Abstract
The present study was undertaken to evaluate the performance of intestinal fatty-acid-binding protein (i-FABP) in the diagnosis of newborn necrotizing enterocolitis (NEC) and prediction of surgical NEC. A comprehensive literature search was performed in PubMed and EMBASE to identify potential relevant studies. QUADAS-2 tool was used to assess the quality of the included studies. The sensitivity, specificity and other measurements of accuracy of i-FABP were pooled. Summary receiver operating characteristic curves (SROC) and area under the curve (AUC) were used to summarize overall diagnostic performance. After screening 150 titles and abstracts, followed by 32 full-text publications, 14 studies were included. The sample size, sensitivity, specificity, diagnostic odds ratio (DOR) and AUC were: plasma i-FABP 217, 0.64(95% CI 0.53 to 0.74), 0.91(95% CI 0.84 to 0.95), 14.22 (95%CI 6.42 to 31.52) and 0.84; urinary i-FABP 211, 0.64(95% CI 0.53 to 0.74), 0.73(95% CI 0.64 to 0.80), 6.35 (95%CI 3.17 to 12.72) and 0.81; urinary i-FABP/Cr 165, 0.78(95% CI 0.65 to 0.88), 0.75(95% CI 0.65 to 0.82), 6.35 (95%CI 3.17 to 12.72) and 0.81; plasma i-FABP for surgical NEC 45, 0.71(95% CI 0.51 to 0.87), 0.76(95% CI 0.50 to 0.93), 7.58 (95%CI 0.87 to 65.82) and 0.80. Plasma i-FABP is a promising biomarker in the diagnosis of NEC with high specificity and DOR; but its usefulness is limited because of medium sensitivity. The urinary i-FABP and urinary i-FABP/Cr add little value in the diagnosis. The findings were somewhat limited by the quality and small size of some of the studies included.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Necrotizing Enterocolitis (NEC) is a common gastrointestinal disorder of newborns associated with high morbidity and mortality. It is also the leading cause of aberrant growth and neurodevelopment among surviving preterm infants [1]. Early diagnosis of NEC is difficult because the initial clinical presentation is undifferentiated with other common situation, such as sepsis. The serum biochemistry is also unreliable and is not always parallel with severity of the disease. The typical appearance of abdominal radiography may be transient or absent in some patients. In order to detect NEC early in preterms with high risk, several biomarkers were investigated in the past decades, including markers of epithelial damage, smooth muscle injures, inflammation and pathogen invasion. Fatty-acid-binding proteins (FABP) comprise a group of cytoplasmic small molecular mass proteins (14–15 kDa) with high organ sensitivity [2]. Intestinal fatty-acid-binding protein (i-FABP) constitutes up to 2% of the cytoplasmic protein content of the mature enterocyte [3]. The plasmic contents, including i-FABP, are liberated into the circulation upon intestinal epithelial injury. I-FABP can passes the glomerular filter with a fractional renal excretion of 28% and a half-life of 11 min [4]. As a consequence, plasma and urinary i-FABP levels may reflect the extent of intestinal epithelial cell damage. Therefore, the plasma i-FABP (i-FABPp)and urinary i-FABP (i-FABPu) are promising biomarkers in early diagnosis of NEC.
Some studies have evaluated the value of i-FABPp or i-FABPu as early detection markers of NEC and predictors of NEC with indications for surgery. But the number of included cases was small in a single study [5–8]. To systematically assess the performance characteristics of i-FABP as a potential suitable marker for NEC and surgical NEC, the authors conducted this systematic review and meta-analysis.
Material and Methods
The systematic review and meta-analysis followed the criteria defined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [9].
The following databases were searched in April, 2015 for relevant articles published in English: PubMed (from January 1980), EMBASE (from January 1989) and the Cochrane Central Register of Controlled Trials (CENTRAL). The search strategy was as follows: necrotizing enterocolitis AND diagnosis AND ((biomarkers) or (fatty-acid-binding protein)). No methodological filters in database searches were used in order to be as sensitive as possible. The titles and abstracts of all articles were read to determine their relevance. Full articles were also scrutinized if the title and abstract were unclear. Reference lists of identified articles were screened for additional publications of interest. If more than one publication appeared in the same study, data from the most inclusive report were used.
