Abstract
Background
Deregulating cellular metabolism is one of the prominent hallmarks of malignancy, with a critical role in tumor survival and growth. However, the role of reprogramming aspartate metabolism in hepatocellular carcinoma (HCC) are largely unknown.
Methods
The multi-omics data of HCC patients were downloaded from public databases. Univariate and multivariate stepwise Cox regression were used to establish an aspartate metabolism-related gene signature (AMGS) in HCC. The Kaplan–Meier and receiver operating characteristic curve analyses were performed to evaluate the predictive ability for overall survival (OS) in HCC patients. Gene set enrichment analysis and immune infiltration analysis were operated to determine the potential mechanisms underlying the AMGS. Single-cell RNA sequencing (scRNA-seq) data of liver cancer stem cells were visualized by t-SNE algorithm. In vivo and in vitro experiments were implemented to investigate the biological function of CAD in HCC. In addition, a nomogram based on the AMGS and clinicopathologic characteristics was constructed by univariate and multivariate Cox regression analyses.
Results
Patients in the high-AMGS subgroup exerted advanced tumor status and poor prognosis. Mechanistically, the high-AMGS subgroup patients had significantly enhanced proliferation and stemness-related pathways, increased infiltration of regulatory T cells and upregulated expression levels of suppressive immune checkpoints in the tumor immune microenvironment. Notably, scRNA-seq data revealed CAD, one of the aspartate metabolism-related gene, is significantly upregulated in liver cancer stem cells. Silencing CAD inhibited proliferative capacity and stemness properties of HCC cells in vitro and in vivo. Finally, a novel nomogram based on the AMGS showed an accurate prediction in HCC patients.
Conclusions
The AMGS represents a promising prognostic value for HCC patients, providing a perspective for finding novel biomarkers and therapeutic targets for HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary liver cancer (PLC), which refers to malignant tumors originated in the liver tissue, is the sixth most common malignancy with the third-highest mortality rate worldwide [1], and approximately 90% of all PLC cases are of the pathological type of hepatocellular carcinoma (HCC). Despite recent advancements in targeted therapy and immunotherapy, the lack of effective long-term treatment still limits the overall survival (OS) of patients with advanced HCC [2]. There are many causes of HCC, including viral hepatitis (primarily HBV/HCV), metabolic disorders, alcoholic hepatitis, and non-alcoholic steatohepatitis (NASH) [3], which implies that the molecular mechanisms of hepatocarcinogenesis are sophisticated. Thus, an in-depth study of the exact molecular mechanisms regulating HCC will facilitate the discovery of new therapeutic approaches for HCC, which is of great scientific and clinical significance.
Deregulating cellular metabolism, also known as reprogramming cellular metabolism, has been identified as one of the hallmarks of cancer [4]. To obtain the energy and substances needed for cell survival and growth, tumor cells alter their metabolic patterns, including glucose, fatty acid and amino acid metabolism [5]. Aspartate is a non-essential amino acid, generated predominantly from glutamate and oxaloacetate by aspartate aminotransferase enzymes [6, 7]. As a precursor substance, aspartate is involved in the synthesis of asparagine and arginine in cells [8]. In addition to protein synthesis, aspartate is the major substrate for de novo purine and pyrimidine synthesis [9, 10]. Furthermore, aspartate metabolism has also been reported to be implicated in the production of NADPH for the elimination of reactive oxygen species [11], maintaining cellular redox homeostasis. Recently, increasing evidence demonstrated the essential role of aspartate in cell growth and survival, and many types of tumors exhibit an aspartate-dependency [12]. However, the role of aspartate metabolism in HCC have not been fully elucidated.
In the present study, we screened and verified the expression levels of aspartate metabolism-related genes and their association with OS in HCC patients of The Cancer Genome Atlas (TCGA) and the International Cancer Genome Consortium (ICGC). These genes were then used to construct an aspartate metabolism-related gene signature (AMGS) which we confirmed can effectively predict prognostic risk for HCC patients. The AMGS-associated biological processes, pathways and immune infiltration status were investigated. We further focused on CAD, one component of the AMGS, and explored its function in HCC. Furthermore, a novel nomogram combining the AMGS and clinicopathologic characteristics is developed to prognosticate the OS of HCC patients. Our study indicates that aspartate metabolism-related genes, especially the AMGS, have a close association with the outcome of HCC patients and stemness properties, providing potential prognostic biomarkers and therapeutic targets for HCC.
Methods
Data acquisition
Bulk mRNA sequencing data {normalized by log2 [fragment per kilobase million (FPKM) + 1]} were obtained from the TCGA and ICGC databases, along with corresponding clinicopathological information. A training cohort of three hundred seventy HCC patients from the TCGA and a validation cohort of two hundred thirty-one HCC patients from the ICGC were included in this study, and clinicopathologic information for each cohort is shown in Supplementary Table 1. Twenty-two aspartate metabolism-related genes were filtered from the KEGG database (hsa00250 Alanine, aspartate and glutamate metabolism), and protein–protein interaction analysis of these aspartate metabolism-related genes was conducted using the STRING online database [13]. The mass spectrometry data were downloaded from the CPTAC, and immunohistochemistry images were obtained from the HPA. Single-cell sequencing (scRNA-seq) data (GSE103866) [14] were downloaded from the Gene Expression Omnibus (GEO) DataSets.
