Abstract
Purpose
This study aimed to evaluate the prognostic significance of changes in inflammatory markers in patients with Hepatitis B virus-related hepatocellular carcinoma (HBV-HCC) treated with first-line lenvatinib plus a programmed cell death protein 1 (PD-1) inhibitor.
Methods
This study retrospectively included 117 HBV-HCC patients treated with first-line lenvatinib in combination with a PD-1 inhibitor. Independent factors affecting progression-free survival (PFS) and overall survival (OS) were explored based on baseline indicators and inflammatory markers changes after one treatment cycle.
Results
Multivariate analysis revealed that an alpha-fetoprotein (AFP) level \(\geqslant\)400 ng/mL [hazard ratio (HR), 1.69; 95% confidence interval (CI), 1.11–2.58; P = 0.01] was identified as an independent risk factor, platelet-to-neutrophil ratio (PNR) \(\leqslant\) 65.43 (HR 0.50; 95% CI 0.30–0.84; P \(< 0.01)\) and SII \(\leqslant\)539.47 (HR 0.54; 95% CI 0.30–0.96; P = 0.03) were identified as independent protective factors for PFS. Additionally, multivariate analysis demonstrated that AFP \(\geqslant\) 400 ng/mL, HBV-HCC patients with diabetes mellitus (DM), and SII \(> 303.66\) were independent risk factors of OS. The patients whose SII had increased after one cycle of treatment showed a poorer PFS (HR 1.61; 95 %CI 1.10–2.37; P = 0.015) and OS (HR 1.76; 95 % CI 1.15–2.70; P = 0.009) than patients whose SII had decreased. The objective response rate (ORR) was higher in the SII-decreased patients (47.5% vs 32.5%, P = 0.11). Mann–Whitney test found a significant difference in therapeutic response between the SII-increased patients and the SII-decreased patients (P = 0.04).
Conclusion
SII can be associated with outcomes in patients with HBV-HCC treated with first-line lenvatinib plus PD-1 inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer-related death in the world [1]. In China, hepatitis B virus (HBV) infection is a crucial cause of HCC, and more than 80% of HCC patients are caused by chronic HBV infection, resulting in a severe disease burden [2, 3]. The prognosis of HBV-HCC is extremely poor, which has been the focus of research [4]. Surgical resection is an effective treatment for early-stage HCC. However, approximately 50% of patients are found at an advanced stage of the disease and miss the opportunity for surgery [5]. In recent years, antiangiogenic drugs or targeted therapy drugs combined with immunotherapy have become an essential treatment for unresectable or advanced HCC. Based on the positive results of the Phase III IMbrave150 trial, the combination of the programmed death-ligand 1 (PD-L1) inhibitor Atezolizumab and the Bevacizumab have become the first-line standard of care for unresectable HCC [6]. Based on the results of the Phase III REFLECT trial, Lenvima (an oral multi-targeted tyrosine kinase inhibitor) was approved by the Food and Drug Administration in 2015 for first-line treatment of unresectable HCC) patients [7]. Lenvatinib has immunomodulatory activity, and compared with the immunodeficient tumor microenvironment, lenvatinib exhibits improved antitumor activity in the immunocompetent tumor microenvironment [8]. Studies have shown that lenvatinib blocks fibroblast growth factor receptor 4, down-regulates tumor PD-L1 levels and Treg differentiation, and improves anti-PD1 efficacy [9]. Preliminary results of several clinical trials have shown that the first-line treatment strategy of Lenvatinib combined with PD-1 inhibitors can improve the survival of patients with unresectable disease [10, 11].
Due to tumor heterogeneity, there is a wide variation in patients’ response to treatment, with only some benefiting from the same treatment strategy. Therefore, it is essential to identify reliable biomarkers to select the population that may benefit from treatment. Inflammatory markers are convenient, fast, inexpensive, and can be dynamically monitored. Studies have shown that immune-inflammatory markers based on peripheral blood neutrophil, lymphocyte, monocyte, and platelet counts are associated with the prognosis of various solid tumors [12, 13]. The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and other inflammatory markers have shown high value in predicting the prognosis of HCC patients [14, 15]. However, the relationship between inflammatory markers and the combination treatment of lenvatinib and PD-1 inhibitors in unresectable HBV-HCC patients is yet to be explored.