Screening of the titles and abstracts were undertaken by two of the authors independently (GY and YW), and the disagreement were solved by consensus. Case-control and prospective cohort studies of humans investigating the performance characteristics of i-FABPp or i-FABPu for the diagnosis of NEC were included. Other criteria were defined as follows: (1) it should be original article; (2) the participants should be neonates, including preterm and term babies. (3) the standards of diagnosis should be stated, including abdominal symptoms, systemic signs, and radiologic findings. (4) the NEC should be Bell II or III stage. (5) it reported sufficient data to calculate sensitivity and specificity. Following information was extracted from the eligible studies: first author, publication year, disease type, research design, number of patients and controls, cutoff values, and true and false positives and negatives. The extracted data were collected with a pre-designed electronic form.
The methodological quality assessment were undertaken by two of the authors independently, and differences in assessment were solved by consensus. The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) was adopted to perform quality assessment of included studies [10]. The QUADAS-2 is composed of four domains: (1) patient selection, (2) index test, (3) reference standard and (4) flow and timing. The authors did not supply any additional domain. For each domain, the risk of bias and concerns were analyzed and rated as low risk, high risk and unclear risk.
The diagnostic value of i-FABPp and i-FABPu was evaluated by calculating the pooled sensitivity, pooled specificity, positive likelihood ratios (PLR), negative likelihood ratios (NLR), diagnostic odds ratio (DOR) and corresponding 95% CI. Summary receiver operator characteristics (SROC) and area under the curve (AUC) were also used to evaluate the performance of the diagnostic tests.
Spearman correlation coefficient were used to check the possible threshold effect. Heterogeneity across studies was checked by the I 2 test and, the Q test P value, which quantifies the proportion of total variation across studies caused by heterogeneity rather than chance. All analyses were performed using RevMan 5.3 (Cochrane Collaboration) and Meta-DiSc 1.4 (Cochrane Colloquium, Barcelona, Spain). All statistical tests were 2-sided, a P value less than 0.05 was considered for statistical significance.
Results
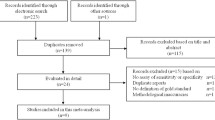
One hundred and fifty articles were obtained by searching the databases. All studies were identified for further evaluation after the titles and abstracts were read. There was one repetitive study [11]. Fourteen measured up to the criteria and were included [2, 5–8, 12–20]. One hundred and thirty-five were excluded because they were animal studies, review articles, or irrelevant to the current study (Fig. 1). Table 1 shows the characteristics of included studies with the optimal cut-off point values identified in the individual studies. They were conducted in Germany [6], USA [8, 12, 14, 18, 20], UK [13, 19], Netherlands [5, 7, 16], Turkey [17], China [15] and Austria [2]. Eleven were finished in the past 5 y [2, 5, 7, 12–19]. Criteria for inclusion or exclusion in each study design were clear. No additional studies were found from references cited in the papers included in the review. The extracted data are displayed in Table 2, including the true-positive, false-positive, false-negative, and true-negative, sensitivities, specificities, PLR and NLR.
The results of evaluation are reported in graphs according to QUADAS-2 (Fig. 2). In the domain of patients selection bias, the designs of 6 studies were case-control [2, 13–15, 17, 19]. The thresholds were pre-specified in only three studies [8, 19, 20]. The others were determined on the basis of the optimum sum of sensitivity and specificity in each study. The majority of studies explicitly state the diagnostic criteria of NEC and the Bell’s stage. The final diagnoses were made without the knowledge of the level of i-FABP. Only five studies had both low risk in bias and low concerns about applicability [5–7, 17, 18].