Consensus cluster
The R package ConsensuClusterPlus [15] was utilized to conduct unsupervised clustering analysis based on the expression of 22 aspartate metabolism-related genes from patients in the TCGA cohort, which was repeated 1000 iterations. Cumulative distribution function (CDF) and delta area curves were analyzed for category number k from 2 to 9 in the consensus clustering model to determine the clustering number. PCA was then conducted to identify the distribution. K-M curve analysis with the log-rank test was used to compare the OS between the clusters.
Construction of a prognostic signature
First, the association between the expression of 22 aspartate metabolism-related genes and OS in HCC patients of the TCGA-LIHC cohort was analyzed using univariate Cox regression. Then, genes with p value <0.05 were selected for further multivariate Cox stepwise regression with the Akaike information criterion method, implemented by R package survival. Finally, the formula for calculating the AMGS risk score was as follows:
where xi is the expression level [log2 (FPKM + 1)] of each aspartate metabolism-related gene and Coefi is the corresponding coefficient obtained from the Cox regression. The AMGS scores for HCC patients were calculated with the median score serving as the cut-off value for classifying patients into the high-AMGS and low-AMGS subgroups. R package timeROC [16] were utilized to assess the prediction accuracy of survival of HCC patients. The ICGC cohort further validated the reliability of the AMGS.
Functional analysis
DEGs with {|Fold change (FC)| > 1 and adjusted p value <0.05} were determined utilizing R package limma [17]. These DEGs were then subjected to Gene Ontology (GO) enrichment analysis, and upregulated or downregulated DEGs in the high-AMGS subgroup were also subjected to KEGG analysis using Metascape tool. Gene set enrichment analysis (GSEA) was employed to investigate hallmark gene sets enrichment [18].
Tumor immunity analysis
The infiltration levels of immune cells in HCC patients were evaluated by the CIBERSORT algorithm [19]. Correlations between infiltration level of T regulatory cells and the AMGS, as well as the expression level of CAD, ASPA and GOT2, were calculated, respectively. Furthermore, violin plots were used to present expression levels of immune checkpoints.
scRNA-seq analysis
The SMART-seq data (GSE103866) [14] for liver Cancer stem cells (CSCs) including 43 huH-1 cells, 55 HuH-7 cells and 20 primary HCC cells were processed utilizing R package Seurat [20]. The t-SNE algorithm was used to visualize cell distribution. Violin plot was applied to compare the expression levels of CAD, ASPA and GOT2 between cells with triple positive or negative CSCs markers.
In vitro experiments
The human HCC cell line HuH-7 cells were cultured in DMEM supplemented with 10% FBS. siRNAs for CAD and negative control were transfected into HuH-7 cells with Lipofectamine RNAiMAX reagent. CCK-8 and EdU Imaging Kits were applied according to the manufacturer’s protocol to assess cell viability and proliferation. Additionally, HuH-7 cells were seeded in 6-well plates for colony formation assay and in 96-well ultra-low attachment plates for sphere formation assay.
In vivo experiments
For the subcutaneous tumor model, 6-week-old male BALB/c nude mice were randomized into two groups. 1 × 106 HuH-7 cells transinfected with sh-CAD or sh-NC were injected subcutaneously into BALB/c nude mice. After 4 weeks, the mice were euthanized. The tumors were fixed in 4% paraformaldehyde and embedded in paraffin. Subsequent immunohistochemical analysis was performed following our previously published procedure [21]. The animal experiment was approved by the Animal Ethics Committee of Sun Yat-sen University.
qRT-PCR and Western blot
qRT-PCR and Western blot were used to evaluate expression levels of CAD and CD133, respectively, with GAPDH as a reference. The experimental procedure is in accordance with our published article [22]. Reagents and antibodies were listed in Supplementary Table 2.
Nomogram establishment
Independent risk factors for the OS of HCC patients were identified by univariate and multivariate Cox regression analyses and then integrated to establish a nomogram. Calibration curves were generated using the R package rms, while ROC curves were plotted using the R package timeROC.
Statistical analysis
Bioinformatic analyses in the present study were carried out utilizing R software (version 4.1.0). All experiments were conducted in independent triplicate, and the data were presented as means ± SD and analyzed by GraphPad Prism software (version 9.4.1) with Student’s t test. A p value <0.05 was considered statistically significant.
Results
Aspartate metabolism-related genes is associated with the prognosis of HCC patients
We screened the KEGG pathway map00250 to obtain aspartate metabolism-related genes, which consisting of ADSL, ADSS, ADSSL1, AGXT, AGXT2, ASL, ASNS, ASPA, ASRGL1, ASS1, CAD, DDO, FOLH1, GOT1, GOT2, GPT, GPT2, IL4I1, NAT8L, NIT2, RIMKLA and RIMKLB. The protein–protein interaction analysis showed a regulatory network of all 22 genes (Fig. 1a). Then, we applied consensus clustering analysis to explore the association between the aspartate metabolism-related genes and subtypes of HCC. Based upon cumulative distribution function (CDF) value and delta area plot, 370 HCC patients from TCGA were classified into two distinct clusters, which was validated by principal component analysis (PCA) (Fig. 1b–e). We found that patients from cluster 1 showed a longer OS than those from cluster 2 (p < 0.001, Fig. 1f). These results indicated a prognostic value of aspartate metabolism-related genes in HCC.
