Abstract
Background
About 50–60% treatment-naïve advanced non-small-cell lung cancers were coexistence of epidermal growth factor receptor (EGFR) and mesenchymal epithelial transition (MET) overexpression. However, few studies demonstrated the prognostic value of MET protein expression in untreated EGFR-mutant lung adenocarcinoma (LUAD).
Methods
A total of 235 EGFR-mutant untreated advanced LUAD patients were retrospectively enrolled. MET expression was determined using immunohistochemistry, and MET positivity was defined as 2 + or 3 + using the METmab scoring algorithm. Progression-free survival (PFS) and overall survival (OS) were analysed according to MET expression status. Independent factors predicting prognosis were identified using multivariate Cox regression analyses.
Results
Of the 235 patients, 113 (48.1%) harboured exon 19 deletion (19_del), 103 (43.8%) had exon 21 L858R mutations, and 19 (8.1%) had other mutation types, including exon 21 L861Q, exon 18 G719A/C, exon 20 S768I, and L858R/19_del double mutations. MET-positive expression was observed in 192 (81.7%) cases. There was no significant difference in baseline clinicopathological characteristics between MET positivity and MET negativity groups. Patients were stratified by different EGFR mutation subtypes. MET-positive patients in the L858R mutation subgroup had markedly shorter PFS and OS than MET-negative patients (median PFS: 13 versus 27.5 months, p < 0.001; median OS: 29 versus not reached, p = 0.008), but no significant difference was observed in the 19_del subgroup. Multivariate Cox regression analyses indicated that MET positivity was an independent predictor for poor PFS and OS in L858R subgroup (PFS: HR = 3.059, 95% CI 1.552–6.029, p = 0.001; OS: HR = 3.511, 95% CI 1.346–9.160, p = 0.010). Additionally, an inferior survival outcome of MET positivity was observed in the L858R mutation subgroup when treated with EGFR–tyrosine kinase inhibitor (TKI) monotherapy as the first-line regimen (median PFS: 13 versus 36.5 months, p < 0.001; median OS: 29 versus not reached, p = 0.012) but not with EGFR–TKI plus platinum doublet chemotherapy.
Conclusions
MET positive expression was an independent predictor of poor outcomes in untreated EGFR L858R mutation advanced LUAD patients treated with first-line EGFR–TKI monotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Non-small cell lung cancers (NSCLCs) represent approximately 80–85% of all lung cancers and are generally diagnosed at an advanced stage with a poor prognosis [1, 2]. The success of small molecular tyrosine kinase inhibitors (TKIs) targeting the epidermal growth factor receptor (EGFR) has initiated an era of precision medicine in lung cancer management [1, 2]. However, increasing acquisition of resistance to EGFR–TKIs occurs via a variety of different mechanisms, including EGFR-dependent and EGFR-independent mechanisms [3, 4]. For EGFR target-independent resistance, the mesenchymal epithelial transition (MET) signalling pathway is one of the most relevant mechanisms following prior EGFR–TKI therapy and includes MET gene amplification, MET mutations and overexpression of MET and/or its ligand (hepatocyte growth factor, HGF) [3,4,5]. Preclinical and clinical studies showed that a close interaction between EGFR and the MET signalling pathway may cooperate in driving tumorigenesis in NSCLC [6,7,8,9].
MET exon 14 skipping mutations and high levels of MET amplification are well recognized valuable biomarkers for predicting the therapeutic efficacy of MET inhibitors, and MET protein expression status correlates poorly with alterations of the MET gene [10]. Despite the high frequency (35–72%) of MET overexpression in NSCLC, its predictive and prognostic significance remains controversial, and results generally differ between scoring algorithms for MET immunohistochemical (IHC) staining, positive threshold criteria, and the study population [11,12,13]. Early clinical trials using MET IHC as a biomarker were primarily performed in unselected populations, which limited the predictive capacity for MET-targeted agents, and greatly limits the clinical application of MET overexpression as a biomarker [14, 15]. MET overexpression is detected in approximately 50–60% (depending on method and cutpoint used) of EGFR-mutant advanced NSCLC [13, 16]. The METmab scoring algorithm is a clinically widespread application compared to the H-score analysis of MET immunoreactivity, and the present study examined the prognosis predictive significance of MET expression assessed using the METmab method in EGFR-mutant untreated advanced LUAD.
Patients and methods
Patient population
Patients diagnosed with locally advanced or metastatic LUAD between July 2015 and December 2020 at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology were retrospectively included in this study. The following patient inclusion criteria were used: age > 18 years; diagnosed with advanced LUAD (stage IIIB/IV, AJCC 8th edition); detected EGFR gene status using molecular testing; MET expression determined using IHC; and treated with the first-generation EGFR–TKIs-based regimen. The following exclusion criteria were used: treated with any neoadjuvant treatment or thoracic radiotherapy prior to definite diagnosis by percutaneous lung biopsy; incomplete clinicopathological data; lost to follow-up; and wild-type EGFR. Clinicopathological information was collected, including age, gender, smoking status, Eastern Cooperative Oncology Group performance status (ECOG PS) score, histopathological differentiation, and first-line treatment regimens. Follow-up evaluations for all patients were performed via outpatient clinic visits or telephone contact and included survival, time of death, cause of death, sites of progression or new metastases, time of disease progression, and the detailed process of treatment.