In this study, we conducted a retrospective study of patients with HBV-HCC who were treated with lenvatinib plus a PD-1 inhibitor as the first-line treatment and analyzed the predictive value of inflammatory markers for the clinical outcome of these patients.
Materials and methods
Case collection
We retrospectively analyzed 117 HBV-HCC patients who received dual therapy of a PD-1 inhibitor plus lenvatinib at Beijing Youan Hospital, Capital Medical University from January 2017 to July 2022. The inclusion criteria were as follows: (1) patients diagnosed with HCC by dynamic computed tomography (CT)/magnetic resonance imaging (MRI) or pathology according to the European Association for the Study of the Liver Clinical Practice Guidelines: Management of Hepatocellular Carcinoma; (2) patients who were positive for hepatitis B surface antigen (HBsAg), negative for hepatitis C virus or HIV antibodies; (3) patients who had not received other immunotherapy before starting PD-1 inhibitor treatment; (4) patients did not have recent infections or autoimmune diseases during the treatment; (5) patients were not diagnosed with malignancy other than HCC. The research received approval from the Hospital Ethics Committee and adhered to the ethical principles of the Declaration of Helsinki.
Clinicopathologic variables and data collection
We collected demographic information of patients (including gender and age), medical history (including history of chronic viral hepatitis B, DM, and history of anti-tumor treatment), and clinical pathological features [including blood chemistry, serum AFP levels, vascular invasion, distant metastasis, Barcelona Clinic Liver Cancer (BCLC) stage] by reviewing electronic medical records. Albumin–bilirubin (ALBI) grades were calculated using the system described by Johnson et al. in 2015 [16]. Child–Pugh scores were calculated according to the method created by Child CG and Pugh RN [17]. The modified ALBI (mALBI) grade criteria were calculated according to the method proposed by Masatoshi Kudo [18]. We also collected routine blood test data on neutrophil count, lymphocyte count, platelet count, and monocyte count from patients before immunotherapy and two to three weeks after the first cycle of immunotherapy. The NLR, PLR, Lymphocyte-to-monocyte ratio (LMR), Platelet-to-albumin ratio (PAR), PNR, and SII were calculated according to the following equations.
NLR = neutrophil count [10\(^9\)/L]/lymphocyte count [10\(^9\)/L]
PLR = platelet count [10\(^9\)/L]/ lymphocyte count [10\(^9\)/L]
LMR = lymphocyte count [10\(^9\)/L]/monocyte count [10\(^9\)/L]
PAR = platelet count [10\(^9\)/L]/ albumin [g/L]
PNR = platelet count [10\(^9\)/L]/neutrophil count [10\(^9\)/L]
SII = neutrophil count [10\(^9\)/L] \(\times\) platelet count [10\(^9\)/L]
The main outcome indicator of the study was PFS, defined as the time from the beginning of immunotherapy to disease progression or last follow-up or death from any cause. The secondary endpoint was OS, defined as the time from the beginning of immunotherapy to death from any cause or last follow-up.
Statistical analysis
Continuous variables that followed a normal distribution were presented as mean ± standard deviation, while those not adhering to a normal distribution were expressed as median and interquartile range (IQR). Using the median value of PFS and OS as the cutoff value, PFS and OS were divided into two subgroups, respectively, and the optimal cutoff values for NLR, PLR, LMR, PNR, PAR, and SII were calculated using receiver operating characteristic (ROC) curves. Prognostic factors affecting PFS and OS were analyzed using Cox proportional hazards models. Initially, variables were subjected to univariate analysis. Subsequently, factors with a P value \(< 0.05\) in the univariate analysis were included in the multivariate analysis. Survival curves were plotted using the Kaplan–Meier method, and intergroup comparisons were made using the log-rank test. A P value less than 0.05 was considered statistically significant in all statistical analyses. Statistical analysis was performed using IBM SPSS Statistics version 25.0 and MedCalc version 22.0 software.