Identification and prognosis of AMGS subtypes in HCC patients. a The protein–protein interaction analysis of 22 aspartate metabolism-related genes. b Cumulative distribution function (CDF) for category number k ranging from 2 to 9 in the consensus clustering model. c Delta area curves for each category number k. d The heatmap of consensus clustering matrix corresponding to k = 2. e PCA of the two clusters of HCC patients. f K-M curve analysis of the OS in the two clusters of HCC patients
Construction of the AMGS of HCC
To further investigate the predictive value of aspartate metabolism-related genes, we first applied univariate Cox regression and found twelve genes associated with the OS of HCC patients in the TCGA cohort (Table 1). Multivariate analysis with stepwise Cox regression identified three genes (CAD, ASPA and GOT2) to construct the AMGS. In comparison to normal liver tissue, the CAD expression was increased in HCC tissue and ASPA and GOT2 expression were decreased (Fig. 2a–c). Accordingly, high expression level of CAD and low expression levels of ASPA and GOT2 associated with a poor OS of HCC patients (Fig. 2d–f).
Construction of the AMGS to predict HCC patient’s prognosis in the TCGA cohort. a–c Comparison of expression levels of CAD, ASPA and GOT2 between normal liver and HCC tissues. d–f K–M curves for OS of patients with high vs. low expression levels of CAD, ASPA and GOT2. g Distribution of the risk score for each patient. h Distribution of the OS for each patient. i PCA of the two subgroups based on high and low risk scores. j K–M curves of the OS of patients in high-AMGS and low-AMGS subgroups. k Receiver operating characteristic (ROC) curves for predicting 1-, 3-, and 5-year OS in HCC patients based on the AMGS. l Heatmap for CAD, ASPA and GOT2 expression, clinicopathological features and the AMGS scores of HCC patients. (****p < 0.0001)
The AMGS was calculated based on CAD, ASPA and GOT2 expression levels and arithmetic coefficients: AMGS score = (0.560479161 × CAD expression) + (−0.35523779 × ASPA expression) + (−0.347769723 × GOT2 expression). According to the median of the AMGS score, HCC patients were categorized into high-AMGS and low-AMGS subgroups (Table 2). The AMGS score and survival status of each patient were shown in Fig. 2g and h. PCA confirmed that the patients of the high-AMGS and low-AMGS subgroups were distinctly clustered (Fig. 2i). Kaplan–Meier (K-M) curve analysis revealed that patients in the high-AMGS subgroup exerted a significantly shorter OS compared with those in the low-AMGS subgroup (Fig. 2j). The area under the curve (AUC) values of the AMGS for predicting 1-, 3-, and 5-year survival reached 0.727, 0.673, and 0.688, respectively (Fig. 2k). We ranked HCC patients from the TCGA database based on AMGS risk scores (Fig. 2l). The heatmap confirmed that the expression level of CAD was increased whereas those of ASPA and GOT2 were decreased in the high-AMGS subgroup (Fig. 2l). Additionally, the AMGS score was positively correlated with higher TNM stage, WHO grade and AFP value (Fig. 2l). In addition, the AMGS has been independently validated in the ICGC cohort. The results also showed a high prognostic predictive value of AMGS (Fig. 3).
Validation of the AMGS in the ICGC cohort. a Distribution of the risk score for each patient. b Distribution of the OS for each patient. c PCA of the two subgroups based on high and low risk scores. d K-M curves of the OS of patients in high-AMGS and low-AMGS subgroups. r ROC curves for predicting 1-, 2-, and 3-year OS in HCC patients based on the AMGS
AMGS-associated biological processes and pathways in HCC
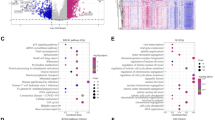
To further investigate potential biological functions and mechanisms of the AMGS in HCC, differential expression gene (DEG) analysis was conducted between the high-AMGS and low-AMGS subgroups of the TCGA cohort. Compared with the low-AMGS subgroup, the high-AMGS subgroup exhibited upregulation of 55 DEGs and downregulation of 176 DEGs, as depicted in Supplementary Fig. 1. Further analysis of these DEGs was conducted through Metascape, which demonstrated a significant enrichment in several biological processes involving amino acid metabolism, lipid catabolism and other organic compound catabolic reactions (Fig. 4a). Moreover, these DEGs of different processes formed a regulatory network (Fig. 4b), suggesting a potential interaction between aspartate metabolism and lipid metabolism in reprogramming the metabolism of HCC. KEGG enrichment analysis of these DEGs is illustrated in Fig. 4c and d. GESA results further demonstrated that several oncogenic hallmarks including PI3K/AKT/mTOR and mTORC1 signaling, proliferation-related pathways including MYC and E2F targets, stemness-related pathways including Notch, Wnt/β-catenin and Hedgehog signaling, along with TGF-β signaling were prominently enriched in the high-AMGS subgroup (Fig. 4e). Together, these results reveal potential biological processes and pathways related to the AMGS in HCC.