Seven studies with 217 patients evaluated the performance of i-FABPp for diagnosis of NEC [6–8, 13, 15, 17, 20]. A forest plot of sensitivity and specificity and SROC is shown in Fig. 3. Analysis of diagnostic threshold showed that Spearman correlation coefficient was 0.739 (P = 0.058). Pooled sensitivity, specificity, PLR and NLR were 0.64(95% CI 0.53 to 0.74), 0.91(95% CI 0.84 to 0.95), 4.82(95% CI 2.83 to 8.23) and 0.45 (95% CI 0.34 to 0.58). The I 2 value of sensitivity, specificity, PLR and NLR were 5.1% (P = 0.388), 19.1% (P = 0.284), 0.0% (P = 0.644) and 0.0% (P = 0.848), indicating no significant heterogeneity in the meta-analyses. Diagnostic accuracy was evaluated by the pooled DOR and AUC, which were 14.22 (95% CI 6.42 to 31.52) and 0.84.
Three studies evaluated the diagnostic accuracy of i-FABPu in diagnosis of NEC [7, 14, 18]. Pooled sensitivity, specificity, PLR, NLR were 0.64 (95% CI 0.53 to 0.74), 0.73 (95% CI 0.64 to 0.80), 2.60 (95% CI 1.93 to 3.49) and 0.49 (95% CI 0.33 to 0.71). The I 2 value of sensitivity, specificity, PLR and NLR were 76.9% (P = 0.013), 65.0% (P = 0.058), 0.0% (P = 0.636) and 8.8% (P = 0.334). There is significant heterogeneity between studies. The pooled DOR and AUC were 6.35 (95%CI 3.17 to 12.72) and 0.81 (Fig. 4).
In order to compensate for variations in urine concentration, values of i-FABPu were expressed as ratio of I-FABPu to creatinine in some studies. Four studies evaluated the diagnostic accuracy of i-FABPu/Cr in diagnosis of NEC [12, 16, 18, 19]. Pooled sensitivity, specificity, PLR and NLR are displayed in Fig. 5. There is significant heterogeneity between studies. The pooled DOR and AUC were 6.35 (95%CI 3.17 to 12.72) and 0.81.
Three studies evaluated the diagnostic accuracy of i-FABPp in diagnosis of surgical NEC [7, 13, 15]. Pooled sensitivity, specificity, PLR and NLR are shown in Fig. 6. There is no significant heterogeneity between studies. The pooled DOR and AUC were 7.58 (95%CI 0.87 to 65.82) and 0.80.
Discussion
I-FABP is released into the blood and urine when intestinal mucosa is injured. It has been shown to be a sensitive marker for mucosa injury caused by ischemia and reperfusion [21], low-flow [22] and NEC [11]. In this systematic review, authors evaluated the results of all the published studies that have examined the performance characteristics of i-FABP in NEC. Meta-analysis were performed in 7 studies with 217 patients to explore the potential value of i-FABPp in NEC diagnosis. The i-FABPp displayed good performance characteristics with medium sensitivity (0.64) and high specificity (0.91), suggesting a relative high rate of missed diagnosis (36%). The AUC of SROC curve was 0.87, suggesting i-FABPp as a potential biomarker for diagnosis of NEC. PLR and NLR are easier to understand in clinical practice. The pooled PLR value of 4.82 suggests that NEC patients have an approximately 5-fold higher chance of elevated i-FABPp than do patients without NEC. At the same time, the pooled NLR was 0.45, indicating that low level i-FABPp is 45% likely to be a false negative, which is not low enough to rule out NEC. This may be explained by the fact that totally necrotic gut may no longer result in uptake of released i-FABP into the circulation [5]. Therefore, i-FABP can be used as a diagnostic method of NEC combined with other parameters, such as clinical profile and radiograph. I-FABP also has a limited value in early detection of NEC.
I-FABPu is a noninvasive test to identify gut wall integrity loss and gut wall inflammation in infants with gastrointestinal symptoms suspected of NEC. Three studies with 211 patient and four studies with 165 patients were included in the meta-analysis for the performance of i-FABPu and i-FABPu/Cr in diagnosis of NEC respectively. The results suggest that i-FABPu and i-FABPu/Cr may be non-invasive methods for NEC diagnosis. However, the positive LR (2.60 and 4.94 respectively) and negative LR (0.49 and 0.27 respectively) suggest that i-FABPu and i-FABPu/Cr may not be adequate to rule in and rule out NEC patients. Since the included studies and sample size were small, more high quality studies are needed. Some authors have reported that combination of noninvasive measurement of I-FABP and fecal calprotectin seem promising for diagnosing NEC at an early time point [16].