Results
Clinical characteristics
During the study period, a total of 117 patients met the study conditions. The baseline clinicopathological features of the patients are shown in Table 1. Ninety patients (76.9%) were younger than 65 years of age, and most were male (n = 92, 78.6%). Twenty-five (21.4%) patients had DM. Seventy-two patients (61.5%) had baseline AFP levels of less than 400 ng/mL. Most patients were BCLC stage C (75.2%). There were 67 cases (57.3%) with vascular invasion and 50 cases (42.7%) with extrahepatic metastasis. ALBI grade of 1, 2, and 3 were recorded in 15 (12.8%), 84 (71.8%), and 18 (15.4%), respectively. Within ALBI grade 2, there were 22 (18.8%) cases with mALBI grade 2a and 62 (53.0%) cases with grade 2b. The Child–Pugh score was categorized as class A in 81 (69.2%) and class B in 36 (30.8%) patients. The most used antiviral drug was entecavir (59 cases, 50.4%), followed by tenofovir alafenamide (32 cases, 27.4%) and tenofovir disoproxil fumarate (26 cases, 22.2%). The most used PD-1 inhibitor was tislelizumab (46 cases, 39.3%), followed by cedilimumab (43 cases, 36.8%) and camrelizumab (28 cases, 23.9%).
The optimal cutoff values for predicting the PFS and OS
ROC curve analysis showed that the optimal cutoff values of NLR, PLR, LMR, PAR, PNR, and SII for predicting PFS were 4.20, 127.52, 2.72, 3.13, 65.43, and 539.47, with respective areas under the curve (AUC) of 0.724, 0.658, 0.608, 0.574, 0.627 and 0.706. The optimal cutoff values of NLR, PLR, LMR, PAR, PNR, and SII for predicting OS were 3.56, 88.12, 3.50, 3.13, 31.43, and 303.66, with respective AUC of 0.808, 0.682, 0.689, 0.600, 0.677 and 0.762 (Table 2).
Factors affecting PFS and OS
Univariate analysis of baseline clinicopathological features indicated that AFP (P = 0.03), DM (P = 0.02), NLR (P \(< 0.01\) ), PLR (P \(< 0.01\)), LMR (P \(< 0.01\)), PNR (P \(< 0.01\)), and SII (P < 0.01) were significantly associated with PFS. Multivariable analysis with these factors revealed that AFP \(\geqslant\) 400 ng/mL (HR 1.69; 95% CI 1.11–2.58; P = 0.01) was an independent risk factor of PFS. The PNR \(\leqslant\) 65.43 (HR 0.50; 95% CI 0.30–0.84; P \(< 0.01\)) and the SII \(\leqslant\) 539.47 (HR 0.54; 95% CI 0.30–0.96; P = 0.03) were independent protective predictors of PFS (Table 3).
Univariate analysis of baseline clinicopathological features indicated that AFP (P = 0.04), DM (P = 0.04), Extrahepatic metastasis (P = 0.03), NLR (P \(< 0.01\)), PLR (P \(< 0.01\)), LMR (P = 0.03), PAR (P = 0.02), PNR (P \(< 0.01\)), and SII (P \(< 0.01\)) were significantly associated with OS. Multivariable analysis with these factors revealed that AFP\(\geqslant\) 400 ng/mL (HR 1.97; 95% CI 1.22–3.17; P \(< 0.01\)) and DM = yes (HR 2.43 95% CI 1.38–4.27; P = 0.02) were independent risk factors of OS. The SII \(\leqslant\) 303.66 (HR 0.40; 95% CI 0.19–0.85; P = 0.02) was an independent protective predictor of OS (Table 4).