Enrichment of biological function and pathway in the two subgroups. a The GO enriched terms for DEGs, colored by p value. b Network of the GO enriched terms, colored by cluster. c Bubble chart of the KEGG enriched terms for unregulated DEGs in the high-AMGS subgroup. d Bubble chart of the KEGG enriched terms for downregulated DEGs in the high-AMGS subgroup. e The GSEA enriched hallmarks in the high-AMGS subgroup
Relationship between the AMGS and tumor immune microenvironment in HCC
TGF-β signaling is widely believed to exert regulatory effects on immune cells within the tumor immune microenvironment (TIME), which induces immune tolerance or evasion during tumor progression [23]. To investigate whether AMGS influence the infiltration degree of miscellaneous immune cells, the CIBERSORT algorithm was performed in high-AMGS and low-AMGS subgroups. Our analyses indicated that the high-AMGS subgroup of both TCGA and ICGC cohorts showed significant higher infiltration of Tregs, M0 macrophages, and activated myeloid dendritic cells (Fig. 5a, Supplementary Fig. 2). Further analyses revealed a positive correlation between Treg infiltration and the AMGS score as well as expression level of CAD, but negative correlations with expression levels of ASPA and GOT2 (Fig. 5b–e). Furthermore, we assessed the differential expression of several immune checkpoint genes between the two subgroups, and found that PDCD1, CTLA-4, TIGIT, VISTA, TIM-3 and HMGB1 were markedly upregulated in the high-AMGS subgroup (Fig. 5f). Altogether, our results reveal that HCC patients in the high-AMGS subgroup prone to evolve suppressive TIME.
Landscape of the TIME in the two subgroups in the TCGA cohort. a Box plots of the fraction of infiltrated immune cells in the two subgroups, estimated by the CIBERSORT algorithm. b Correlation analysis of the AMGS risk score and infiltration of Tregs. c–e Correlation analysis of expression levels of CAD, APSA and GOT2 and infiltration of Tregs. f Comparison of expression levels of immune checkpoint genes between the two subgroups. (ns not significant. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001)
Proteomic Analysis and ScRNA-seq Data of CAD, ASPA and GOT2 in HCC
We obtained proteomic data of HCC and non-tumor liver tissues from the Clinical Proteomic Tumor Analysis Consortium (CPTAC) database. As shown in Fig. 6a–c, the CAD expression is higher in HCC tissues and the ASPA and GOT2 expression were higher in non-tumor liver tissue. Moreover, consistent dysregulated expression of CAD, ASPA and GOT2 were further validated by IHC staining from the Human Protein Atlas (HPA) database (Fig. 6d). Given the active stemness-related pathways in the high-AMGS subgroup, we further analyzed a scRNA-seq dataset (GSE103866) containing cancer stem cell (CSC) marker positive liver CSCs (CD133 + EpCAM + CD24+) and CSC marker negative HCC cells (CD133-EpCAM-CD24-) [14]. The results showed that CAD, ASPA and GOT2 were expressed in the samples of this dataset (Fig. 6e–h). Further analysis showed that CAD was significantly upregulated in liver CSCs in comparison with non-stem HCC cells, while there was no significant difference between two cells in the expression levels of ASPA and GOT2 (Fig. 6i–k). These data suggested that CAD is a potential oncogenic gene in HCC that is associated with stemness properties.
Upregulation of CAD in liver CSCs. a–c Comparison of expression levels of CAD, ASPA and GOT2 between HCC and non-tumor liver tissues in the CPTAC cohort. d Representative IHC images of CAD, ASPA and GOT2 in normal liver and HCC tissues from the HPA database. e t-Distributed Stochastic Neighbor Embedding (tSNE) plot of cancer cells from huH-1, HuH-7 and patient-derived primary HCC cells. f–h tSNE plots showing the expression level of CAD, ASPA and GOT2 in all cells. i–k Comparison of expression levels of CAD, ASPA and GOT2 between the CSCs marker triple negative (TN) and positive (TP) cells. (ns, not significant. **p < 0.01; ****p < 0.0001)
Knockdown of CAD attenuated cell proliferation and stemness properties
We then implemented CAD knockdown to investigate exact functions of CAD in HCC (Fig. 7a). CCK-8 and colony formation assays demonstrated that transfection of CAD siRNAs significantly suppressed HuH-7 cells proliferation (Fig. 7b–d), which was further verified by EdU assay (Fig. 7e and f). Silencing CAD significantly attenuated stem cell sphere formation of HuH-7 cells (Fig. 7g–h). Moreover, Western blot indicated that the expression of CD133 was decreased when CAD was silenced (Fig. 7i). To further investigate the oncogenic effect of CAD in HCC, subcutaneous tumor models in nude mice were generated with stably silenced CAD or control HuH-7 cells (Fig. 8a). Knockdown of CAD significantly inhibited HCC tumor growth (Fig. 8b and c). Immunohistochemical staining showed that the percentage of Ki67-positive cells was reduced in the sh-CAD group compared to sh-NC group (Fig. 8d and e). Together, the data illustrate the significant role of CAD in promoting cell proliferation and stemness properties in HCC.
