Abstract
Background
It is well established that smoking is the most significant risk factor for bladder cancer, yet the impact of smoking on the recurrence and progression of non-muscle-invasive bladder cancer (NMIBC) remains a contentious issue.
Objective
To review all relevant literature published to date, providing a comprehensive assessment of the effects of smoking on the recurrence and progression of NMIBC, thereby offering a basis for smoking cessation management in NMIBC patients.
Methods
A search was conducted for all relevant literature published up to April 2024 in PubMed, Web of Science, and Embase databases. The existing literature results and deficiencies were analyzed, and the gaps in understanding between different studies were highlighted, with recommendations for future research.
Results
A total of 24 studies were included in this work. Among them, 14 studies suggested that smoking promotes the recurrence and progression of NMIBC, while another 10 studies concluded that smoking has no effect on the recurrence and progression of NMIBC patients.
Conclusions
Our research indicates that smoking increases the risk of recurrence and progression in NMIBC patients, and quitting smoking can improve health-related quality of life. High-quality, large-sample prospective cohort studies (or randomized controlled studies) are still needed in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With more than 6,10,000 new cases expected to be diagnosed in 2022—more than 3.1% of all cancer-related fatalities globally—bladder cancer (BCa) is the ninth most frequent malignancy, according to GLOBOCAN [1]. Non-muscle-invasive bladder cancer (NMIBC) accounts for 75% to 85% of bladder cancer cases [2]. Within 5 years, 10% to 15% of tumors advance to muscle-invasive bladder cancer (MIBC), and recurrence rates for NMIBC patients range from 50 to 70% [3,4,5]. BCa is the most financially demanding cancer due to its costly surgical care and ongoing monitoring [6].
As it increases the risk by two to four times, smoking is now the most well-established risk factor for BCa [7]. It is unknown, however, how smoking affects relapse and progression in NMIBC patients. According to recent research, smoking has a detrimental effect on oncologic outcomes for individuals who have non-smoking-related cancers such as prostate cancer [8] as well as other smoking-related cancers, including lung [9] and renal cell carcinoma [10]. A growing amount of literature has examined the impact of smoking on the recurrence and progression of NMIBC; however, the findings have been mixed as research continues to be conducted. We examined pertinent research up until April 2024, discussed and evaluated the findings of previous studies, and studied pertinent literature in order to completely evaluate the effects of smoking and quitting on recurrence and progression in patients with NMIBC.
There is conflicting evidence about the relationship between smoking and patient outcomes. While some studies have linked heavy, long-term smoking to lower relapse-free survival [11,12,13,14,15] and invasiveness [11, 15,16,17,18,19,20]. In addition, other research indicates that there is no connection between smoking and patient outcomes [21,22,23]. These investigations were constrained by retrospective cohort studies, unreliable exposure evaluations, and small sample sizes. There are few prospective cohort studies, which are also limited by the small sample size and short follow-up time. Furthermore, different studies observed different outcome measures and defined relapse and progression differently. This makes it challenging to compare the outcomes consistently. Generally speaking, the majority of research supports the idea that smoking contributes to the development and recurrence of NMIBC. Future research must be backed by high-quality, large-sample randomized controlled trials due to the current lack of strong evidence.
Next, we discussed the connection between smoking and BCa based on the etiology of the disease, followed by the description of our strategy for searching the literature, an analysis of the literature’s findings and limitations based on the type of study (retrospective cohort study, prospective cohort study, randomized controlled study (RCT), systematic review, and meta-analysis), and lastly an inductive summary of the body of known research. Future research recommendations are also suggested.
The epidemiology of bladder cancer
Incidence and mortality of bladder cancer
BCa is sixth in males and thirteenth in death among all cancers worldwide, with women ranking tenth in terms of cancer incidence. There were 2,20,000 bladder cancer deaths and 6,14,000 new cases reported globally in 2022 [1]. The age-standardized incidence and mortality rates were 2.4/100,000 for women and 3.1/100,000 for men, respectively [1]. Regional differences were seen in age-standardized morbidity and mortality rates; mortality rates were greater in developing regions than in developed regions, and the highest incidence rates were found in southern Europe, western Europe, and North America, in that order [1].
Etiology of bladder cancer
Smoking
Approximately half of BCa cases are related to smoking, making it the most well-established risk factor for the disease [24,25,26]. The risk of BCa is directly correlated with time and can increase by two to three times with smoking [7]. Still, increasing the intensity of smoking did not substantially raise the risk of BCa once it reached 15 cigarettes per day (or 50 packs annually). In addition, the risk was much lower for smokers who had stopped more than 20 years prior to the diagnosis, although it did not go down immediately after stopping. However, the risk of BCa remained elevated by 50% even for individuals who stopped smoking more than 20 years earlier [26].
Chemical product exposure
A significant risk factor for BCa is prolonged exposure to industrial chemicals such as chlorinated hydrocarbons, polycyclic aromatic hydrocarbons, and aromatic hydrocarbons [27]. Workplace exposure to chemicals, dyes, rubber, pharmaceutical preparations, textiles, paint, and pesticides is responsible for about 20% of BCa cases [28]. Paint workers are also at a heightened risk of developing bladder cancer. Studies on the epidemiology of BCa have shown that the incidence of the disease is lower in farmers, gardeners, teachers, and other jobs, and greater in business and administrative staff, male electricians, and electronics workers [28, 29].
Race
BCa is also significantly influenced by race; non-Hispanic Caucasians have the highest prevalence, around twice as high as African Americans; however, racial disparities were only observed in non-muscle-invasive tumors, which had comparable rates [30]. Adverse pathology is more common in African Americans, and their disease-specific survival is lower [31, 32].
Other factors
Other potential risk factors include a history of pelvic radiation therapy [33], use of the chemotherapy medication cyclophosphamide [24], drinking water contaminated with high amounts of arsenic or arsenic over an extended period of time [34], and chronic infections (bacterial, schistosomiasis, and HPV infections) [24, 35]. Furthermore, a family history of the condition doubles the risk of BCa [36], which may possibly be linked to genetic factors. There was no statistically significant correlation found between drinking alcohol and developing BCa. Although the exact relationship between a high fruit and vegetable diet and a low risk of BCa is unknown [33, 37, 38], a high intake of fat, cholesterol, fried meals, and red meat may raise the risk of BCa [28].
Genomic variation
DNA mutations in healthy bladder cells are the first step toward malignant alterations. BCa is frequently caused by chemical carcinogens, such as 2-naphthylamine, 4-aminobenzene, and others. Urine contains carcinogenic substances that cause malignant alterations in bladder epithelial cells, and tobacco metabolites are discharged in it. HER-2, HRAS, Bcl-2, FGFR3, C-myc, MSH2, APE1, GTSEI, and other bladder-related oncogenes are among the oncogenes that are currently the subject of the majority of research on the genesis of BCa [39,40,41,42,43,44,45].
