Abstract
Objectives
To investigate the presence of Y-chromosome sequences and determine their frequency in patients with Turner syndrome.
Methods
The study included 23 patients with Turner syndrome from Brazil, who gave written informed consent for participating in the study. Cytogenetic analyses were performed in peripheral blood lymphocytes, with 100 metaphases per patient. Genomic DNA was also extracted from peripheral blood lymphocytes, and gene sequences DYZ1, DYZ3, ZFY and SRY were amplified by Polymerase Chain Reaction.
Results
The cytogenetic analysis showed a 45,X karyotype in 9 patients (39.2 %) and a mosaic pattern in 14 (60.8 %). In 8.7 % (2 out of 23) of the patients, Y-chromosome sequences were found. This prevalence is very similar to those reported previously. The initial karyotype analysis of these patients did not reveal Y-chromosome material, but they were found positive for Y-specific sequences in the lymphocyte DNA analysis.
Conclusion
The PCR technique showed that 2 (8.7 %) of the patients with Turner syndrome had Y-chromosome sequences, both presenting marker chromosomes on cytogenetic analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Turner syndrome (TS) is caused by one of the most common chromosomal abnormalities of humans, its incidence being of 1:2500 live female births. The phenotypic features of TS vary widely, but usually include a low hairline, triangular facies, high arched palate, small mandible, webbed neck, broad chest with widely spaced nipples, cubitus valgus, short fourth metacarpal and/or metatarsal, multiple pigmented nevi, congenital lymphedema of hands and feet, gonadal dysgenesis, primary or secondary amenorrhea, short stature and cardiac and renal anomalies [1].
The etiology of the Turner syndrome has been associated with total or partial monosomy of the X-chromosome. About 60 % of TS patients have a 45,X karyotype, while the remaining patients have other karyotypes [2, 3]. Overall, patients with X-monosomy tend to have more severe phenotypic features and a higher morbidity, showing the importance of a chromosome analysis in TS prognosis [3].
The diagnosis of TS must take into consideration both phenotypic features and karyotype findings, i.e., chromosome analysis is necessary for a definitive diagnosis of TS. In Brazil, previous studies have demonstrated that TS is usually diagnosed late, the mean age at TS diagnosis still being higher than that reported in developed countries. Some typical characteristics of TS are already present at birth, which indicates the importance of making the information required for early TS diagnosis available to pediatricians and non-pediatricians of the newborn care team [4, 5].
TS patients who carry Y-chromosome material are at high risk of developing gonadoblastoma, a benign gonadal tumor but with a high potential of malignant transformation, the incidence of which is higher between the second and third decades of life. Prophylactic gonadectomy is currently recommended in all TS patients with Y-chromosome material [6].
For the detection of Y-chromosome sequences, cytogenetic analysis may be insufficient, as it can miss cases in which Y-chromosome material is present only in a small proportion of cells or in very small amounts, or even as part of marker chromosomes containing Y-specific regions [7]. Therefore, more reliable techniques, such as molecular analysis, should be used to improve diagnostic accuracy in TS patients.
The aim of this study was to investigate the presence of Y-chromosome sequences and to determine their frequency in Brazilian patients with TS.
Material and Methods
The study included 23 patients with TS from the outpatient Genetics clinic of the Federal University of Triângulo Mineiro (UFTM), Uberaba, State of Minas Gerais, Brazil. Karyotyping was done in the Cytogenetics Laboratory of the Discipline of Genetics of UFTM. Chromosome analysis was performed in cultured peripheral blood lymphocytes and the number of metaphases analyzed by Giemsa-Trypsin-Giemsa (GTG) banding was 100 for each patient. The karyotype distribution is presented in Table 1.
This study was approved by the local Research Ethics Committee (CEP/UFTM no. 927) and written informed consent was obtained from all patients and/or their parents.
Genomic DNA was extracted from lymphocytes using a phenol-chloroform method and amplified using four primer sets encompassing the Y-chromosome regions DYZ1 (Yq12), DYZ3 (Ycen), ZFY (Yp11.31) and SRY (Yp11.32). An internal control of the reaction consisting of the OPN (4q21-q25) gene was used to confirm the presence and availability of DNA in the sample. Primer sequences, annealing temperatures and expected size of the PCR products are described in Table 2.