Twenty percent to 40% of NEC will require operative intervention [23]. Mortality increases up to 50% when surgery is necessary [24]. Ideally, surgery should be performed when intestinal gangrene is imminent but before perforation or necrosis actually occurs. However, this time is difficult to identify. Three studies with 45 patients evaluated i-FABPp in the prediction of surgical NEC. Though medium sensitivity and specificity were shown in combined analysis, the PLR and NLR were only 2.55 and 0.49 respectively. One possible explanation is that elevated level of i-FABPp is suggestive of mucosal damage rather than damage extending into the muscle layers.
The cut-off values used in the studies included in this review were different; the authors may have defined optimal cut-off value according their own study design and ROC. Various techniques were used for measuring i-FABP among the studies, including ELISA and radioimmunoassay. And the completion time of these included studies spans over 10 y. There is intrinsic deficiency of meta-analysis for diagnostic accuracy test so that optimal cut-off value could not be identified [25]. The authors performed threshold analysis and heterogeneity caused by the threshold effect was only found in the combined analysis for i-FABPp in surgical NEC. Also, the value of a meta-analysis for diagnostic accuracy with PLR, NLR and AUC under SROC has been depicted globally [26]. Even so, further work should aim to identify the cut-off value that provides optimal diagnostic accuracy. The published bias should be analyzed in a systematic review. But the number of the included studies is small and thus the value of the funnel plots is decreased. So the authors did not perform the published bias analysis.
The index systematic review is the first analysis of all published studies of i-FABP in diagnosis of NEC and summarizes the diagnostic and prognostic performance of i-FABPp and i-FABPu. The other strength of this systemic review is that QUADAS-2 was used to assess the quality of included studies. This tool will allow for more transparent rating of bias and applicability of primary diagnostic accuracy studies [10].
This study also has some limitations. First, the number of the studies and the cases included in the review is small. Especially for i-FABPu and i-FABPu/Cr in NEC, investment in high-quality comparative and confirmatory studies are needed in the future. Furthermore, the small studies did not allow for examination of accuracy and detection of the heterogeneity in different subgroups of patients. Second, the case-control studies were not excluded in the present review because of limited number of studies. The inclusion criteria were inconsistent. Finally, the results may be biased by the unpublished studies, studies published in other languages, and studies published in journals not indexed in the databases authors’ searched.
In conclusion, i-FABP, in serum or urine, is a promising biomarker in the diagnosis of NEC. The techniques for measurement of i-FABP are simple and accessible in most institutions. But the value should be clarified by larger size, well-designed study in newborns with NEC. I-FABP combined with other biochemistry markers may have greater value in the future for possible clinical application in high-risk preterms.
References
Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2007;92:F193–8.
Benkoe TM, Mechtler TP, Weninger M, Pones M, Rebhandl W, Kasper DC. Serum levels of interleukin-8 and gut-associated biomarkers in diagnosing necrotizing enterocolitis in preterm infants. J Pediatr Surg. 2014;49:1446–51.
Pott J, Hornef M. Innate immune signalling at the intestinal epithelium in homeostasis and disease. EMBO Rep. 2012;13:684–98.
van de Poll MC, Derikx JP, Buurman WA, et al. Liver manipulation causes hepatocyte injury and precedes systemic inflammation in patients undergoing liver resection. World J Surg. 2007;31:2033–8.
Thuijls G, Derikx JP, van Wijck K, et al. Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Ann Surg. 2010;251:1174–80.
Guthmann F, Borchers T, Wolfrum C, Wustrack T, Bartholomaus S, Spener F. Plasma concentration of intestinal- and liver-FABP in neonates suffering from necrotizing enterocolitis and in healthy preterm neonates. Mol Cell Biochem. 2002;239:227–34.
