. Eur J Pediatr. 2011;170:493–501.
Buckmiller LM. Propranolol treatment for infantile hemangiomas. Curr Opin Otolaryngol Head Neck Surg. 2009;17:458–9.
Ji Y, Chen S, Xu C, Li L, Xiang B. The use of propranolol in the treatment of infantile haemangiomas: an update on potential mechanisms of action. Br J Dermatol. 2015;172:24–32.
Jian D, Chen X, Babajee K, et al. Adverse effects of propranolol treatment for infantile hemangiomas in China. J Dermatolog Treat. 2014;25:388–90.
Kunzi-Rapp K. Topical propranolol therapy for infantile hemangiomas. Pediatr Dermatol. 2012;29:154–9.
Bonifazi E, Colonna V, Mazzotta F, Balducci G, Laforgia N. Propranolol in rapidly growing hemangiomas. Eur J Pediatr Dermatol. 2008;18:185–92.
Xu G, Lv R, Zhao Z, Huo R. Topical propranolol for treatment of superficial infantile hemangiomas. J Am Acad Dermatol. 2012;67:1210–3.
Zaher H, Rasheed H, Esmat S, et al. Propranolol and infantile hemangiomas: different routes of administration, a randomized clinical trial. Eur J Dermatol. 2013;23:646–52.
Kovacevic M, Lukinovic Skudar V, Maricic G, Krnjevic-Pezic G, Stanimirovic A. Topical propranolol cream in treatment of superficial infantile hemangiomas: a literature review and 4 years of clinical experience. Acta Dermatovenerol APA. 2014;23:75–8.
Contributions
YW and XZ: Wrote the paper; YadY and JZ: Analyzed the data; YunY: Collected the pictures; YL: Reviewed the whole paper and will act as guarantor for the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
The study is supported by Natural Science Foundation of China (No. 81201244).
Additional information
Yuanyuan Wang and Xingcun Zhang contributed equally to this study and share first authorship.
Electronic supplementary material
ESM 1
(DOCX 128 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, X., Yang, Y. et al. Efficacy and Safety of 2% Topical Propranolol Cream for the Treatment of Proliferating Infantile Strawberry Hemangiomas. Indian J Pediatr 84, 425–429 (2017). https://doi.org/10.1007/s12098-017-2303-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-017-2303-7