PCR was performed in a final reaction volume of 30 μL containing approximately 100 μg of genomic DNA, 20 pmol of each forward (F) and reverse (R) primer, 1 unit of Taq DNA polymerase, 1X PCR buffer, 1.5 mM of MgCl2, and 200 μM of each dNTP. In all PCR experiments, normal female DNA, normal male DNA and a blank reaction (with no DNA) were used as controls in all runs. The PCR products were submitted to electrophoresis on 10 % polyacrylamide gel and stained with silver nitrate.
All DNA extractions and PCR reactions were performed by a female technician, to avoid the risk of male DNA contamination.
Results
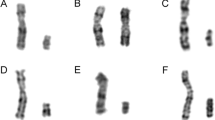
In this study, two out of the 23 patients (8.7 %) had Y-specific sequences detected by PCR analysis. These patients also had a mosaic cell line with a marker chromosome, detected by cytogenetic analysis. The cytogenetic and molecular results and clinical features of these patients are showed in the Table 3. The cytogenetic techniques did not allow the identification of the precise origin, whether X or Y, of the marker chromosomes. However, molecular analysis demonstrated that the markers were derived from a Y chromosome.
None of these patients had shown any Y-chromosome fragments when they were analyzed by classical cytogenetic techniques only. The cytogenetic analysis showed a 45,X karyotype in 9 of them (39.2 %) and mosaics in 14 (60.8 %).
In one patient, a previous cytogenetic analysis performed in another laboratory had reported a plain 45,X karyotype (25 metaphases analyzed). The authors' evaluation, done in 100 peripheral blood lymphocyte metaphases revealed a 45,X[90]/46,X,mar[10] karyotype. These diverging findings may be explained by improved identification techniques and changes in clinical screening practices. In authors' laboratory, the diagnosis of TS is based on the chromosome analysis of 100 peripheral blood lymphocyte metaphases. In most of the studies, the diagnosis of TS is based on 30 analyzed cells only, and therefore, they may not detect low-grade mosaicism.
Discussion
In the index study, two patients that showed marker chromosomes on conventional karyotype analysis actually carried hidden Y-chromosome material, detectable by DNA analysis of blood cells. In fact, about half of the unidentifiable marker chromosomes that occur in TS at an estimated frequency of 3 % are Y-chromosome-derived [8].
The prevalence of Y-chromosome sequences in the present sample was 8.7 %, which is in agreement with previous studies [9–11]. A study done in Italy investigated 171 TS patients for sequences SRY and DYZ3 showed a prevalence of 8.2 % [9]. FISH analysis of buccal smears revealed five patients (7.9 %) with Y-chromosome material [11]. In another, recently published study on 32 Mexican patients with TS, the prevalence of Y-chromosome sequences was 9.4 % (3/32) [10].
According to the literature, TS patients with marker chromosomes identified by cytogenetic analysis have demonstrated a frequency of Y-chromosome sequences ranging from 6.9 % to 60 % (Table 4). The present results are also consistent with other studies conducted in Brazilian patients that reported the presence of Y-chromosome material in 4.8 % [18], 6.8 % [19], and 10.4 %, respectively [14]. According to the literature, the frequency of hidden Y-chromosome material ranges between 0 % and 61.1 % [10]. Several factors contribute to these different findings, such as: patient selection, number of metaphases analyzed in the cytogenetic study, sample size, methodology, and the Y-chromosome sequence markers used [9–19].
It has traditionally been recommended that a search for Y-chromosome fragments in TS should only be performed under two circumstances: when there are signs of virilization and/or when there is a marker chromosome not identified by classical cytogenetics [20, 21]. Thus, patients with TS and a marker chromosome detected by conventional cytogenetic analysis may benefit from molecular screening analysis to detect the presence of Y-chromosome material. PCR is a rapid and low-cost technique that is more sensitive than karyotyping for detecting hidden Y-chromosome material.