Schurink M, Kooi EM, Hulzebos CV, et al. Intestinal fatty acid-binding protein as a diagnostic marker for complicated and uncomplicated necrotizing enterocolitis: a prospective cohort study. PLoS One. 2015;10:e0121336.
Lieberman JM, Sacchettini J, Marks C, Marks WH. Human intestinal fatty acid binding protein: report of an assay with studies in normal volunteers and intestinal ischemia. Surgery. 1997;121:335–42.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36.
Derikx JP, Evennett NJ, Degraeuwe PL, et al. Urine based detection of intestinal mucosal cell damage in neonates with suspected necrotising enterocolitis. Gut. 2007;56:1473–5.
Gollin G, Stadie D, Mayhew J, et al. Early detection of impending necrotizing enterocolitis with urinary intestinal fatty acid-binding protein. Neonatology. 2014;106:195–200.
Evennett N, Cerigioni E, Hall NJ, Pierro A, Eaton S. Smooth muscle actin as a novel serologic marker of severe intestinal damage in rat intestinal ischemia-reperfusion and human necrotising enterocolitis. J Surg Res. 2014;191:323–30.
Gregory KE, Winston AB, Yamamoto HS, et al. Urinary intestinal fatty acid binding protein predicts necrotizing enterocolitis. J Pediatr. 2014;164:1486–8.
Ng EW, Poon TC, Lam HS, et al. Gut-associated biomarkers L-FABP, I-FABP, and TFF3 and LIT score for diagnosis of surgical necrotizing enterocolitis in preterm infants. Ann Surg. 2013;258:1111–8.
Reisinger KW, Van der Zee DC, Brouwers HA, et al. Noninvasive measurement of fecal calprotectin and serum amyloid a combined with intestinal fatty acid-binding protein in necrotizing enterocolitis. J Pediatr Surg. 2012;47:1640–5.
Aydemir C, Dilli D, Oguz SS, et al. Serum intestinal fatty acid binding protein level for early diagnosis and prediction of severity of necrotizing enterocolitis. Early Hum Dev. 2011;87:659–61.
Mannoia K, Boskovic DS, Slater L, Plank MS, Angeles DM, Gollin G. Necrotizing enterocolitis is associated with neonatal intestinal injury. J Pediatr Surg. 2011;46:81–5.
Evennett NJ, Hall NJ, Pierro A, Eaton S. Urinary intestinal fatty acid-binding protein concentration predicts extent of disease in necrotizing enterocolitis. J Pediatr Surg. 2010;45:735–40.
Edelson MB, Sonnino RE, Bagwell CE, Lieberman JM, Marks WH, Rozycki HJ. Plasma intestinal fatty acid binding protein in neonates with necrotizing enterocolitis: a pilot study. J Pediatr Surg. 1999;34:1453–7.
Thuijls G, van Wijck K, Grootjans J, et al. Early diagnosis of intestinal ischemia using urinary and plasma fatty acid binding proteins. Ann Surg. 2011;253:303–8.
Gollin G, Zieg PM, Cohn SM, Lieberman JM, Marks WH. Intestinal mucosal injury in critically ill surgical patients: preliminary observations. Am Surg. 1999;65:19–21.
Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;23:278–85.
Luig M. Lui K; NSW & ACT NICUS group. Epidemiology of necrotizing enterocolitis–part II: risks and susceptibility of premature infants during the surfactant era: a regional study. J Paediatr Child Health. 2005;41:174–9.
Walter SD, Sinuff T. Studies reporting ROC curves of diagnostic and prediction data can be incorporated into meta-analyses using corresponding odds ratios. J Clin Epidemiol. 2007;60:530–4.
Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. Beta-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis. 2011;52:750–70.
Contributions
GY: Literature search, evaluation of studies, data analysis; YW: Evaluation of studies; GY and YW: Wrote the article; XJ: Guided the research and will act as guarantor for the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
None.
Rights and permissions
About this article
Cite this article
Yang, G., Wang, Y. & Jiang, X. Diagnostic Value of Intestinal Fatty-Acid-Binding Protein in Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Indian J Pediatr 83, 1410–1419 (2016). https://doi.org/10.1007/s12098-016-2144-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-016-2144-9