Abstract
Objective
To determine the correlation between various obesity indices and pulmonary function parameters in obese Thai children and adolescents.
Methods
Obese children and adolescents aged from 8 to 18 y and diagnosed under the criteria of International Obesity Task Force (IOTF) were enrolled. Anthropometric and body composition measurements (bioelectrical impedance analysis) of all eligible participants were recorded. Pulmonary function studies (spirometry and body plethysmography) were also performed on the same day.
Results
Forty-five children and adolescents [84 % boys; mean age 11.9 ± 2.4 y; mean BMI 31.8 ± 5.1 kg/m2; and, mean body mass index (BMI) z-score 3.2 ± 0.5] were studied. Mean body fat percentage, mean fat mass index (FMI), mean fat free mass index, and mean truncal fat percentage were 47.4 ± 10.2 %, 15.2 ± 5.2 kg/m2, 16.3 ± 3.1 kg/m2, and 47.7 ± 11.5 %, respectively. Abnormal lung functions were found in 73.2 % of subjects; the most common was decreased functional residual capacity (FRC) (29 cases; 64.4 %). There was a negative correlation between FRC and BMI z-score (r = −0.32; p 0.03), waist-height ratio (r = −0.32; p 0.02), body fat percentage (r = −0.32; p 0.03), FMI (r = −0.36; p 0.02), and truncal fat percentage (r = −0.32; p 0.04). Obese individuals who had FMI > 17 kg/m2 were 5.7 times more likely to have decreased FRC than those who had lower FMI (95 % CI 1.1–29.7; p 0.016).
Conclusions
Decreased FRC was the most common pulmonary function abnormality in obese children and adolescents. BMI z-score, waist-height ratio, body fat percentage, FMI, and truncal fat percentage were all negatively correlated with FRC. FMI had the highest negative correlation. Obese individuals with FMI > 17 kg/m2 had a 5.7 times increased risk of low FRC. Appropriate planning for respiratory care and follow-up may be required in this population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obesity is associated with increased risk of respiratory symptoms, morbidity, and mortality. Previous studies have reported various abnormal lung functions in obese pediatric populations [1–3]. Many obesity indices determined by anthropometric and body fat measurements were found to have correlations with ventilatory functions in obese and non-obese populations [1, 4–8]. However, most of these studies evaluated lung function by spirometry alone and used indirect methods, such as anthropometric and skinfold measurement, for evaluating volume and distribution of body fat. Few studies have evaluated the impact of body fat [as assessed using direct methods, such as dual energy x-ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA)] on lung volumes [1, 9]. Compared to DXA, BIA is less complicated to perform and does not expose patients to radiation. Based on authors’ review of the literature, there has been no study in an obese pediatric population that included lung volumes and direct assessment of body fat using BIA in determining the impact of obesity on lung function. The objectives of the index study were to: 1) evaluate the correlation between various obesity indices (assessed by anthropometry and BIA) and lung function parameters (assessed by spirometry and lung volume measurement); and, 2) identify the obesity index that correlated most closely with abnormal lung function in obese children and adolescents.
Material and Methods
This was a cross-sectional study. The study protocol was approved by the Institutional Review Board, Faculty of Medicine, Chulalongkorn University. Informed consent and assent (when applicable) were obtained from study participants and/or their legal guardians before being formally enrolled in the study.
Children and adolescents aged from 8 to 18 y diagnosed with obesity, according to International Obesity Task Force (IOTF) criteria for childhood obesity [10], were recruited from the Nutrition and Pulmonology Clinic, King Chulalongkorn Memorial Hospital from August 1, 2013 to January 31, 2014. Exclusion criteria included: any underlying pulmonary diseases or neuromuscular disorders that may affect the results of the pulmonary function tests (PFT); respiratory infection during the past 2 wk; and, poor effort and ability in performing PFTs. Pulmonary function study and obesity indices, assessed by anthropometric and body composition measurement, were evaluated on the same day for each eligible participant.
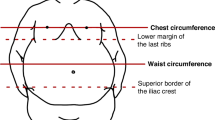
All participants had weight and height measured by standard scale (Detecto™, Cardinal Scale Manufacturing Co., Webb City, MO, USA). Waist circumference was measured at the midpoint between the lowest rib and the iliac crest. Hip circumference was measured at the level of the greater trochanter, while standing. Body mass index (BMI) was calculated and expressed as z-score with adjustments for age and sex, based on WHO growth reference [11, 12].