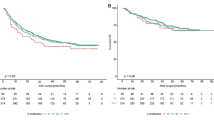
Kaplan–Meier survival analyses showed the relationship between SII and survival results. The patients with an SII of \(> 539.47\)attained poorer PFS (HR = 2.56; 95% CI 1.66–3.94; P \(< \,0.001\)) (Fig. 1) compared with the patients with an SII of \(\leqslant\) 539.47. The patients with an SII of \(> 303.66\) showed a significantly worse OS than those with an SII of \(\leqslant\) 303.66 (HR 2.78; 95% CI 1.80–4.28; P \(< \,0.001)\) (Fig. 2).
The impact of changes in SII before and after immunotherapy on PFS and OS
Kaplan–Meier survival analyses showed that after the first cycle of immunotherapy, the patients with a increased SII attained poorer PFS (HR 1.61; 95% CI 1.10–2.37; P = 0.015) (Fig. 3) and OS (HR 1.76; 95% CI 1.15–2.70; P = 0.009) (Fig. 4) compared with the patients with a decreased SII.
Association between SII and therapeutic response
According to modified Response Evaluation Criteria in Solid Tumors (mRECIST) guidelines, one patient (0.8%) achieved complete response (CR), 43 (36.8%) achieved partial response (PR), 47 (40.2%) achieved stable disease (SD) and 26 (22.2%) achieved progressive disease (PD). The corresponding objective response rate (ORR) was 37.6%. The ORR of the patients whose SII had decreased (47.5%) was higher than patients whose SII had increased (32.5%), but the difference was not statistically significant (P = 0.11). We compared the therapeutic responses of the two groups using the Mann–Whitney test and found that there was a significant difference between the SII-increased patients and the SII-decreased patients (Z = – 2.055, P = 0.04) (Table 5).
Discussion
Globally, liver cancer is one of the most common malignant tumors, with HBV-HCC being the most common type of liver cancer [19]. In recent years, with the rapid development of molecular targeted therapy and immunotherapy, patients’ survival has greatly improved [20]. The strategy of combining targeted drugs with immunotherapy has become one of the most important first-line treatments for advanced HCC. Research on Immune checkpoint inhibitors (ICIs) combined with antiangiogenic drugs has shown promising prospects. Antiangiogenic drugs represented by lenvatinib combined with anti-PD-1/PD-L1 antibodies have shown encouraging ORR in advanced HCC patients. Lenvatinib combined with nivolumab and pembrolizumab achieved ORRs of 54.2% and 36.0%, respectively [10, 21]. The latest Phase III LEAP-002 study further suggests that lenvatinib combined with pembrolizumab provides a survival benefit compared to lenvatinib alone (median OS of 21.2 months vs. 19.0 months) [11]. However, there are significant individual differences between immunotherapy and targeted therapy. Studies have shown that the response to immunotherapy differs between hepatitis B virus-related and non-viral HCC [22]. Therefore, finding reliable predictive biomarkers has always been a major challenge in clinical practice.