Materials and methods
Retrieval strategy
To obtain relevant literature, we searched PubMed, Web of Science, and Embase databases for articles published up to April 2024. The search terms are as follows: “((progression OR progression-free survival OR muscle-invasive) AND (non-muscle invasive bladder cancer OR NMIBC) AND (Risk factors OR Smoking OR smoke OR cigarette OR tobacco)) OR ((progression OR recurrence-free survival OR muscle-invasive OR recurrence) AND (non-muscle invasive bladder cancer OR NMIBC) AND (Smoking OR smoke OR cigarette OR tobacco))” and “(recurrence OR progression OR survival) AND (non-muscle invasive bladder cancer OR NMIBC) AND (Smoking OR smoke OR cigarette OR tobacco)”.
Research selection
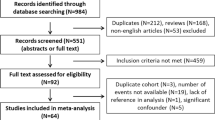
Once redundant literature has been eliminated, the two writers (VV and MDR) individually examine the entire text to assess eligibility; if there is disagreement, the third researcher (SC) is consulted before a consensus is resolved. For additional pertinent references, we then searched through all of the identified papers, reviews that had already been published, and meta-analyses. Listed below are the inclusion criteria: Works addressing how smoking affects relapse and progression in NMIBC patients that provide measures of statistical uncertainty (such as standard error, variance, or exact P-values) or hazard ratios (HR) and 95% confidence intervals (CI). Excluded from consideration was any literature that disclosed smoking status alone, without mentioning smoking intensity or duration, or that disclosed smoking intensity or duration alone. The risk of tumor development or recurrence is the main outcome. The first time a bladder tumor returns is known as a disease recurrence. The upper urinary tract tumor is considered a second primary tumor rather than a tumor recurrence [13]. Any bladder tumor that reaches stage T2 or above is considered to have progressed [13].
Smoking-related indicators and their concepts
The variables related to smoking included: smoking status; average number of cigarettes smoked per day (CPD; that is, amount: 1–9, 10–19, 20–29, ≥ 30); length of smoking (≤ 9.9, 10–19.9, 20–29.9, 30–39.9, ≥ 40 years); and time to stop smoking (≤ 4.9, 5–9.9, ≥ 10 years). The categories of smoking status include smokers (> 100 cigarettes, non-negligible lifetime number of cigarettes) and never smokers (< 100 cigarettes) [13]. Smokers can continue to be classified as quitters (who stopped smoking 1 year before diagnosis), former smokers (who quit between 1 year before diagnosis and 3 months after diagnosis), and current smokers (who continue to smoke after diagnosis). Former smokers and current smokers are the most common categories of smokers.
At the time of diagnosis, smoking history was frequently evaluated through self-report [13]. Cumulative smoking exposure is the result of CPD after years of smoking. Smokers were categorized into four categories of lifetime cumulative smoking exposure based on the amount and duration of their smoking: mild short-term (< 20 CPD and < 20 years), mild long-term (< 20 CPD and ≥ 20 years), heavy short-term (≥ 20 CPD and < 20 years), and heavy long-term (≥ 20 CPD and ≥ 20 years) [8, 13, 46].
Result
Retrospective cohort studies
Smoking promoted the recurrence and progression of NMIBC (the difference was statistically significant)
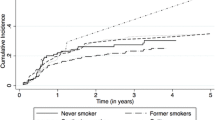
In Rink et al.’s report, 91 non-smokers, 192 former smokers, and 107 current smokers were enrolled, with a median follow-up of 66 months [47]. Univariate analysis revealed that among ever smokers, increasing smoking intensity (p ≤ 0.015), duration (p < 0.001), and cumulative exposure (p < 0.001) were associated with an increased risk of illness recurrence and progression [47]. In multivariate analysis, cumulative smoking exposure showed an independent risk factor for progression and relapse (p < 0.003). When compared to present smoking, quitting smoking more than ten years before treatment was independently linked to a lower rate of relapse (HR 0.4, p < 0.001). In addition, current smokers had a lower survival rate than former smokers, and former smokers had a lower survival rate than never smokers (p > 0.05). In a related study, there were 2043 primary NMIBC patients with a median follow-up of 49 months (24% never smokers, 47% former smokers, and 29% current smokers, respectively) [13]. In multivariate analysis, smoking status was also linked to the cumulative incidence of illness progression (p = 0.003). In smokers who are currently or were previously smokers, cumulative smoking exposure was linked to both disease progression and relapse (p < 0.001).Giving up smoking for more than ten years decreased the chance of advancement (HR: 0.42; 95% CI 0.22–0.83; p = 0.036) and relapse (HR: 0.66; 95% CI 0.52–0.84; p < 0.001). According to Kashif et al., smokers and non-smokers were equally divided among the 64 patients, and they were followed for an average of 28.36 months. The likelihood of tumor growth was four times higher in smokers than in non-smokers [48]. There were 64 non-smokers, 64 former smokers, 59 ex-smokers, and 78 continuing smokers. The median follow-up was 38 months. For current and former smokers, the 3-year relapse-free survival rates were 45% and 70%, respectively, in Chen et al.’s findings [49]. One hundred sixty-eight patients (34.7%) had never smoked, 121 patients (25%) were current smokers, and 195 patients (40.3%) were former smokers. The median follow-up was 25 months. In addition, cumulative smoking exposure was found to be significantly (p < 0.001) linked with tumor recurrence in both present and past smokers by Li et al [50]. Patients who had quit smoking for at least 10 years had a decreased risk of tumor recurrence compared to current smokers (HR: 0.456, p = 0.007) [50]. According to Ogihara et al., 181 patients (28.5%) were classified as current smokers, 154 (24.3%) as former smokers, and 299 (47.2%) as non-smokers. The rates of tumor recurrence were considerably lower in non-smokers than in smokers (p < 0.001 and p < 0.001, respectively) than in smokers [51]. Patients with a 15-year or longer smoking cessation history had significantly decreased tumor recurrence rates (p < 0.001). According to Andrade et al.’s report [52], 132 patients with pT1 NMIBC were followed for an average of 76 months. Smoking load had a substantial impact on progression (HR: 1.034, 95% CI 1.016–1.052; p = 0.0002) and relapse (HR: 1.019, 95% CI 1.008–1.030; p = 0.0004) (Table 1).