CAD silencing inhibits cell proliferation and sphere formation in HCC cells. a qRT-PCR analysis of CAD expression in HuH-7 cells transfected with CAD and negative control (NC) siRNAs. b Cell viability assessed by the CCK-8 assay in HuH-7 cells with indicated treatment. c–d Representative images and quantification of colony formation assay in HuH-7 cells with indicated treatment. e–f Representative images and quantification of EdU assay in HuH-7 cells with indicated treatment. g–h Representative images and quantification of sphere formation assay in HuH-7 cells with indicated treatment. i Western blot analysis determined expression levels of CAD and CD133 in HuH-7 cells with indicated treatment. (*p < 0.05; **p < 0.01; ***p < 0.001)
CAD silencing inhibits HCC growth in vivo. a and b Images of the subcutaneous tumor models of HuH-7 cells transfected with sh-NC or sh-CAD. c Tumor weights of subcutaneous tumors of indicated groups. e and f Representative images and quantification of Ki-67 staining of indicated groups. (*p < 0.05; **p < 0.01)
Establishment and validation of the AMGS-based nomogram for HCC
To ascertain whether the AMGS could function as an independent prognostic factor, univariate and multivariate analyses were implemented on the AMGS and other clinicopathologic characteristics in both the TCGA and ICGC cohorts. Impressively, the results showed that the AMGS and TNM stage were independent prognostic factors in both cohorts (Figs. 9a and b and 10a and b), underscoring their clinical significance. We then selected the AMGS and TNM stage to construct an AMGS-based nomogram in the TCGA cohort (Fig. 9c). Gratifyingly, calibration curves validated the reliability and precision of the nomogram’s predicted rates for 1-, 2-, 3-, and 5-year survival in the TCGA cohort (Fig. 9d–g). Moreover, the AUC values of the AMGS-based nomogram reached 0.756, 0.708,0.745 and 0.722 at 1-, 2-, 3- and 5-year intervals, surpassing that of the AMGS and individual clinical predictors (Fig. 9h–k). Additionally, the improved prediction accuracy and efficiency of the AMGS-based nomogram were further confirmed in the ICGC cohort (Fig. 10c–h). Collectively, these results demonstrate that the AMGS-based nomogram represents an accurate and robust predictive ability for the OS of HCC patients, highlighting its practicality and translational value.
Development and evaluation of the AMGS-based nomogram for HCC in the TCGA cohort. a–b Forest plots of univariate and multivariate Cox regression analyses of the AMGS and clinicopathologic characteristics in terms of the OS. c The AMGS-based nomogram combining the TNM stage and AMGS for predicting the OS of HCC patients. d–g Calibration curves of the nomogram for 1-, 2-, 3-, and 5-year predicted probabilities of the OS. h–k ROC curves for predicting 1-, 2-, 3-, and 5-year survival of HCC patients based on the nomogram, the AMGS and TNM stage, respectively
Validation of the AMGS-based nomogram for HCC in the ICGC cohort. a–b Forest plots of univariate and multivariate Cox regression analyses of the AMGS and clinicopathologic characteristics in terms of the OS. c–e Calibration curves of the nomogram for 1-, 2-, and 3-year predicted probabilities of the OS. f–h ROC curves for predicting 1-, 2-, and 3-year survival of HCC patients based on the nomogram, the AMGS and TNM stage, respectively
Discussion
Deregulating cellular metabolism has been recognized as an essential biological hallmark of tumors and aspartate metabolism reprogramming exerts a prominent role in regulating tumor cell survival and proliferation [12]. However, the role of aspartate metabolism in HCC remains to be elucidated. In this study, we found that the expression of aspartate metabolism-related genes was associated with patient survival in two independent HCC cohorts. CAD, ASPA and GOT2 were screened to construct a novel prognostic signature, which could classify HCC patients into the high-AMGS and low-AMGS subgroups. Proliferation and stemness-related pathways and TGF-β signaling were activated, along with Tregs infiltration was increased in the TIME in the high-AMGS subgroup. ScRNA-seq analysis demonstrated that CAD was upregulated in liver CSCs, and further in vitro experiments confirmed that silencing CAD inhibited proliferation and stemness properties of HCC cells. Moreover, a novel nomogram incorporating the AMGS with clinicopathological characteristics was established and showed effective predictive ability for the OS in HCC patients. Our study reveals a critical role of aspartate metabolism-related genes in HCC, which highlights that targeting aspartate metabolism could be a promising therapeutic approach for HCC.