HBV-HCC arises in the context of chronic inflammation and within a fibrotic liver and is an inflammation-driven carcinoma [23]. Increasing evidence suggests that inflammatory responses are associated with the efficacy of anti-PD-1 therapy in cancers, and systemic inflammatory markers can be used to determine prognosis [24, 25]. However, the inflammatory biomarkers that best predict prognosis in anti-PD-1 therapy, especially in HBV-HCC patients, remain unclear. Many studies in hematological markers have shown that NLR can reflect inflammation and host immune response, and high NLR level before treatment is a poor prognostic factor for patients with a variety of malignant tumors receiving immunotherapy [26,27,28] (27–29). In HCC, this idea was also confirmed [29]. Although NLR can help to assess the prognosis of immunotherapy, it only integrates the two cell types. SII combines the three key indicators of neutrophil, lymphocyte, and platelet to reflect the complex immune response and inflammatory state. Can it better predict the prognosis of patients? Our study found that the SII outperformed other inflammatory markers regarding prediction. Our findings are consistent with previous studies. Irsan Hasan et al. [30] found that when evaluated for their role as predictors of 1-year survival in untreated patients with advanced HCC, the NLR had a discriminatory ability based on the area under receiving operator curve of 0.667 (95% CI 0.536–0.798; P = 0.044), with the SII of 0.766 (95% CI 0.643–0.889; P = 0.001). SII had superiority in discriminatory ability (p = 0.0415). Yang et al. [31] also reported that an increase in SII with a cutoff value of 300 was negatively associated with OS in HBV-HCC, and in our study, this cutoff value was 303.66. Another study in patients with advanced HCC treated with PD-1 inhibitors found that NLR at six weeks was a predictor of PFS and OS, whereas SII at six weeks was only a predictor of PFS [32]. The study included patients receiving both anti-PD-1 combination therapy and ICI monotherapy, which may lead to differences in the findings. In conclusion, SII is an effective biomarker and results in better survival with low SII compared with high SII in HBV-HCC patients receiving immunotherapy. Although SII can reflect a patient’s response to treatment, few studies have focused on the dynamic changes of SII in HBV-HCC patients. Our study shows for the first time that SII decrease after treatment is significantly associated with prolonged PFS and OS in HBV-HCC patients receiving first-line lenvatinib plus a PD-1 inhibitor.
DM is widely recognized as a risk factor for cancer. Studies have shown that DM increases the risk of liver cancer [33], and HCC patients with DM have a higher risk of tumor progression [34]. The mechanism by which DM leads to the progression of HCC is multifaceted, involving inflammation, insulin resistance, abnormal lipid metabolism, oxidative stress, and other aspects [35]. In addition, in our study, AFP \(\geqslant\)400 was also established as an independent risk factor for PFS and OS, consistent with the results of previous studies [36, 37]. The combination of AFP and SII can better predict the prognosis of such patients.
This study has several limitations. First, it is a small-scale, single-center clinical study with a small sample size, which may lead to selection bias and insufficient representativeness of the research results. Future research should focus on validating SII in larger cohorts of patients, exploring their interactions with other prognostic factors, and elucidating the precise mechanisms underlying their prognostic significance.
Conclusion
In routine clinical practice, SII is a simple and practical blood marker that can help predict the prognosis of HBV-HCC patients treated with first-line lenvatinib plus PD-1 inhibitors.
Data availability
The data are available upon request from the corresponding author.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–108. https://doi.org/10.1002/hep.27406.
Liu Z, Mao X, Jiang Y, Cai N, Jin L, Zhang T, Chen X. Changing trends in the disease burden of primary liver cancer caused by specific etiologies in China. Cancer Med. 2019;8(12):5787–99. https://doi.org/10.1002/cam4.2477.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Prim. 2021;7(1):6. https://doi.org/10.1038/s41572-020-00240-3.
Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, Wang C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20(4):203–22. https://doi.org/10.1038/s41575-022-00704-9.
Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, Kudo M, Breder V, Merle P, Kaseb A, Li D, Mulla S, Verret W, Xu DZ, Hernandez S, Ding B, Liu J, Huang C, Lim HY, Cheng AL, Ducreux M. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(7):991–1001. https://doi.org/10.1016/s1470-2045(21)00151-0.
Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: a review in hepatocellular carcinoma. Drugs. 2019;79(6):665–74. https://doi.org/10.1007/s40265-019-01116-x.
Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y, Matsuki M, Matsuoka Y, Ghosh S, Kitano H, Nomoto K, Matsui J, Funahashi Y. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS ONE. 2019;14(2):0212513. https://doi.org/10.1371/journal.pone.0212513.
Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, Lin J, Zhang J, Zhu W, Jia H, Qin L, Lu L, Chen J. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. 2021;74(5):2544–60. https://doi.org/10.1002/hep.31921.
Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–70. https://doi.org/10.1200/jco.20.00808.