Smoking promotes the recurrence and progression of NMIBC (no statistically significant difference)
Although the difference was not statistically significant, the study by Fleshner et al. found that current smokers had lower relapse-free survival than either quitters or ex-smokers (127 former smokers, 51 ex-smokers, and 108 current smokers) [11]. Similarly, Matulewicz et al. found that of the 723 NMIBC patients included, 34.7% were nonsmokers, 52.6% were past smokers, and 12.7% were current smokers. Smoking status was not substantially linked with relapse during the three-year trial period [23]. The study population included 181 never smokers (18.8%), 490 former smokers (50.9%), and 292 current smokers (30.3%) at diagnosis. Follow up for 5 years. The risk of relapse or progression was not shown to differ statistically significantly across never, former, or current smokers, according to Grotenhuis et al [53]. The categories of smoking quantity, duration, and cumulative exposure linked to the prognosis of NMIBC also showed no dose–response correlations. The likelihood of a relapse and its advancement were not significantly affected by the quit date [53]. Three huyndred eighty-six patients (62.0%) were former smokers, 97 patients (15.6%) were current smokers, and 140 patients (22.5%) had never smoked. The median follow-up was 80.9 months. Any definition of smoking status did not correlate with relapse or advancement in the Sfakianos et al.’s report [54]. Similar results were found by Michalek et al., who found that of 302 patients, 32% were non-smokers, 22% were past smokers, and 46% were current smokers. Twenty-three months was the median follow-up time. Neither the number of tumor recurrences nor recurrence-free survival were correlated with smoking status [55] (Table 1).
Result and deficiency
Seven of the aforementioned twelve retrospective cohort studies found a statistically significant increase in the risk of recurrence and/or advancement in patients with NMIBC associated with smoking [13, 47,48,49,50,51,52]. According to five reports [11, 23, 53,54,55], smoking had no impact on the rate of recurrence or advancement in NMIBC patients. The constraints inherent in retrospective cohort studies cannot be overcome by any of the aforementioned investigations. Treatment postponement, TURBT repetition, TURBT quality, and adjuvant regimen following TURBT inability to be regulated. Furthermore, it was unable to regulate other tobacco products or other tobacco-exposure scenarios (such as smoking marijuana, cigars, or secondhand smoke). Despite difficulties in data collection, tobacco products of different types and brands differ in tar volume, smoke nicotine volume, and carbon monoxide volume, which may lead to differences in tumor recurrence and progression between patients. Patients self-report their smoking history, making it susceptible to recall bias and fraudulent reporting. Ultimately, people who stop smoking and then pick up again or who smoke continuously cannot be controlled.
Smoking is a dynamic process that takes exposure to smoke into account in addition to smoking status. The only factors that differed between current smokers and never smokers and ex-smokers, respectively, were smoking and quitting. It is inappropriate to compare ex-smokers to never-smokers since there is a dual variable—smoking and quitting—rather than a single variable separating the two groups. Therefore, when follow-up is too short, it is typical to find no differences in recurrence and advancement rates among never-smokers, ex-smokers, and current smokers. Because of this, the Rink et al.’s study continues to be the best retrospective study [13]. Numerous other studies have not encountered a dead end when it comes to qualitative analysis, and their findings also have some guidance and reference value [47,48,49,50,51,52]. It is astounding that in Chen et al.’s study [49], quitting smokers had a lower chance of relapsing than did continuing smokers and nonsmokers. Although the authors provide explanations, it is possible that people who stop smoking avoid being exposed to more smoking-related carcinogens. Nonsmokers have been exposed to other, mainly unknown carcinogens, or nonsmokers’ genetic makeup predominates in carcinogenic environments. It goes without saying that this explanation is implausible. The small sample size and brief follow-up period of Andrade et al.’s study limit the application of its findings [52]. Furthermore, all of this research is ensnared in the qualitative analysis trap [11, 23, 53,54,55]. Homogeneous analysis from the source was not possible in Matulewicz et al.’s report because initial NMIBC accounted for only 11.5% and recurrent NMIBC accounted for 88.5% [23]. The impact of smoking on relapse rates is primarily obscured by the high risk, recurrent nature of the disease, and a significant selection bias. People who stopped smoking within a year after receiving a diagnosis were classified as quitters in Grotenhuis et al.’s report [53], but only smoking exposure before the diagnosis was taken into account for current smokers. This means that constant smokers and those who are trying to quit almost exactly coincide. It is obviously illogical to confuse quitting and chronic smokers, even though the simple qualitative analysis has little reference significance.
Prospective cohort studies (or RCTs)
Smoking promoted the recurrence and progression of NMIBC (the difference was statistically significant)
According to Lammers et al.’s [12], of the 718 NMIBC patients included, 121 were non-smokers, 359 were former smokers, and 238 were current smokers. The average follow-up was 2.5 years. In univariate analysis, past and present smokers had considerably shorter RFS (p = 0.005). When predicting RFS using multivariate analysis, smoking status remained a significant predictor. 97 patients (24.6%) had never smoked, and 298 patients (75.4%) were smokers. The median follow-up was 48 months. 69.1% and 74.2% (p = 0.16), 13.6 and 14.2 months (p = 0.27), and 64.0% and 71.3% (p = 0.08) were the median times to 3-year RFS, RFR, and first relapse for smokers and never-smokers, respectively, in Serretta et al.’s RCT [22]. Smoking was found to be the primary predictor of relapse (p = 0.04) in multivariate analysis [22]. However, in the study by Kwan et al., 874 patients (59.4%) were former smokers, and 111 patients (7.5%) were current smokers. Sixty-seven patients (13.7%) used only pipes and/or cigars; 65 patients (4.4%) used e-cigarettes; and 363 patients (24.7%) used marijuana [56]. The follow-up was 26.4 months. The risk of relapse rose dose-dependently with smoking duration and the number of pack-years smoked. Patients who smoked for more than 40 years (HR, 2.36; 95% CI 1.43–3.91) or more than 40 pack-years (HR, 1.97; 95% CI 1.32–2.95) had the highest risk of recurrence [56] (Table 2).
Smoking promotes the recurrence and progression of NMIBC (no statistically significant difference)
According to Furberg et al., biochemical tests were performed to verify smoking exposure in 354 patients with NMIBC. Of these, 78% were former smokers and 22% were current smokers, with a median follow-up of 3.6 years. The study found that there is no correlation between smoking after diagnosis and the likelihood of relapsing (HR: 0.73, 95% CI 0.45–1.20) [57]. Of the 722 patients with NMIBC included, 103 were never smokers, 266 were former smokers, 186 were current smokers, 150 were former smokers who resumed smoking, and 17 were smokers who quit after diagnosis. During a median follow-up period of 4.21 years, 403 pathologically confirmed NMIBC recurrences occurred in 210 patients. Compared to continuing smokers, only 25 current smokers at diagnosis quit smoking during follow-up (14%) [58]. Relapsing was not less likely in van Osch et al.’s report if smoking was stopped following diagnosis than if it was continued (p = 0.352) [58]. In line with the findings of Serretta et al., a total of 194 patients were diagnosed; 67 (34.5%) of them stopped smoking, while 127 (65.5%) did not. At the 38-month median follow-up, the recurrence rates for former and continuing smokers were 49.2% and 60.3%, respectively, and the 3-year RFS was 50.7% and 42.3%, respectively (p = 0.55) [59]. Recurrence, the clinical characteristics of the original tumor, and the patients’ post-diagnosis smoking behaviors did not show any statistically significant correlation. The number of cigarettes smoked per day and the length of time (years) had no statistically significant impact on the results. Multivariate analysis showed no significant reduction in tumor recurrence following smoking cessation at diagnosis (Table 2).