The institutional ethics committee of Tongji Medical College, Huazhong University of Science and Technology approved the present study (Number: S377), and all patients signed informed consent forms at the beginning of the study. All procedures performed in this study were performed in accordance with the Declaration of Helsinki.
EGFR mutation analysis
The EGFR mutations in biopsy tumour tissues were identified using amplification–refractory mutation system–polymerase chain reaction (ARMS–PCR)-based multiplexed allele-specific assays (AmoyDx, China) or next-generation sequencing (NGS). Based on the results of EGFR mutation analysis, the patients were classified into three groups: exon 19 deletion (19_del), exon 21 L858R point mutation, and other mutation subtypes.
MET immunohistochemistry analysis
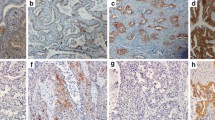
Immunohistochemical staining of MET protein was performed on 4-μm-thick formalin-fixed, paraffin-embedded tissue samples using CONFIRM anti-total MET (SP44) rabbit monoclonal primary antibody (Ventana Medical Systems). IHC staining was performed on a Roche BenchMark XT fully automated immunostainer (Ventana Medical Systems, Tucson, AZ, USA) following the manufacturers’ instructions. The expression of MET was assessed using the METmab scoring algorithm, which involved evaluation of the staining intensity (negative, weak, moderate, or strong) and the prevalence of the intensities in tumour cells. Four MET diagnostic subgroups were scored: 3 + (≥ 50% of the tumour cells staining with strong intensity); 2 + (≥ 50% of the tumour cells with moderate or higher staining, but < 50% with strong intensity); 1 + (≥ 50% of the tumour cells with weak or higher staining, but < 50% with moderate or higher intensity); or 0 (no staining, or < 50% of the tumour cells stained with any intensity) (Fig. 1). Negative expression of MET protein was defined as a score of 0 or 1 + , and positive expression was defined as a score of 2 + or 3 + as previously published [17]. Two pathologists independently evaluated the IHC findings, and a third specialist was consulted in cases of uncertainty or disagreement.
Statistical analysis
Categorical variables are presented as absolute values with percentages, and quantitative variables are presented as medians with ranges. Differences in the clinicopathological parameters between the groups were evaluated using independent t test or chi-squared tests. Progression-free survival (PFS) was determined from the date of diagnosis to the date of the first occurrence of disease progression or death or the last follow-up time when patients were progression free. Overall survival (OS) was defined as the interval from the time of diagnosis to the time of death from any cause or the last follow-up time. Survival analysis was performed using the Kaplan–Meier method, and differences between groups were compared using the log-rank test. Univariate and multivariate Cox proportional-hazards models were used to identify independent predictors of prognosis. Factors with p < 0.2 in univariate Cox regression were included in the multivariate model [18]. All tests were two-sided, and a p value < 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism (GraphPad Software Inc., California, USA) and SPSS (version 22; IBM, Armonk, NY, USA) software.
Results
Patient characteristics
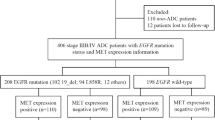
A total of 235 treatment-naïve advanced LUAD patients harbouring EGFR mutations were enrolled in the present study, and a flow diagram illustrating the patient selection process and study design is presented in Fig. 2. Among the 235 patients, 84 (35.7%) were male, and the median age of patients was 60 years (32–88 years). The PS score was 0–1 in 220 (93.6%) patients and 2–3 in 15 (6.4%) patients. For the EGFR mutation subtypes, 113 (48.1%) harboured exon 19 deletion (19_del), 103 (43.8%) had exon 21 L858R mutations, and 19 (8.1%) had an uncommon subtype of mutation, including exon 21 L861Q, exon 18 G719A/C, exon 20 S768I, and L858R/19_del double mutations. Eighty-seven (37.0%) cases were assayed for EGFR status using NGS, and 148 (63.0%) cases were assayed using ARMS–PCR. All patients harbouring EGFR mutations were treated with the first-generation EGFR–TKI-based regimen (gefitinib, erlotinib or icotinib) as the first-line therapy, as a single agent (48.8%) or in combination with platinum doublet chemotherapy (pemetrexed or paclitaxel plus platinum) (51.1%). The baseline clinicopathological characteristics of the study population are presented in Table 1.