Aspartate, an amino acid crucial for tumor cell survival and proliferation, is therefore an attractive target for cancer therapy [24]. In light of this, exploration of dysregulated genes underlying aspartate metabolism may pave the way for the identification of new biomarkers and therapeutic approaches for HCC. Herein, we demonstrated the dysregulation of aspartate metabolism-related genes in HCC and developed a novel AMGS, composed of three genes CAD, ASPA and GOT2, that demonstrated strong predictive power for HCC prognosis. Our findings corroborate with previous reports [25, 26] that CAD was upregulated in HCC and associated with poor prognosis, while the expression level of GOT2 was reduced and associated with a favorable prognosis. Despite recent evidence linking ASPA expression with tumor progression [27, 28], its role in HCC remains largely unsuspected. Our study revealed that the expression level of ASPA was downregulated in HCC and associated with good patient prognosis. Therefore, our study suggests that CAD, GOT2, and ASPA, as important members of the AMGS, play crucial roles in the development and progression of HCC and warrant further in-depth research.
Malignant tumors are cellularly heterogeneous and cells of different differentiation have specific biological functions [29]. CSCs belong to a special category of tumor cells with self-renewal capacity and differentiation potential, and are generally recognized as playing a critical role in tumorigenesis, treatment sensitivity, metastasis and recurrence [30]. Liver CSCs exhibit a more proliferative phenotype and tumorigenicity and are linked to early recurrence and shorter survival time in HCC patients [31]. Previous studies indicated that dysregulation of glucose metabolism and cholesterol biosynthesis maintains the stemness properties of liver CSCs [32, 33], but it remains unknown whether reprogramming of aspartate metabolism regulates the stemness properties of HCC. In this study, we found stemness-related pathways, including Notch, Hedgehog and Wnt/β-catenin signaling, were active in the high-AMGS subgroup. Moreover, single-cell transcriptomics analysis revealed that in comparison with non-stem HCC cells, CAD was upregulated in liver CSCs. Previous studies suggested that CAD serves as a key regulator of glioblastoma stem cells [34], and aspartate accumulation could activate CAD signaling [10]. Importantly, we found that silencing CAD inhibited the spheroid formation and proliferation ability of HCC cells. Our findings provide evidence demonstrating that aspartate metabolism-related genes are associated with stemness-related pathways in liver CSCs, and targeting CAD could be a potential therapeutic approach for HCC.
Immune checkpoint inhibitors (ICIs) have shown significant efficacy in extending survival time of patients with advanced HCC [35], but only a small subset of patients respond positively to this treatment [36, 37]. One possible reason for this is that HCC has a suppressive TIME with increased infiltration of immunosuppressive cells such as Tregs and upregulated expression of multiple immune checkpoint molecules [38]. Aberrant tumor metabolism shapes the suppressive TIME [39], but the role of aspartate metabolism in this process is largely unknown. Herein, we found that high AMGS scores and high CAD expression were positively correlated with more Tregs infiltration, while low ASPA and GOT2 expression were linked to increased Tregs infiltration in HCC. Previous studies have revealed that increased intratumoral infiltration of Tregs was associated with unfavorable survival in HCC patients [40, 41], which partially explains the shorter patient OS in the high-AMGS subgroup. Additionally, we observed upregulation of immune checkpoint molecules, such as PDCD1, CTLA4, TIGIT and TIM3, in the high-AMGS subgroup, which have been implicated in poor response to ICIs and unfavorable patient prognosis in HCC [42,43,44,45]. Our findings suggest the potential association between aspartate metabolism-related genes and the suppressive TIME of HCC, providing a novel perspective for investigating mechanisms of aspartate metabolic reprogramming in molding the TIME of HCC.
Conclusions
In conclusion, our study presents a novel AMGS that effectively predicts the prognosis of HCC patients. Bulk mRNA-seq and scRNA-seq analyses suggest a potential association between aspartate metabolism-related genes and the activation of stemness-related pathways, shaping of the suppressive immune microenvironment. In addition, CAD knockdown inhibits the proliferation and stemness properties of HCC cells. Our study provides a clue for the identification of novel biomarkers and therapeutic targets for HCC.
Data availability
The datasets used in this study can be downloaded in the TCGA (https://gdc.cancer.gov/), ICGC (https://dcc.icgc.org/projects/), GEO (https://www.ncbi.nlm.nih.gov/gds/), CPTAC (https://proteomic.datacommons.cancer.gov/pdc/) and KEGG database (https://www.kegg.jp/).
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- AMGS:
-
Aspartate metabolism-related gene signature
- TCGA:
-
The cancer genome atlas
- ICGC:
-
International cancer genome consortium
- GEO:
-
Gene expression omnibus
- scRNA-seq:
-
Single-cell sequencing
- CSCs:
-
Cancer stem cells
- TIME:
-
Tumor immune microenvironment
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca: A Cancer J Clinicians. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660
Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022. https://doi.org/10.1038/s41575-022-00704-9.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nature reviews. Dis Primers. 2021;7(1):6. https://doi.org/10.1038/s41572-020-00240-3
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. https://doi.org/10.1158/2159-8290.CD-21-1059.
Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21(10):669–80. https://doi.org/10.1038/s41568-021-00378-6.
Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. 2015;162(3):540–51. https://doi.org/10.1016/j.cell.2015.07.016.
Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell. 2015;162(3):552–63. https://doi.org/10.1016/j.cell.2015.07.017.