The present study showed that one patient with TS, initially reported to have a 45,X karyotype, actually carried a mosaic cell line. Another, recently published study showed that: 1) all 45,X individuals with Turner syndrome are cryptic mosaics; 2) absence of the X chromosome in 45,X embryos is caused primarily by mitotic factors; and 3) the placenta is a strong candidate for the location of the rescue line in apparently non-mosaic 45,X individuals [22]. In addition, in routine laboratory diagnosis, the classical cytogenetic analysis is performed in thirty metaphases, which allows detection of 10 % mosaicism with a 95 % confidence interval [23–25]. If the hypothesis that all patients with TS carry mosaicism is true, it is likely that cell lines that are present in blood at frequencies below 10 % go undetected by this method. Molecular studies present the advantages of not requiring cell culture, can be done with a rather small amount of material, and increase the sensitivity of the cytogenetic methods [26]. In the present study, molecular investigation revealed that 8.7 % patients (2/23) carried Y-chromosome sequences. These sequences are localized on the Y centromere region and short arm Y chromosome, but not amplification was observed to Yq.
Moreover, the clinical guideline for TS advises testing for Y-chromosome material in any TS patient (or fetus) with a marker chromosome (a sex chromosome fragment of unknown origin, i.e., X vs. Y), which can be achieved by DNA studies or FISH [24]. Therefore, based on the index results and data from the literature, the authors recommend that a search of hidden Y-chromosome mosaicism should be carried out by molecular techniques in all TS patients, regardless of the karyotype found.
Conclusions
Thus, it was found that 8.7 % of the patients with TS studied had Y-chromosome sequences detected by the PCR method, with the presence of marker chromosomes found in the cytogenetic analysis. So, it is indicated that in patients suspected of TS, at least 100 metaphases should be analyzed cytogenetically, in order to allow the detection of low-grade mosaicism. Moreover, a molecular investigation for Y-chromosome sequences should be done routinely in patients with a cytogenetic diagnosis of TS, regardless of their karyotype.
References
Sybert VP, McCauley E. Turner's syndrome. N Engl J Med. 2004;351:1227–38.
de Araújo C, Galera BB, Galera MF, de Medeiros SF. Clinical and cytogenetic aspects of the turner syndrome in the Brazilian western region. [Article in Portuguese]. Rev Bras Ginecol Obstet. 2010;32:381–5.
Bispo AV, Dos Santos LO, Burégio-Frota P, et al. Effect of chromosome constitution variations on the expression of turner phenotype. Genet Mol Res. 2013;12:4243–50.
Carvalho AB, Guerra-Junior G, Baptista MT, Marques-de-Faria AP, Lemos-Marini SH, Maciel-Guerra AT. Turner syndrome: a pediatric diagnosis frequently made by non-pediatricians. J Pediatr. 2010;86:121–5.
Jung MP, Amaral JL, Fontes RG, Costa AT, Wuillaume SM, Cardoso MHCA. Diagnóstico da síndrome de turner: a experiência do instituto estadual de diabetes e endocrinologia – Rio de Janeiro, de 1970 a 2008. Rev Bras Mater Infant. 2010;10:117–24.
Lipay MV, Bianco B, Verreschi IT. Gonadal dysgenesis and tumors: genetic and clinical Features. [Article in Portuguese]. Arq Bras Endocrinol Metabol. 2005;49:60–70.
Cortés-Gutiérrez EI, Cerda-Flores RM, Silva-Cudish JB, Dávila-Rodríguez MI, Hernández-Herrera R, Leal-Garza CH. Evaluation of sex chromosome aneuploidies in women with turner's syndrome by G-banding and FISH. A serial case study. J Reprod Med. 2003;48:804–8.
Patsalis PC, Hadjimarcou MI, Velissariou V, et al. Supernumerary marker chromosomes (SMCs) in turner syndrome are mostly derived from the Y chromosome. Clin Genet. 1997;51:184–90.
Mazzanti L, Cicognani A, Baldazzi L, et al. Gonadoblastoma in turner syndrome and Y-chromosome-derived material. Am J Med Genet A. 2005;135:150–4.
Cortés-Gutiérrez EI, Herrera-Bartolo R, Dávila-Rodríguez MI, Palacios-Saucedo GC, Vargas-Villarreal J, Romero-Villarreal JB. Molecular detection of cryptic Y-chromosomal material in patients with turner syndrome. Oncol Rep. 2012;28:1205–10.