Result and deficiency
Three of these six Prospective cohort studies showed statistically significant differences in the effects of smoking on relapse and/or progression in patients with NMIBC [12, 22, 56]. Smoking had no effect on recurrence or advancement in patients with NMIBC, according to three trials [57,58,59]. Although it is a Prospective cohort study, Lammers et al.’s report is very representative [12], with the questionnaire being the primary source of its shortcomings. Due to a lack of control over passive smoking and other factors, smoking status was only assessed at the time of patient recruitment. Furthermore, it does not account for those who quit smoking or those who restart after a period of time. False and covert reporting cannot be completely ruled out either, as smoking data is obtained via the questionnaire. It can be more obvious whether the patient has smoked recently if biochemical indications are employed for verification. Like Serretta et al.’s RCT [22], the sample size was modest, and it only looked at smoking status at the time of diagnosis, ignoring any changes in tobacco usage during follow-up and beyond. Furthermore, a high percentage of patients were lost to follow-up, and the trial lacked rigorous prospective control. In contrast, there are very few e-cigarette users in the Kwan et al.’s report [56], and recall bias regarding smoking behavior in participant self-reports cannot be ruled out. The results solely pertain to the relationship between smoking behavior and short-term (≤ 2 years) risk of relapse and progression; they do not address long-term risk.
Three hundred fifty-four NMIBC patients had a history of smoking, according to a paper by Furberg et al [57]. The key is to employ biochemical markers to determine “people who are smoking” with accuracy, prevent patients from disclosing that they smoke, and prevent misreporting by non-smokers who may be exposed to nicotine from other sources. In addition to having small sample numbers, brief follow-up periods, and succumbing to the traps of qualitative analysis, the study shows no correlation between smoking exposure and the likelihood of relapse. It is thought that when the sample size is large enough, the follow-up period is long enough, and the cumulative smoking exposure is the same in quitters and current smokers, stratified investigations of smoking cessation time will produce more acceptable results. As only 14% of the NMIBC group stopped smoking after receiving a diagnosis, there was sampling bias, and the findings in the van Osch et al.’s research were not representative [58]. Furthermore, the study did not standardize postoperative adjuvant treatment or tumor characteristics (stage, grade, size, and number of tumors). Lastly, the data revealed that almost one-third of those who had quit were certain to start smoking again after receiving a diagnosis. This just serves to emphasize the importance of ambulatory monitoring and long-term follow-up. As shown by Serretta et al., quitting smoking at the time of diagnosis did not significantly lower the risk of tumor recurrence [59]. It makes sense to restrict the study participants to quitting and long-term smokers to examine the impact of quitting on relapse and the advancement of NMIBC. The sample size is too small to support a stratified investigation, and the conclusions’ application is constrained.
Systematic reviews and meta-analysis
Smoking promoted the recurrence and progression of NMIBC (the difference was statistically significant)
In a meta-analysis involving 7885 patients with NMIBC, Ślusarczyk et al. found that smokers (current or past) had a greater risk of relapse [60]. (95% CI 1.34–2.09; p < 0.0001). Relapse risk was 1.24 times greater in current smokers than in former smokers (OR = 1.24; 95% CI 1.02–1.50; p = 0.03), according to a subgroup analysis of 2967 patients. When compared to never smokers, smokers had a greater chance of relapse (HR = 1.31; 95% CI 1.15–1.48; p < 0.0001) and progression (HR = 1.18; 95% CI 1.08–1.29; p < 0.001), according to a meta-analysis of survival ratios. The risk of UCB increases by 2–4 times for current smokers; however, this risk can be lowered by giving up smoking. There was little correlation seen between smoking and other outcomes in patients with TURBT and RC, and smoking status, exposure, and quitting had a substantial effect on disease recurrence for patients undergoing TURBT, according to Rink et al.’s report [61]. Current smokers had a higher chance of a local recurrence of NMIBC, according to Van Osch et al. (HR 1.27, 95% CI 1.09–1.46) [62]. Comparable to the findings of Hou et al.’s report, smoking status had a positive correlation with the chance of bladder cancer recurrence (SRRE = 1.23; 95% CI 1.05–1.45) as well as history (SRRE = 1.22; 95% CI 1.09–1.37) [63]. Still, there was no statistically significant correlation between smoking status (SRRE = 1.11; 95% CI 0.71–1.75) or history (SRRE = 1.16; 95% CI 0.92–1.46) and the risk of BCa development (Table 3).
Smoking promotes the recurrence and progression of NMIBC (no statistically significant difference)
Smoking lifelong or persistently is suggestive evidence of moderate risk factors for relapse, which can be positively modified by quitting. This was found in a systematic review by Aveyard et al [15]. Still, the majority of the research’s findings lack statistical significance, and methodological flaws make the evidence basis flimsy. Caini et al. conducted an analysis of the nine included studies and found no statistically significant difference between the quitters’ and the continuing smokers’ risk of relapse (SHR 0.99, 95% CI 0.61–1.61) [64] (Table 3).
Result and deficiency
The six aforementioned systematic reviews and meta-analyses shared the same issue, and the original research—mostly retrospective cohort studies—limited the meta-analysis’s quality [15, 60,61,62,63,64]. The shortcomings of retrospective research are unavoidable for these publications, and by accounting for factors like real smoking, postoperative treatment methods, tumor characteristics, demographic characteristics, and quality of repeat TURBT, they may even exacerbate this shortcoming. The inclusion of 7885 patients in the Ślusarczyk et al.’s analysis allowed for the amplification and statistical differentiation of the differences between non-smokers and smokers (including ex-smokers and current smokers) [60]. However, we advise against drawing straight parallels between quitting and non-smokers. This simply explains why quitting smoking increases the chance of relapsing; it does not explain why quitting smoking alone or in combination with other factors contributes less to recurrence. There are also a lot of important restrictions on the study. Six prospective cohort studies were included out of 64 original investigations. Only two of these six prospective trials, nevertheless, examined the impact of smoking on the development and recurrence of NMIBC. The remaining investigations included smoking as a possible confounder in univariate or multivariate analysis; however, since smoking status was not the primary focus of these studies, reporting bias was unavoidably present. Inconsistencies also existed in baseline attributes such as patient age, tumor stratification, and postoperative adjuvant therapy. Similar issues plagued the other five meta-analyses [15, 61,62,63,64]. Here, we will not go through them one by one.