Choi B, Coloff JL. The diverse functions of non-essential amino acids in cancer. Cancers (Basel). 2019;11(5):675. https://doi.org/10.3390/cancers11050675.
De Falco P, Lazzarino G, Felice F, Desideri E, Castelli S, Salvatori I, et al. Hindering nat8l expression in hepatocellular carcinoma increases cytosolic aspartate delivery that fosters pentose phosphate pathway and purine biosynthesis promoting cell proliferation. Redox Biol. 2023;59:102585. https://doi.org/10.1016/j.redox.2022.102585.
Rabinovich S, Adler L, Yizhak K, Sarver A, Silberman A, Agron S, et al. Diversion of aspartate in ass1-deficient tumours fosters de novo pyrimidine synthesis. Nature. 2015;527(7578):379–83. https://doi.org/10.1038/nature15529.
Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, et al. Glutamine supports pancreatic cancer growth through a kras-regulated metabolic pathway. Nature. 2013;496(7443):101–5. https://doi.org/10.1038/nature12040.
Helenius IT, Madala HR, Yeh JJ. An asp to strike out cancer? Therapeutic possibilities arising from aspartate’s emerging roles in cell proliferation and survival. Biomolecules. 2021;11(11):1666. https://doi.org/10.3390/biom11111666.
Mering CV. String: a database of predicted functional associations between proteins. Nucleic Acids Res. 2003;31(1):258–61. https://doi.org/10.1093/nar/gkg034.
Zheng H, Pomyen Y, Hernandez MO, Li C, Livak F, Tang W, et al. Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology. 2018;68(1):127–40. https://doi.org/10.1002/hep.29778.
Wilkerson MD, Hayes DN. Consensusclusterplus: a class discovery tool with confidence assessments and item tracking. Bioinformatics. 2010;26(12):1572–3. https://doi.org/10.1093/bioinformatics/btq170.
Blanche P, Dartigues J, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med. 2013;32(30):5381–97. https://doi.org/10.1002/sim.5958.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for rna-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7): e47. https://doi.org/10.1093/nar/gkv007.
Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database hallmark gene set collection. Cell Syst. 2015;1(6):417–25. https://doi.org/10.1016/j.cels.2015.12.004.
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–7. https://doi.org/10.1038/nmeth.3337.
Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36(5):411–20. https://doi.org/10.1038/nbt.4096.
He X, Peng Y, He G, Ye H, Liu L, Zhou Q, et al. Increased co-expression of pd1 and tim3 is associated with poor prognosis and immune microenvironment heterogeneity in gallbladder cancer. J Transl Med. 2023;21(1):717. https://doi.org/10.1186/s12967-023-04589-3.
Zhou Q, Lin J, Yan Y, Meng S, Liao H, Chen R, et al. Inpp5f translocates into cytoplasm and interacts with asph to promote tumor growth in hepatocellular carcinoma. J Exp Clin Cancer Res. 2022;41(1). https://doi.org/10.1186/s13046-021-02216-x
Tauriello DVF, Sancho E, Batlle E. Overcoming tgfβ-mediated immune evasion in cancer. Nat Rev Cancer. 2022;22(1):25–44. https://doi.org/10.1038/s41568-021-00413-6.
Gorgoglione R, Impedovo V, Riley CL, Fratantonio D, Tiziani S, Palmieri L, et al. Glutamine-derived aspartate biosynthesis in cancer cells: role of mitochondrial transporters and new therapeutic perspectives. Cancers (Basel). 2022;14(1):245. https://doi.org/10.3390/cancers14010245.
Ridder DA, Schindeldecker M, Weinmann A, Berndt K, Urbansky L, Witzel HR, et al. Key enzymes in pyrimidine synthesis, cad and cps1, predict prognosis in hepatocellular carcinoma. Cancers (Basel). 2021;13(4):744. https://doi.org/10.3390/cancers13040744.
Li Y, Li B, Xu Y, Qian L, Xu T, Meng G, et al. Got2 silencing promotes reprogramming of glutamine metabolism and sensitizes hepatocellular carcinoma to glutaminase inhibitors. Cancer Res. 2022;82(18):3223–35. https://doi.org/10.1158/0008-5472.CAN-22-0042.
Long PM, Moffett JR, Namboodiri AMA, Viapiano MS, Lawler SE, Jaworski DM. N-acetylaspartate (naa) and n-acetylaspartylglutamate (naag) promote growth and inhibit differentiation of glioma stem-like cells. J Biol Chem. 2013;288(36):26188–200. https://doi.org/10.1074/jbc.M113.487553.
Sun C, Gu Y, Chen G, Du Y. Bioinformatics analysis of stromal molecular signatures associated with breast and prostate cancer. J Comput Biol. 2019;26(10):1130–9. https://doi.org/10.1089/cmb.2019.0045.
Clarke MF. Clinical and therapeutic implications of cancer stem cells. N Engl J Med. 2019;380(23):2237–45. https://doi.org/10.1056/NEJMra1804280.
Lytle NK, Barber AG, Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nat Rev Cancer. 2018;18(11):669–80. https://doi.org/10.1038/s41568-018-0056-x.