Freriks K, Timmers HJLM, Netea-Maier RT, et al. Buccal cell FISH and blood PCR-Y detect high rates of X chromosomal mosaicism and Y chromosomal derivatives in patients with turner syndrome. Eur J Med Genet. 2013;56:497–501.
Bianco B, Nunes Lipay MV, Guedes AD, Verreschi IT. Clinical implications of the detection of Y-chromosome mosaicism in Turner's syndrome: report of 3 cases. Fertil Steril. 2008; 90:1197.e17-20.
Bianco B, Lipay M, Guedes A, Oliveira K, Verreschi IT. SRY gene increases the risk of developing gonadoblastoma and/or nontumoral gonadal lesions in turner syndrome. Int J Gynecol Pathol. 2009;28:197–202.
Barros BA, Maciel-Guerra AT, De Mello MP, et al. A inclusão de novas técnicas de análise citogenética aperfeiçoou o diagnóstico cromossômico da síndrome de turner. Arq Bras Endocrinol Metabol. 2009;53:1137–42.
Bianco B, Oliveira KC, Guedes AD, Barbosa CP, Lipay MV, Verreschi IT. OCT4 gonadal gene expression related to the presence of Y-chromosome sequences in turner syndrome. Fertil Steril. 2010;94:2347–9.
Sallai A, Sólyom J, Dobos M, et al. Y-chromosome markers in turner syndrome: screening of 130 patients. J Endocrinol Investig. 2010;33:222–7.
Barros BA, Moraes SG, Coeli FB, et al. OCT4 immunohistochemistry may be necessary to identify the real risk of gonadal tumors in patients with turner syndrome and Y chromosome sequences. Hum Reprod. 2011;26:3450–5.
Araujo C, Galera MF, Galera BB, Silvestre FG, Medeiros SF. Molecular identification of chromosome Y sequences in Brazilian patients with turner syndrome. Gynecol Endocrinol. 2008;24:713–7.
Bispo AV. Burégio-frota P, Oliveira dos santos L, et al. Y chromosome in turner syndrome: detection of hidden mosaicism and the report of a rare X;Y translocation case. Reprod Fertil Dev. 2014;26:1176–82.
Saenger P, Wikland KA, Conway GS, et al. Recommendations for the diagnosis and management of turner syndrome. J Clin Endocrinol Metab. 2001;86:3061–9.
Frías JL. Davenport ML; Committee on genetics and section on endocrinology. Health supervision for children with turner syndrome. Pediatrics. 2003;111:692–702.
Hook EB, Warburton D. Turner syndrome revisited: review of new data supports the hypothesis that all viable 45,X cases are cryptic mosaics with a rescue cell line, implying an origin by mitotic loss. Hum Genet 2014;133:417–424.
Hook EB. Exclusion of chromosomal mosaicism: tables of 90 %, 95 % and 99 % confidence limits and comments on use. Am J Hum Genet. 1977;29:94–7.
Bondy CA; Turner syndrome study group. Care of girls and women with Turner syndrome: a guideline of the Turner syndrome study group. J Clin Endocrinol Metab. 2007;92:10–25.
Wolff DJ. Van dyke DL, Powell CM; Working group of the ACMG laboratory quality assurance committee. Laboratory guideline for turner syndrome. Genet Med. 2010;12:52–5.
Oliveira RM, Verreschi IT, Lipay MV, Eça LP, Guedes AD, Bianco B. Y chromosome in turner syndrome: review of the literature. Sao Paulo Med J. 2009;127:373–8.
Contributions
RLSG and MASB: Design of the study, collection, analysis and interpretation of data and revision of the manuscript; ABTM: Analysis and interpretation of data, literature review, drafting and revision of the manuscript; TAS and LDC: Collection, PCR analysis and interpretation of data. MASB will act as guarantor for this paper.
ᅟ
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
None.
Rights and permissions
About this article
Cite this article
da Silva-Grecco, R.L., Trovó-Marqui, A.B., de Sousa, T.A. et al. Identification of Y-Chromosome Sequences in Turner Syndrome. Indian J Pediatr 83, 405–409 (2016). https://doi.org/10.1007/s12098-015-1929-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-015-1929-6