Llovet JM, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, Xu R, Edeline J, Ryoo BY, Ren Z, Masi G, Kwiatkowski M, Lim HY, Kim JH, Breder V, Kumada H, Cheng AL, Galle PR, Kaneko S, Wang A, Mody K, Dutcus C, Dubrovsky L, Siegel AB, Finn RS. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(12):1399–410. https://doi.org/10.1016/s1470-2045(23)00469-2.
Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, Vanella V, Simeone E, Paone M, Palmieri G, Cavalcanti E, Caracò C, Ascierto PA. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6(1):74. https://doi.org/10.1186/s40425-018-0383-1.
Kubo H, Murayama Y, Arita T, Kuriu Y, Nakanishi M, Otsuji E. The prognostic value of preoperative neutrophil-to-lymphocyte ratio in colorectal cancer. World J Surg. 2016;40(11):2796–802. https://doi.org/10.1007/s00268-016-3595-x.
Xu Y, Yuan X, Zhang X, Hu W, Wang Z, Yao L, Zong L. Prognostic value of inflammatory and nutritional markers for hepatocellular carcinoma. Med (Baltim). 2021;100(25):26506. https://doi.org/10.1097/md.0000000000026506.
Wenpei G, Yuan L, Liangbo L, Jingjun M, Bo W, Zhiqiang N, Yijie N, Lixin L. Predictive value of preoperative inflammatory indexes for postoperative early recurrence of hepatitis B-related hepatocellular carcinoma. Front Oncol. 2023;13:1142168. https://doi.org/10.3389/fonc.2023.1142168.
Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O’Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–8. https://doi.org/10.1200/jco.2014.57.9151.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–9. https://doi.org/10.1002/bjs.1800600817.
Kudo M. Newly developed modified ALBI grade shows better prognostic and predictive value for hepatocellular carcinoma. Liver Cancer. 2022;11(1):1–8. https://doi.org/10.1159/000521374.
Chen CH, Huang GT, Yang PM, Chen PJ, Lai MY, Chen DS, Wang JD, Sheu JC. Hepatitis B- and C-related hepatocellular carcinomas yield different clinical features and prognosis. Eur J Cancer. 2006;42(15):2524–9. https://doi.org/10.1016/j.ejca.2006.06.007.
Liu Z, Lin Y, Zhang J, Zhang Y, Li Y, Liu Z, Li Q, Luo M, Liang R, Ye J. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):447. https://doi.org/10.1186/s13046-019-1412-8.
Kudo M, Ikeda M, Motomura K, Okusaka T, Kobayashi M.J.J.o.C.O. A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC). Study 117. 2020;38(4_suppl): 513
Guven DC, Erul E, Sahin TK, Dizdar O, Yalcin S, Sahin IH. The benefit of immunotherapy in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Fut Oncol. 2022. https://doi.org/10.2217/fon-2022-0642.
Cabillic F, Corlu A. Regulation of transdifferentiation and retrodifferentiation by inflammatory cytokines in hepatocellular carcinoma. Gastroenterology. 2016;151(4):607–15. https://doi.org/10.1053/j.gastro.2016.06.052.
Nakamura K, Smyth MJ. Targeting cancer-related inflammation in the era of immunotherapy. Immunol Cell Biol. 2017;95(4):325–32. https://doi.org/10.1038/icb.2016.126.
Hill M, Segovia M, Russo S, Girotti MR, Rabinovich GA. The paradoxical roles of inflammation during PD-1 blockade in cancer. Trends Immunol. 2020;41(11):982–93. https://doi.org/10.1016/j.it.2020.09.003.
Nakamoto S, Ohtani Y, Sakamoto I, Hosoda A, Ihara A, Naitoh T. Systemic immune-inflammation index predicts tumor recurrence after radical resection for colorectal cancer. Tohoku J Exp Med. 2023;261(3):229–38. https://doi.org/10.1620/tjem.2023.J074.