Lee TK, Guan X, Ma S. Cancer stem cells in hepatocellular carcinoma—from origin to clinical implications. Nat Rev Gastroenterol Hepatol. 2022;19(1):26–44. https://doi.org/10.1038/s41575-021-00508-3.
Loong JHC, Wong T, Tong M, Sharma R, Zhou L, Ng K, et al. Glucose deprivation–induced aberrant fut1-mediated fucosylation drives cancer stemness in hepatocellular carcinoma. J Clin Invest. 2021;131(11): e143377. https://doi.org/10.1172/JCI143377.
Mok EHK, Leung CON, Zhou L, Lei MML, Leung HW, Tong M, et al. Caspase-3–induced activation of srebp2 drives drug resistance via promotion of cholesterol biosynthesis in hepatocellular carcinoma. Cancer Res. 2022;82(17):3102–15. https://doi.org/10.1158/0008-5472.CAN-21-2934.
Wang X, Yang K, Wu Q, Kim LJY, Morton AR, Gimple RC, et al. Targeting pyrimidine synthesis accentuates molecular therapy response in glioblastoma stem cells. Sci Transl Med. 2019;11(504):eaau4972. https://doi.org/10.1126/scitranslmed.aau4972
Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–72. https://doi.org/10.1038/s41571-021-00573-2.
Yau T, Park J, Finn RS, Cheng A, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (checkmate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. https://doi.org/10.1016/S1470-2045(21)00604-5.
Verset G, Borbath I, Karwal M, Verslype C, Van Vlierberghe H, Kardosh A, et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: data from the open-label, phase ii keynote-224 trial. Clin Cancer Res. 2022;28(12):2547–54. https://doi.org/10.1158/1078-0432.CCR-21-3807.
Donne R, Lujambio A. The liver cancer immune microenvironment: therapeutic implications for hepatocellular carcinoma. Hepatology. 2022:n/a–n/a. https://doi.org/10.1002/hep.32740
Li X, Wenes M, Romero P, Huang SC, Fendt S, Ho P. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat Rev Clin Oncol. 2019;16(7):425–41. https://doi.org/10.1038/s41571-019-0203-7.
Zhou J, Ding T, Pan W, Zhu L, Li L, Zheng L. Increased intratumoral regulatory t cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125(7):1640–8. https://doi.org/10.1002/ijc.24556.
Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of garp+ctla-4+foxp3+ t regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired t-cell functionality. Cancer Res. 2013;73(8):2435–44. https://doi.org/10.1158/0008-5472.CAN-12-3381.
Chang H, Jung W, Kim A, Kim HK, Kim WB, Kim JH, et al. Expression and prognostic significance of programmed death protein 1 and programmed death ligand-1, and cytotoxic t lymphocyte-associated molecule-4 in hepatocellular carcinoma. APMIS. 2017;125(8):690–8. https://doi.org/10.1111/apm.12703.
Liu X, Li M, Wang X, Dang Z, Jiang Y, Wang X, et al. Pd-1+ tigit+ cd8+ t cells are associated with pathogenesis and progression of patients with hepatitis b virus-related hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68(12):2041–54. https://doi.org/10.1007/s00262-019-02426-5.
Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, et al. Tim-3/galectin-9 signaling pathway mediates t-cell dysfunction and predicts poor prognosis in patients with hepatitis b virus-associated hepatocellular carcinoma. Hepatology. 2012;56(4):1342–51. https://doi.org/10.1002/hep.25777.
Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu Y, et al. Human cd14+ ctla-4+ regulatory dendritic cells suppress t-cell response by cytotoxic t-lymphocyte antigen-4-dependent il-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59(2):567–79. https://doi.org/10.1002/hep.26694.
Acknowledgements
We thank all the databases that provided public data downloads and the researchers who proposed the algorithms used in this study.
Funding
This research was funded by National Natural Science Foundation of China (No. 82073045, 82103090), Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515012107, 2022A1515012391, 2020A1515010232), Beijing Xisike Clinical Oncology Research Foundation (Y-MSDPU2022-0826), Science and Technology Program of Guangzhou (No.202201020311), China Postdoctoral Science Foundation (2020TQ0384, 2021M703742), the Guangdong Science and Technology Department (2020B1212060018), the Key Laboratory of Malignant Tumour Gene Regulation and Target Therapy of Guangdong Higher Education Institutes, Sun Yat‐sen University (Grant KLB09001), and the Key Laboratory of Malignant Tumour Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology ([2013]163).
Author information
Authors and Affiliations
Contributions
Conceptualization: ZZ and ZX. Methodology: JS and YY. Software: KW. Data collection: JS. Manuscript preparation: HL, HL and CH. Manuscript editing: SM and ZZ. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The study was approved by the Animal Ethics Committee of Sun Yat-sen University and conducted in accordance with institutional guidelines.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, J., Wen, K., Mui, S. et al. Integrated analysis reveals an aspartate metabolism-related gene signature for predicting the overall survival in patients with hepatocellular carcinoma. Clin Transl Oncol 26, 2181–2197 (2024). https://doi.org/10.1007/s12094-024-03431-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-024-03431-6