Discussion
In terms of mechanisms, single nucleotide polymorphisms in cytochrome P450 reductase, N-acetyltransferase, glutathione S-transferase, genes linked to inflammation, STK12 genes, and DNA-repair genes, as well as DNA damage brought on by tobacco carcinogens, are all signific`ant contributors to the development of cancer [65,66,67,68]. UCB patients’ prognosis and HPV DNA presence are significantly correlated, according to recent studies [69]. Tumor recurrence was reported by 47.3% of 19 HPV-positive patients (n = 9) and 36.8% of 38 HPV-negative patients (n = 14), with no significant differences in age, follow-up period, smoking status, or tumor grade (p = 0.445). During the 2-year follow-up period, there was a correlation found between the presence of HPV DNA and an increased susceptibility to relapse. According to a number of studies [70,71,72], lower e-cadherin expression encourages BCa metastasis and progression and is linked to a poor prognosis. Restoring E-cadherin expression can stop tumor invasion, metastasis, and progression. Li et al. conducted more research and discovered that the unique dsRNA-mediated up-regulation of E-cadherin expression impeded the expansion and metastasis of BCa cells by blocking the genes that target β-catenin/TCF [73]. Furthermore, a stronger correlation was found between decreased BCa outcomes and elevated miR-155 levels [74, 75]. Lu et al.’s additional research revealed that the expression of miR-155 varied between RT4 and T24 BCa cells [76]. Via the intercellular transfer of TNTs, miR-155 can activate the Deptor-mTOR signaling pathway, thereby facilitating the invasion and growth of BCa cells. Numerous signaling pathways, including the MAPK/ERK, PI3K/Akt, and JAK/STAT pathways, have been shown to be activated by nicotine through nicotinic acetylcholine receptors. These pathways are linked to tumor development and acquired treatment resistance in addition to tumor formation [77,78,79]. The PI3K/Akt/mTOR pathway may be activated by nicotine exposure in vitro and in vivo in research to promote tumor cell proliferation. Conversely, blocking this system may decrease BCa cell line T24 viability [80]. These initial results offer foundational ideas for mitigating nicotine exposure and decreasing the aggressiveness of bladder cancerous growths.
According to the number of included study populations and references, the majority of research (14 references) revealed that smoking significantly accelerated the progression and recurrence of NMIBC in patients [12, 13, 22, 47,48,49,50,51,52, 56, 60,61,62,63]. Smoking had no effect on the recurrence or advancement of NMIBC in patients, according to less than half of the investigations (10 papers) [11, 15, 23, 53,54,55, 57,58,59, 64]. Meta-analyses utilizing randomized controlled studies or prospective cohort studies as the primary research studies offer the strongest level of evidence. But unfortunately, no such evidence is available. Certain meta-analyses that rely on retrospective cohort studies [61, 62], prospective cohort studies [12, 22, 56], and retrospective cohort studies [13, 47, 49, 51] still hold some guiding significance. Almost all of the research also supports stopping smoking, which enhances quality of life in relation to health. The pathological types of BCa mentioned in the above 24 articles are all urothelial carcinoma. While there are gender disparities in bladder cancer incidence, men are more likely than women to develop BCa. But none of the 24 publications mentioned above examined how smoking affected NMIBC patients’ progression and recurrence from a gender standpoint. Gender did not distinguish between current smokers, former smokers, or never smokers. One of the publications provided a plausible explanation [49]: it was not possible to segment the study by gender because the prevalence of smoking among women during the same period was just 4%. Therefore, we cannot know whether there is a gender difference in the "effect of smoking on the recurrence and progression of non-muscle-invasive bladder cancer".
These investigations were constrained by retrospective cohort studies, unreliable exposure evaluations, and small sample sizes. Due to their small sample size and short follow-up period, prospective cohort studies are few. Of the twenty-four articles, a large number focused on the relapse and progression outcomes of non-smokers, ex-smokers, and chronic smokers, falling into the trap of qualitative analysis [11, 23, 53,54,55]. There are not many literary works that have managed to escape the confines of qualitative analysis and examine smoking exposure as well as status. The impact of smoking on the development and recurrence of NMIBC was examined by contrasting never-smokers with current smokers. Cumulative smoking exposure was the only variable. The study should only include smokers who are currently active to better examine the impact of smoking intensity and duration on relapse and progression. To examine the impact of quitting on the recurrence and progression of NMIBC, current and former smokers were matched. When cumulative smoking exposure was equal, the effects of quitting on relapse and progression were evaluated. Former smokers should be the only ones included in the study to more thoroughly examine the impact of smoking intensity and time prior to quitting on relapse and progression. Comparing never-smokers to ex-smokers is inappropriate since there are two variables to consider: smoking and quitting. The sole variable is not smoking or stopping. It is also not appropriate to compare smokers (both past and present) with those who have never smoked. Releasing oneself from smoking reduces the harmful consequences of prior smoking to some extent, even though smokers are still exposed to smoking. The people who smoke now are most affected by smoking, followed by those who have smoked in the past, and lastly, those who have never smoked. A former smoker’s cumulative smoking exposure is not always smaller than a smoker’s present exposure, though when looking at it quantitatively.
Compared to other tobacco-related diseases, bladder cancer is not as well known to the general public [81,82,83]. When BCa was first discovered in about 30% of patients, they were still heavy smokers [84]. Roughly 40% of smokers continue to smoke while receiving treatment for BCa [22]. Furthermore, few urologists provide their patients with any therapies to assist in quitting [17, 83] because they may not think that quitting smoking is crucial to the clinical care of BCa [85]. A lack of clinical proof could be the cause of this [85]. Positively, with medical guidance, most individuals with BCa are willing to give up smoking [86]. According to a prospective experiment, patients’ rates of quitting smoking increased dramatically (from 2.6% to 12.1%) after receiving a smoking cessation intervention for as little as five minutes. Clearly, higher rates of smoking cessation may result from improved clinician and patient understanding of the value of quitting smoking in the management of illness [87].
Prospective cohort studies (or RCTs) are better equipped to address the limitations of retrospective cohort studies, including an inability to account for genuine smoking status, postoperative treatment, tumor features, population factors, and repeated TURBT and TURBT quality. Among them [57], recollection bias and purposeful concealment can be significantly mitigated by employing biochemical markers to reliably identify “people who are smoking.” Furthermore, dynamic monitoring and follow-up over an extended period of time are crucial. Smokers who have altered over time can be identified through dynamic monitoring. Excessive overlap between present and previous smokers was more likely to occur during shorter follow-up periods. The entire scope of disparities between present and previous smokers will be revealed by long-term follow-up [22, 63]. The following factors must be carefully controlled: smoking status must be authentic and reliable (questionnaire survey combined with biochemical verification); demographic characteristics (age, gender); tumor characteristics (stage, grade, tumor size, tumor number); and postoperative treatment plan (whether perfusion therapy, chemotherapy drug infusion therapy, or repeated surgery). Stratified studies must be conducted to clearly understand the effects of smoking cessation years, smoking time, and smoking exposure on relapse and progression of NMIBC patients. Large samples are the basic and necessary conditions for completing stratified analysis.
We should increase public awareness of smoking-related hazards and focus on improving the prognosis of other tobacco-related systemic diseases and improving quality of life, even though the conclusion that smoking promotes relapse and progression in patients with NMIBC is not supported by high-quality, large-sample prospective cohort studies (or randomized controlled studies). Simultaneously, there is an increased focus on deterring nonsmokers from starting to smoke as well as helping current smokers to give up as soon as feasible [57].