Ng SP, Bahig H, Jethanandani A, Sturgis EM, Johnson FM, Elgohari B, Gunn GB, Ferrarotto R, Phan J, Rosenthal DI, Frank SJ, Fuller CD, Garden AS. Prognostic significance of pre-treatment neutrophil-to-lymphocyte ratio (NLR) in patients with oropharyngeal cancer treated with radiotherapy. Br J Cancer. 2021;124(3):628–33. https://doi.org/10.1038/s41416-020-01106-x.
Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18(1):360. https://doi.org/10.1186/s12916-020-01817-1.
Mouchli M, Reddy S, Gerrard M, Boardman L, Rubio M. Usefulness of neutrophil-to-lymphocyte ratio (NLR) as a prognostic predictor after treatment of hepatocellular carcinoma. Review article. Ann Hepatol. 2021;22: 100249. https://doi.org/10.1016/j.aohep.2020.08.067.
Hasan I, Lutfie L, Rinaldi I, Kurniawan J, Loho IM. Comparison between neutrophil-lymphocyte ratio and systemic immune-inflammation index as predictors of one-year survival in patients with untreated advanced hepatocellular carcinoma. J Gastrointest Cancer. 2023;54(1):135–46. https://doi.org/10.1007/s12029-021-00796-7.
Yang Z, Zhang J, Lu Y, Xu Q, Tang B, Wang Q, Zhang W, Chen S, Lu L, Chen X. Aspartate aminotransferase-lymphocyte ratio index and systemic immune-inflammation index predict overall survival in HBV-related hepatocellular carcinoma patients after transcatheter arterial chemoembolization. Oncotarget. 2015;6(40): 43090–43098. https://doi.org/10.18632/oncotarget.5719
Jia G, Qiu L, Zheng H, Qin B, Sun Z, Shao Y, Yang Z, Shao J, Zhou Y, Jiao S. Nomogram for predicting survival in patients with advanced hepatocellular carcinoma treated with PD-1 inhibitors: incorporating pre-treatment and post-treatment clinical parameters. BMC Cancer. 2023;23(1):556. https://doi.org/10.1186/s12885-023-11064-1.
Li X, Wang X, Gao P. Diabetes mellitus and risk of hepatocellular carcinoma. Biomed Res Int. 2017;2017:5202684. https://doi.org/10.1155/2017/5202684.
Wang YG, Wang P, Wang B, Fu ZJ, Zhao WJ, Yan SL. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: a systematic review and meta-analysis. PLoS ONE. 2014;9(5):95485. https://doi.org/10.1371/journal.pone.0095485.
Zhang C, Liu S, Yang M. Hepatocellular carcinoma and obesity, type 2 diabetes mellitus, cardiovascular disease: causing factors, molecular links, and treatment options. Front Endocrinol (Lausanne). 2021;12: 808526. https://doi.org/10.3389/fendo.2021.808526
Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, Schelman WR, Chintharlapalli S, Abada PB, Sherman M, Zhu AX. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39(12):2214–29. https://doi.org/10.1111/liv.14223.
Chan MY, She WH, Dai WC, Tsang SHY, Chok KSH, Chan ACY, Fung J, Lo CM, Cheung TT. Prognostic value of preoperative alpha-fetoprotein (AFP) level in patients receiving curative hepatectomy—an analysis of 1182 patients in Hong Kong. Transl Gastroenterol Hepatol. 2019;4:52. https://doi.org/10.21037/tgh.2019.06.07
Funding
This work was supported by the Capital Health Research and Development of Special Fund (No. shoufa, 2022-2-2186) and the "Sailing" Program Medical Capacity Improvement project of Beijing Hospital Management Center (No: ZLRK202332).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was approved by the Ethical Committee of the Beijing Youan Hospital Capital Medicine University (approval LL-2022-060-K).
Informed consent
Patient consent was waived, as approved by the Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, Y., Zhang, M., Liu, D. et al. Changes in systemic immune-inflammation index (SII) predict the prognosis of patients with hepatitis B-related hepatocellular carcinoma treated with lenvatinib plus PD-1 inhibitors. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03596-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03596-0