Body composition (body fat percentage, fat mass, fat-free mass, and truncal fat percentage) was measured using a bioelectrical impedance analyzer (BIA) (Tanita™ BC-418, Tanita Corporation, Tokyo, Japan) by the same investigator throughout the study. Measurement was performed in accordance with the instructions provided in the manufacturer’s instruction manual. For the determination of body fat percentage, fat mass, and fat-free mass (for entire body and specific body parts), the body composition analyzer uses data acquired by DXA from both Japanese and Western subjects, as well as a regression calculation data derived from repeated regression analyses using height, weight, age, and impedance between right hand, right foot, and individual body parts. Fat mass index (FMI) and fat-free mass index were calculated from the ratio of fat mass and fat-free mass, relative to height [2].
Spirometry and lung volumes (measured by body plethysmography) were evaluated by Vmax 6200 Autobox™ (SensorMedics, Yorba Linda, CA, USA) diagnostic system. PFT parameters included forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1/FVC ratio, forced expiratory flow rate within 25–75 % of vital capacity (FEF25 %–75 %), total lung capacity (TLC), and functional residual capacity (FRC). All parameters, excluding FEV1/FVC, were expressed as percentage of predicted value, as calculated from the normal values for the Asian population (European Respiratory Society, 1993). Obstructive defect was defined as FEV1 < 80 % predicted (and/or FEF25 %–75 % < 70 % predicted) with FEV1/FVC < 0.80. Restrictive defect was defined as TLC < 80 % predicted and low FRC was defined as FRC < 80 % predicted.
Collected data included age, sex, obesity indices (BMI, BMI z-score, % weight for height, waist-to-hip ratio, waist-to-height ratio, body fat percentage, truncal fat percentage, fat mass index, and fat free mass index), and pulmonary function variables (FVC, FEV1, FEF25–75 %, FRC, and TLC). The most common lung function abnormality was then identified. Spearman’s rank correlation test was used to identify correlations between various obesity indices and pulmonary function variables that were most commonly found to be abnormal. A two-tailed P < 0.05 was considered statistically significant. Analyses were performed using SPSS version 16.0 (IBM, SPSS, Inc., Chicago, IL, USA).
To investigate correlation between obesity indices and lung function variables, the authors used the following formula for calculating sample size:
where α = 0.05, β = 0.2, and r represented the estimated correlation coefficient between any lung function parameters and any obesity indices. With a clinical effect size (r) of 0.5, the calculated sample size was 29 cases.
Results
There were 45 children and adolescents (mean age 11.9 ± 2.4 y; 84 % boys) who were eligible to participate in this study. Demographic data, lung functions, and body composition variables are presented in Table 1. Abnormal PFT was found in 33 (73 %) cases. The most common abnormality was decreased FRC (29 cases; 64.4 %). Other abnormal lung functions were obstructive defect (3 cases; 7 %) and restrictive defect (1 case; 2 %).
Given that decreased FRC was the most common lung function abnormality found, the authors analyzed the correlations between obesity indices and FRC and found that BMI z-score, waist-to-height ratio, body fat percentage, FMI, and truncal fat percentage had statistically negative correlations with FRC (r = −0.32, −0.32, −0.32, −0.36, and −0.32, respectively; p 0.03, 0.02, 0.03, 0.02, and 0.04, respectively) (Table 2). FMI had the highest negative correlation with FRC (r = −0.36). To identify the FMI cut-off value that is statistically associated with low FRC, the authors serially tested the association between each FMI value and low FRC value using chi-square test and found that FMI > 17 kg/m2 is statistically associated with low FRC (odds ratio 5.7; 95 % CI 1.1-29.7; p 0.016).
Discussion
In the index study, authors found a high prevalence (73 %) of abnormal lung function in obese children and adolescents. The mean BMI z-score of the study population was also quite high, suggesting that most of the study cases were moderately to severely obese. As such, this high prevalence may not be associated with the less obese population. Decreased FRC was the most common lung function abnormality found in the index study (64.4 % of cases). This finding is comparable to the previous pediatric study reported by Li et al [1]. However, a higher frequency of decreased FRC was found, as compared to the Li et al. study (64.4 % vs. 46 %). This may be attributable to higher levels of obesity (higher BMI z-score) in the index study, as compared to the Li et al. study (BMI z-scores 3.2 vs. 2.4, respectively). It has been proposed that the mechanism of decreased FRC results from a decrease in expiratory reserve volume (ERV) due to fat deposition in the chest wall and displacement of the diaphragm into the thorax by the obese abdomen [13]. Decreased FRC causes many deleterious effects to the respiratory system, including increased airway and pulmonary vascular resistance and worsening lung compliance and alveolar ventilation. These effects increase the risk of atelectasis, ventilation/perfusion mismatching, and hypoxemia in obese individuals [14]. Early detection of lung function abnormality would be helpful in planning for appropriate respiratory care that helps to prevent respiratory complications in this population.