Conclusion
When combined with the information now available on NMIBC, smoking is widely acknowledged to have a negative impact on patient outcomes. It is also known to accelerate the progression and recurrence of BCa. Reducing tobacco use lowers the risk of cardiovascular events, all-cause mortality, and second primary malignancies while also improving health-related quality of life and making procedures safer. Encouragement and assistance in quitting smoking should be provided to all patients who smoke. Large-scale, high-quality Prospective cohort study (or RCT) evidence is desperately needed to support the weak evidence that smoking causes the recurrence and progression of BCa in clinical practice. These limitations stem from the study type, sample size, and design. This has substantial clinical implications for urologists’ scientific cognition, increasing smoking cessation counseling, bolstering smoking cessation publicity, and lessening patient financial load while enhancing patient prognosis.
Data availability
Given that no new data were created or analyzed for this study, data sharing is not applicable to this publication.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2024;74(3):229–63.
Oddens J, Brausi M, Sylvester R, Bono A, van de Beek C, van Andel G, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63(3):462–72.
Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59(6):997–1008.
Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Gonzalez M, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol. 2009;182(5):2195–203.
Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–5.
Stenzl A, Hennenlotter J, Schilling D. Can we still afford bladder cancer? Curr Opin Urol. 2008;18(5):488–92.
Zeegers MP, Tan FE, Dorant E, van Den Brandt PA. The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer. 2000;89(3):630–9.
Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA. 2011;305(24):2548–55.
Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ (Clinical research ed). 2010;340:b5569.
Kroeger N, Klatte T, Birkhäuser FD, Rampersaud EN, Seligson DB, Zomorodian N, et al. Smoking negatively impacts renal cell carcinoma overall and cancer-specific survival. Cancer. 2012;118(7):1795–802.
Fleshner N, Garland J, Moadel A, Herr H, Ostroff J, Trambert R, et al. Influence of smoking status on the disease-related outcomes of patients with tobacco-associated superficial transitional cell carcinoma of the bladder. Cancer. 1999;86(11):2337–45.
Lammers RJ, Witjes WP, Hendricksen K, Caris CT, Janzing-Pastors MH, Witjes JA. Smoking status is a risk factor for recurrence after transurethral resection of non-muscle-invasive bladder cancer. Eur Urol. 2011;60(4):713–20.
Rink M, Furberg H, Zabor EC, Xylinas E, Babjuk M, Pycha A, et al. Impact of smoking and smoking cessation on oncologic outcomes in primary non-muscle-invasive bladder cancer. Eur Urol. 2013;63(4):724–32.
Wyszynski A, Tanyos SA, Rees JR, Marsit CJ, Kelsey KT, Schned AR, et al. Body mass and smoking are modifiable risk factors for recurrent bladder cancer. Cancer. 2014;120(3):408–14.
Aveyard P, Adab P, Cheng KK, Wallace DM, Hey K, Murphy MF. Does smoking status influence the prognosis of bladder cancer? A systematic review. BJU Int. 2002;90(3):228–39.
Sturgeon SR, Hartge P, Silverman DT, Kantor AF, Linehan WM, Lynch C, et al. Associations between bladder cancer risk factors and tumor stage and grade at diagnosis. Epidemiology. 1994;5(2):218–25.
Strope SA, Montie JE. The causal role of cigarette smoking in bladder cancer initiation and progression, and the role of urologists in smoking cessation. J Urol. 2008;180(1):31–7.
Hirao Y, Kim WJ, Fujimoto K. Environmental factors promoting bladder cancer. Curr Opin Urol. 2009;19(5):494–9.
Jiang X, Castelao JE, Yuan JM, Stern MC, Conti DV, Cortessis VK, et al. Cigarette smoking and subtypes of bladder cancer. Int J Cancer. 2012;130(4):896–901.
Reznikoff CA, Sarkar S, Jülicher KP, Burger MS, Puthenveettil JA, Jarrard DF, et al. Genetic alterations and biological pathways in human bladder cancer pathogenesis. Urol Oncol. 2000;5(5):191–203.
Ogihara K, Kikuchi E, Yuge K, Yanai Y, Matsumoto K, Miyajima A, et al. The preoperative neutrophil-to-lymphocyte ratio is a novel biomarker for predicting worse clinical outcomes in non-muscle invasive bladder cancer patients with a previous history of smoking. Ann Surg Oncol. 2016;23(Suppl 5):1039–47.
Serretta V, Altieri V, Morgia G, Di Lallo A, Carrieri G, Allegro R. Cigarette smoking status at diagnosis and recurrence in intermediate-risk non-muscle-invasive bladder carcinoma. Urology. 2013;81(2):277–81.
Matulewicz RS, Ravvaz K, Weissert JA, Porten S, Steinberg GD. Association of smoking status and recurrence of non-muscle invasive bladder cancer among patients managed with blue light cystoscopy. Urologic oncology. 2021;39(12):833.e19-e26.
Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234–41.
Chavan S, Bray F, Lortet-Tieulent J, Goodman M, Jemal A. International variations in bladder cancer incidence and mortality. Eur Urol. 2014;66(1):59–73.
van Osch FH, Jochems SH, van Schooten FJ, Bryan RT, Zeegers MP. Quantified relations between exposure to tobacco smoking and bladder cancer risk: a meta-analysis of 89 observational studies. Int J Epidemiol. 2016;45(3):857–70.
Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol. 2017;198(3):552–9.
Al-Zalabani AH, Stewart KF, Wesselius A, Schols AM, Zeegers MP. Modifiable risk factors for the prevention of bladder cancer: a systematic review of meta-analyses. Eur J Epidemiol. 2016;31(9):811–51.
Colt JS, Friesen MC, Stewart PA, Donguk P, Johnson A, Schwenn M, et al. A case-control study of occupational exposure to metalworking fluids and bladder cancer risk among men. Occup Environ Med. 2014;71(10):667–74.
Yee DS, Ishill NM, Lowrance WT, Herr HW, Elkin EB. Ethnic differences in bladder cancer survival. Urology. 2011;78(3):544–9.
Wang Y, Chang Q, Li Y. Racial differences in urinary bladder cancer in the United States. Sci Rep. 2018;8(1):12521.
Gild P, Wankowicz SA, Sood A, von Landenberg N, Friedlander DF, Alanee S, et al. Racial disparity in quality of care and overall survival among black vs white patients with muscle-invasive bladder cancer treated with radical cystectomy: A national cancer database analysis. Urol Oncol. 2018;36(10):469.e1-e11.
Abern MR, Dude AM, Tsivian M, Coogan CL. The characteristics of bladder cancer after radiotherapy for prostate cancer. Urol Oncol. 2013;31(8):1628–34.
Fernández MI, López JF, Vivaldi B, Coz F. Long-term impact of arsenic in drinking water on bladder cancer health care and mortality rates 20 years after end of exposure. J Urol. 2012;187(3):856–61.
Zaghloul MS, Gouda I. Schistosomiasis and bladder cancer: similarities and differences from urothelial cancer. Expert Rev Anticancer Ther. 2012;12(6):753–63.
Egbers L, Grotenhuis AJ, Aben KK, Alfred Witjes J, Kiemeney LA, Vermeulen SH. The prognostic value of family history among patients with urinary bladder cancer. Int J Cancer. 2015;136(5):1117–24.
Liu H, Wang XC, Hu GH, Guo ZF, Lai P, Xu L, et al. Fruit and vegetable consumption and risk of bladder cancer: an updated meta-analysis of observational studies. Eur J Cancer Prev : Off J Eur Cancer Prev Organ (ECP). 2015;24(6):508–16.
Buckland G, Ros MM, Roswall N, Bueno-de-Mesquita HB, Travier N, Tjonneland A, et al. Adherence to the Mediterranean diet and risk of bladder cancer in the EPIC cohort study. Int J Cancer. 2014;134(10):2504–11.
Guo G, Sun X, Chen C, Wu S, Huang P, Li Z, et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat Genet. 2013;45(12):1459–63.
Kriegmair MC, Balk M, Wirtz R, Steidler A, Weis CA, Breyer J, et al. Expression of the p53 Inhibitors MDM2 and MDM4 as outcome predictor in muscle-invasive bladder cancer. Anticancer Res. 2016;36(10):5205–13.
Skeldon SC, Semotiuk K, Aronson M, Holter S, Gallinger S, Pollett A, et al. Patients with Lynch syndrome mismatch repair gene mutations are at higher risk for not only upper tract urothelial cancer but also bladder cancer. Eur Urol. 2013;63(2):379–85.
Guey LT, García-Closas M, Murta-Nascimento C, Lloreta J, Palencia L, Kogevinas M, et al. Genetic susceptibility to distinct bladder cancer subphenotypes. Eur Urol. 2010;57(2):283–92.
Liu A, Zeng S, Lu X, Xiong Q, Xue Y, Tong L, et al. Overexpression of G2 and S phase-expressed-1 contributes to cell proliferation, migration, and invasion via regulating p53/FoxM1/CCNB1 pathway and predicts poor prognosis in bladder cancer. Int J Biol Macromol. 2019;123:322–34.
Corral R, Lewinger JP, Van Den Berg D, Joshi AD, Yuan JM, Gago-Dominguez M, et al. Comprehensive analyses of DNA repair pathways, smoking and bladder cancer risk in Los Angeles and Shanghai. Int J Cancer. 2014;135(2):335–47.
van der Post RS, Kiemeney LA, Ligtenberg MJ, Witjes JA, Hulsbergen-van de Kaa CA, Bodmer D, et al. Risk of urothelial bladder cancer in Lynch syndrome is increased in particular among MSH2 mutation carriers. J Med Genet. 2010;47(7):464–70.
Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306(7):737–45.
Rink M, Xylinas E, Babjuk M, Hansen J, Pycha A, Comploj E, et al. Impact of smoking on outcomes of patients with a history of recurrent nonmuscle invasive bladder cancer. J Urol. 2012;188(6):2120–7.
Kashif Khan M, Ahmed I, Raza SJ. Factors effecting recurrence and progression of high grade non invasive bladder cancer treated by intravesical BCG. Pak J Med Sci. 2014;30(2):326–30.
Chen CH, Shun CT, Huang KH, Huang CY, Tsai YC, Yu HJ, et al. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer. BJU Int. 2007;100(2):281–6.
Li HM, Azhati B, Rexiati M, Wang WG, Li XD, Liu Q, et al. Impact of smoking status and cumulative smoking exposure on tumor recurrence of non-muscle-invasive bladder cancer. Int Urol Nephrol. 2017;49(1):69–76.
Ogihara K, Kikuchi E, Yuge K, Ito Y, Tanaka N, Matsumoto K, et al. Refraining from smoking for 15 years or more reduced the risk of tumor recurrence in non-muscle invasive bladder cancer patients. Ann Surg Oncol. 2016;23(5):1752–9.
Andrade DL, Moretti TBC, Neto WA, Benedetti J, Reis LO. Smoke load prognostic impact on bacillus Calmette-Guérin (BCG) treated non-muscle invasive bladder cancer. Int Urol Nephrol. 2020;52(8):1471–6.
Grotenhuis AJ, Ebben CW, Aben KK, Witjes JA, Vrieling A, Vermeulen SH, et al. The effect of smoking and timing of smoking cessation on clinical outcome in non-muscle-invasive bladder cancer. Urol Oncol. 2015;33(2):65.e9-17.
Sfakianos JP, Shariat SF, Favaretto RL, Rioja J, Herr HW. Impact of smoking on outcomes after intravesical bacillus Calmette-Guérin therapy for urothelial carcinoma not invading muscle of the bladder. BJU Int. 2011;108(4):526–30.
Michalek AM, Cummings KM, Pontes JE. Cigarette smoking, tumor recurrence, and survival from bladder cancer. Prev Med. 1985;14(1):92–8.
Kwan ML, Haque R, Young-Wolff KC, Lee VS, Roh JM, Ergas IJ, et al. Smoking behaviors and prognosis in patients with non-muscle-invasive bladder cancer in the be-well study. JAMA Netw Open. 2022;5(11):e2244430.
Furberg H, Petruzella S, Whiting K, Stein E, Orlow I, Kenney J, et al. Association of biochemically verified post-diagnosis smoking and nonmuscle-invasive bladder cancer recurrence risk. J Urol. 2022;207(6):1200–6.
van Osch FHM, Jochems SHJ, Reulen RC, Pirrie SJ, Nekeman D, Wesselius A, et al. The association between smoking cessation before and after diagnosis and non-muscle-invasive bladder cancer recurrence: a prospective cohort study. Cancer Causes Control : CCC. 2018;29(7):675–83.
Serretta V, Di Maida F, Baiamonte D, Vella M, Pavone C, Cacciatore L, et al. Does smoking cessation at primary diagnosis reduce the recurrence risk of nonmuscle-invasive bladder cancer? Results of a prospective study. Urol Int. 2020;104(5–6):396–401.
Ślusarczyk A, Zapała P, Zapała Ł, Radziszewski P. The impact of smoking on recurrence and progression of non-muscle invasive bladder cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2023;149(6):2673–91.
Rink M, Crivelli JJ, Shariat SF, Chun FK, Messing EM, Soloway MS. Smoking and bladder cancer: a systematic review of risk and outcomes. Eur Urol Focus. 2015;1(1):17–27.
van Osch FH, Jochems SH, van Schooten FJ, Bryan RT, Zeegers MP. Significant role of lifetime cigarette smoking in worsening bladder cancer and upper tract urothelial carcinoma prognosis: a meta-analysis. J Urol. 2016;195(4 Pt 1):872–9.