Many previous pediatric studies have investigated the correlation between various obesity indices and lung functions [4–8]. However, those studies evaluated lung functions by spirometry, without lung volume assessment. In addition, body fat distribution was determined by skinfold measurement, an indirect method that is associated with substantial prediction errors [15]. In the index study, the authors specifically analyzed the correlations between obesity indices and FRC, the most common lung function abnormality found. They directly measured body composition and fat distribution using BIA, a widely-used technology for the clinical assessment of body composition and nutritional status. BIA has been studied for benefit as a prognostic tool in dialysis and cancer patients [16]. BIA has also been used in many research studies to determine the effect of body composition on pulmonary functions in adult populations [9]. Compared to DXA, BIA is slightly less accurate, but less complicated to use for body composition measurement. BIA is also less expensive and does not expose the patient to radiation.
In the present study, authors found five obesity indices that were negatively correlated with FRC, including BMI z-score, waist-to-height ratio, body fat percentage, FMI, and truncal fat percentage. Weight, height, and BMI showed no correlation with FRC. The present findings suggest that it is the amount and central distribution of body fat, not the entire body weight or height, which negatively impacts lung volumes. This confirms the findings of the previous studies [1, 4–8] and implies that anthropometric measurements routinely used in clinical practice (weight, height, weight-for-height percentage, and BMI) are not adequate for assessing visceral adiposity and identifying the obese individual who is at risk for respiratory complications.
Waist circumference can be easily measured in clinical practice. The ratio between waist circumference and other anthropometric measurements, such as height and hip circumference, have been used to identify truncal obesity. Previous studies have reported a negative correlation between waist-to-hip ratio and lung functions [7, 8]. The effect of waist-to-hip ratio on lung function seems to be sex dependent. In adults, waist-to-hip ratio had a more pronounced effect on lung function in males, as compared to females [17]. This may be due to differences in body fat distribution between the two genders. Waist-to-height ratio has been proposed to be less influenced by sexual maturation. Previous studies have shown that waist-to-height ratio is better than BMI and waist circumference in determining central fat distribution and cardio-metabolic risk [18, 19]. In the present study, it was the waist-to-height ratio, not waist-to-hip ratio that had a negative correlation with FRC. In adolescents and adults, patterns of body fat distribution are different between males and females. Males have fat accumulation around the abdomen, while females tend to accumulate fat around the hips [20]. Therefore, waist-to-height ratio might be less influenced by sex, as compared to waist-to-hip ratio. As such, the waist-to-height ratio may be superior to the waist-to-hip ratio in identifying the obese individual who is at risk for pulmonary complications.
The present study is limited by the small sample size, which did not allow the authors to categorize and compare the cases by gender or preadolescent vs. adolescent. As a result, the power of the correlation coefficient could be affected by age and sex. In the present study, FMI showed the highest negative correlation to FRC, when compared to other obesity indices. FMI is calculated using body fat and height, which makes this measurement less likely to be affected by age and sex, as compared to the other indices (BMI z-score, waist-to-height ratio, body fat percentage, and truncal fat percentage). The authors also found that obese individuals who had FMI > 17 kg/m2 were 5.7 times more likely to have decreased FRC. This cut-off value might be considered as a screening tool for identifying obese children and adolescents who are at risk for having low FRC. It should be noted that this cut-off value was identified after the study was conducted (post-hoc). Further study is still needed to determine its validity in predicting low FRC in this population.
Conclusions
Decreased FRC was the most common lung function abnormality found in obese children and adolescents. BMI z-score and obesity indices that determined central fat distribution, such as waist-to-height ratio, body fat percentage, FMI, and truncal fat percentage all showed negative correlation with FRC. FMI had the highest negative correlation. These obesity indices may be helpful in identifying a low FRC and should be included in the clinical assessment of obese children and adolescents. FMI > 17 kg/m2 may be considered as a screening tool to identify obese individuals who are at risk for low FRC and may require appropriate respiratory care to prevent pulmonary complications.
Abbreviations
- BIA:
-
Bioelectrical impedance analysis
- BMI:
-
Body mass index
- DXA:
-
Dual energy x-ray absorptiometry
- ERV:
-
Expiratory reserve volume
- FEV1 :
-
Forced expiratory volume in 1 second
- FEF25–75% :
-
Forced expiratory flow between 25 % and 75 % of forced vital capacity
- FMI:
-
Fat mass index
- FRC:
-
Functional residual capacity
- FVC:
-
Forced vital capacity
- RV:
-
Residual volume
- TLC:
-
Total lung capacity
References
Li AM, Chan D, Wong E, Yin J, Nelson EA, Fok TF. The effect of obesity on pulmonary function. Arch Dis Child. 2003;88:361–3.
Spathopoulos D, Paraskakis E, Trypsianis G, et al. The effect of obesity on pulmonary lung function of school aged children in Greece. Pediatr Pulmonol. 2009;44:273–80.
Inselman LS, Milanese A, Deurloo A. Effect of obesity on pulmonary function in children. Pediatr Pulmonol. 1993;16:130–7.
Lazarus R, Colditz, Berkey CS, Speizer FE. Effect of body fat on ventilator function in children and adolescents: cross-sectional finding from a random population sample of school children. Pediatr Pulmonol. 1997;24:187–94.
Gonzalez-Barcala FJ, Takkouche B, Valdes L, et al. Body composition and respiratory function in healthy non-obese children. Pediatr Int. 2007;49:553–7.
Chen Y, Rennie D, Cormier Y, Dosman JA. Waist circumference associated with pulmonary function in children. Pediatr Pulmonol. 2009;44:216–21.
Paralikar SJ, Kathrotia RG, Pathak NR, Jani MB. Assessment of pulmonary functions in obese adolescent boys. Lung India. 2012;29:236–40.
Shaheen AA, El-Sobeky SB, Ibrahim AHM. Anthropometric measurements and ventilatory function in obese and non-obese female college students. Middle-East J Sci Res. 2011;7:634–42.
Park JE, Chung JH, Lee KH, Shin KC. The effect of body composition on pulmonary function. Tuberc Respir Dis. 2012;72:433–40.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–5.
World Health Organization. Growth reference data for 5–19 years: BMI for age (5–19 years) Z score: girls. Available at http://www.who.int/growthref/bmifa_girls_5_19years_z.pdf Accessed on January 10, 2013.
World Health Organization. Growth reference data for 5–19 years: BMI for age (5–19 years) Z score: boys. Available at http://www.who.int/growthref/bmifa_boys_5_19years_z.pdf Accessed on January 10, 2013.
Sood A. Altered resting and exercise respiratory physiology in obesity. Clin Chest Med. 2009;30:445–54.
Salome CM, King GG, Berend N. Physiology of obesity and effects of lung function. J Appl Physiol. 2010;108:206–11.
Slaughter M, Lohman T, Boileau R, et al. Skinfold estimations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–23.
Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis – part 1: review of principles and methods. Clin Nutr. 2004;23:1226–43.
Maiolo C, Mohamed EI, Carbonelli MG. Body composition and respiratory function. Acta Diabetol. 2003;40:S32–8.
Kuba VM, Leone C, Damiani D. Is waist-to-height ratio a useful indicator of cardio-metabolic risk in 6–10-year-old children? BMC Pediatr. 2013;13:91.
Hsieh SD, Yoshinaga H, Muto T. Waist-to-height ratio, a simple and practical index for assessing central fat distribution and metabolic risk in Japanese men and women. Int J Obes Relat Metab Disord. 2003;27:610–6.
Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab. 2007;21:415–30.
Conflict of Interest
None.
Source of Funding
This research was supported by a grant from the Ratchadapiseksompotch Research Fund, Faculty of Medicine, Chulalongkorn University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kongkiattikul, L., Sritippayawan, S., Chomtho, S. et al. Relationship between Obesity Indices and Pulmonary Function Parameters in Obese Thai Children and Adolescents. Indian J Pediatr 82, 1112–1116 (2015). https://doi.org/10.1007/s12098-015-1777-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-015-1777-4