Hou L, Hong X, Dai M, Chen P, Zhao H, Wei Q, et al. Association of smoking status with prognosis in bladder cancer: A meta-analysis. Oncotarget. 2017;8(1):1278–89.
Caini S, Del Riccio M, Vettori V, Francolini G, D’Ecclesiis O, Cai T, et al. Prognostic impact of post-diagnosis smoking cessation among bladder cancer patients: a systematic literature review and meta-analysis. Cancers. 2022;14(16):4022. https://doi.org/10.3390/cancers14164022.
Xiao X, Ma G, Li S, Wang M, Liu N, Ma L, et al. Functional POR A503V is associated with the risk of bladder cancer in a Chinese population. Sci Rep. 2015;5:11751.
de Maturana EL, Ye Y, Calle ML, Rothman N, Urrea V, Kogevinas M, et al. Application of multi-SNP approaches Bayesian LASSO and AUC-RF to detect main effects of inflammatory-gene variants associated with bladder cancer risk. PLoS ONE. 2013;8(12):e83745.
Xing J, Dinney CP, Shete S, Huang M, Hildebrandt MA, Chen Z, et al. Comprehensive pathway-based interrogation of genetic variations in the nucleotide excision DNA repair pathway and risk of bladder cancer. Cancer. 2012;118(1):205–15.
Rothman N, Garcia-Closas M, Chatterjee N, Malats N, Wu X, Figueroa JD, et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat Genet. 2010;42(11):978–84.
Sarier M, Usta SS, Turgut H, Öztürk SA, Soylu A, Emek M, et al. Prognostic value of HPV DNA in urothelial carcinoma of the bladder: a preliminary report of 2-year follow-up results. Urol J. 2021;19(1):45–9.
Carneiro P, Figueiredo J, Bordeira-Carriço R, Fernandes MS, Carvalho J, Oliveira C, et al. Therapeutic targets associated to E-cadherin dysfunction in gastric cancer. Expert Opin Ther Targets. 2013;17(10):1187–201.
Daugaard I, Sanders KJ, Idica A, Vittayarukskul K, Hamdorf M, Krog JD, et al. miR-151a induces partial EMT by regulating E-cadherin in NSCLC cells. Oncogenesis. 2017;6(7):e366.
Yuan Z, Wong S, Borrelli A, Chung MA. Down-regulation of MUC1 in cancer cells inhibits cell migration by promoting E-cadherin/catenin complex formation. Biochem Biophys Res Commun. 2007;362(3):740–6.
Li C, Liu J, Zhang Q, Cui K, Ge Q, Wang C, et al. Upregulation of E-cadherin expression mediated by a novel dsRNA suppresses the growth and metastasis of bladder cancer cells by inhibiting β-catenin/TCF target genes. Int J Oncol. 2018;52(6):1815–26.
Catto JW, Miah S, Owen HC, Bryant H, Myers K, Dudziec E, et al. Distinct microRNA alterations characterize high- and low-grade bladder cancer. Can Res. 2009;69(21):8472–81.
Wang H, Men CP. Correlation of increased expression of MicroRNA-155 in bladder cancer and prognosis. Lab Med. 2015;46(2):118–22.
Lu JJ, Yang WM, Li F, Zhu W, Chen Z. Tunneling nanotubes mediated microRNA-155 intercellular transportation promotes bladder cancer cells’ invasive and proliferative capacity. Int J Nanomed. 2019;14:9731–43.
Chernyavsky AI, Shchepotin IB, Grando SA. Mechanisms of growth-promoting and tumor-protecting effects of epithelial nicotinic acetylcholine receptors. Int Immunopharmacol. 2015;29(1):36–44.
Momi N, Ponnusamy MP, Kaur S, Rachagani S, Kunigal SS, Chellappan S, et al. Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through α7nAChR-mediated MUC4 upregulation. Oncogene. 2013;32(11):1384–95.
Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res: MCR. 2014;12(1):14–23.
Yuge K, Kikuchi E, Hagiwara M, Yasumizu Y, Tanaka N, Kosaka T, et al. Nicotine induces tumor growth and chemoresistance through activation of the PI3K/Akt/mTOR pathway in bladder cancer. Mol Cancer Ther. 2015;14(9):2112–20.
Bjurlin MA, Cohn MR, Freeman VL, Lombardo LM, Hurley SD, Hollowell CM. Ethnicity and smoking status are associated with awareness of smoking related genitourinary diseases. J Urol. 2012;188(3):724–8.
Sell V, Ettala O, Perez IM, Järvinen R, Pekkarinen T, Vaarala M, et al. Awareness of smoking as a risk factor in bladder cancer: results from the prospective FinnBladder 9 trial. Eur Urol Focus. 2022;8(5):1246–52.
Guzzo TJ, Hockenberry MS, Mucksavage P, Bivalacqua TJ, Schoenberg MP. Smoking knowledge assessment and cessation trends in patients with bladder cancer presenting to a tertiary referral center. Urology. 2012;79(1):166–71.
Grotenhuis AJ, Dudek AM, Verhaegh GW, Witjes JA, Aben KK, van der Marel SL, et al. Prognostic relevance of urinary bladder cancer susceptibility loci. PLoS ONE. 2014;9(2):e89164.
Bjurlin MA, Goble SM, Hollowell CM. Smoking cessation assistance for patients with bladder cancer: a national survey of American urologists. J Urol. 2010;184(5):1901–6.
Bassett JC, Gore JL, Chi AC, Kwan L, McCarthy W, Chamie K, et al. Impact of a bladder cancer diagnosis on smoking behavior. J Clin Oncol : Off J Am Soc Clin Oncol. 2012;30(15):1871–8.
Michael J, Matulewicz RS, Bjurlin MA. Assessment of tobacco screening and smoking cessation recommendations among bladder cancer guidelines: a call to action. J Urol. 2022;207(3):490–2.
Acknowledgements
We are grateful to all researchers for their enrolled studies.
Funding
This research was supported by the National Natural Science Foundation of China (grant no. 82060459).
Author information
Authors and Affiliations
Contributions
Chaohu Chen: Conceptualization, Methodology, Investigation, Writing—Original Draft, Visualization. Guangrui Fan: Conceptualization, Investigation, Writing—Original Draft. Pan Li: Resources, Methodology, Investigation, Writing—Original Draft. Enguang Yang: Resources, Methodology, Investigation. Suoshi Jing: Resources, Methodology, Investigation. Yuwen Gong: Conceptualization, Methodology, Investigation. Luyang Zhang: Resources, Methodology, Conceptualization. Yibo Shi: Conceptualization, Methodology, Investigation. Zhiping Wang: Supervision, Project administration, Funding acquisition, Writing—Review & Editing. Approval of the manuscript: All authous.
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts of interest have been disclosed by the authors.
Ethical approval
Ethical approval does not apply because this work is not research involving humans and/or animals.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, C., Fan, G., Li, P. et al. Effect of smoking on the recurrence and progression of non-muscle-invasive bladder cancer. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03694-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03694-z