MET protein expression
Among the 235 patients with EGFR mutations, 192 (81.7%) had MET-positive expression, and 43 (18.3%) were MET-negative. According to EGFR-sensitive mutation subtypes, MET positivity was observed in 85.8% (97/113) of patients in the 19_del subgroup, 79.6% (82/103) of patients in the L858R mutation subgroup, and 68.4% (13/19) of patients in other mutation subtypes (p = 0.147). There were no statistically significant correlations of MET expression with baseline clinicopathological features or EGFR mutation subtypes (Table 1).
Survival according to MET expression and EGFR mutation subtypes
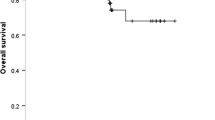
A total of 129 (54.9%) patients had cancer progression, and 71 (30.2%) died by July 15, 2021. The median follow-up time was 28 months (3–70 months). Of all patients harbouring EGFR mutations, there was a trend towards worse PFS and OS with MET-positive expression, but the differences were not statistically significant (PFS: median PFS: 19 versus 27 months, p = 0.070; median OS: 38 versus not reached, p = 0.054) (Fig. 3 A and B). Patients were stratified into the 19_del subgroup and L858R subgroup according to different EGFR-sensitive mutation subtypes. Notably, patients with MET positivity had a markedly shorter PFS and OS than patients with MET-negative expression in the L858R mutation subgroup (median PFS: 13 versus 27.5 months, p < 0.001; median OS: 29 versus not reached, p = 0.008) (Fig. 3 C and D). No significant difference was found between MET expression and survival prognosis in the 19_del mutation subgroup (Fig. 3 E and F). The results from univariate and multivariate Cox proportional-hazards regression analyses indicated that MET-positive expression was an independent factor predicting poor PFS and OS in the L858R mutation subgroup (PFS: HR = 3.059, 95% CI 1.552–6.029, p = 0.001; OS: HR = 3.511, 95% CI 1.346–9.160, p = 0.010) but not in the 19_del subgroup (Tables 2 and 3). Multivariate Cox regression analysis showed that MET positivity was also an independent risk factor for OS in the EGFR mutation group (HR = 1.990, 95% CI: 1.015–3.900, p = 0.045) (Table 3).
Kaplan–Meier survival analysis of MET expression in EGFR-mutant advanced lung adenocarcinoma according to different EGFR mutation subtypes. Progression-free survival and overall survival outcomes in patients with EGFR mutations (A, B), L858R mutations (C, D), and 19_del mutations (E, F), respectively
Survival according to MET expression and first-line EGRF–TKI-based treatment
All patients were treated with first-generation EGFR–TKI monotherapy (icotinib, gefitinib or erlotinib) (48.8%) or in combination with platinum doublet chemotherapy (pemetrexed or paclitaxel plus platinum) (51.1%) as a first-line therapy. The first-line treatment regimens were not significantly associated with survival outcomes in EGFR-mutant patients regardless of mutation subtype (Supplementary Fig. 1; Tables 2 and 3). For the 115 patients treated with single-agent EGFR–TKIs, MET positivity was significantly associated with poor PFS and OS in the L858R mutation subgroup (median PFS: 13 versus 36.5 months, p < 0.001; median OS: 29 versus not reached, p = 0.012) (Fig. 4 E and F). However, among the patients who received EGFR–TKIs plus chemotherapy as their first-line therapy, no significant differences for PFS and OS were found between MET-positive and MET-negative expression regardless of subgroup (Fig. 4). Subsequent univariate and multivariate Cox regression analyses for PFS and OS further confirmed the independence of MET positivity in predicting poor prognosis of patients treated with EGFR–TKI monotherapy (PFS: HR = 4.685, 95% CI 1.725–12.721, p = 0.002; OS: HR = 5.893, 95% CI 1.334–26.042, p = 0.019) (Supplementary Table 1 and Supplementary Table 2) rather than EGFR–TKIs + chemotherapy.
Kaplan–Meier survival analysis of MET expression in EGFR-mutant advanced lung adenocarcinoma, stratified by different EGFR-sensitive mutation subtypes and first-line treatment regimens. For patients treated with first-line EGFR–TKI monotherapy, progression-free survival and overall survival outcomes in patients with EGFR mutations (A, B), L858R mutations (E, F), and 19_del mutations (I, J), respectively. For patients treated with first-line EGFR–TKI + chemotherapy, progression-free survival and overall survival outcomes in patients with EGFR mutations (C, D), L858R mutations (G, H), and 19_del mutations (K, L), respectively
Additionally, we conducted Kaplan–Meier survival curve analysis to evaluate the survival discrepancy between patients with MET-positive expression who received EGFR–TKIs only and those who received EGFR–TKIs plus chemotherapy, based on EGFR mutation types. Our results showed that there was no statistically significant difference in PFS and OS between the two groups, irrespective of the EGFR mutation subtype (Supplementary Fig. 2). For patients with concurrent L858R mutation and MET-positive expression, the prognosis for PFS and OS were equivalent between the two first-line therapy groups (median PFS: 13 versus 14 months, p = 0.948; median OS: 29 versus 28 months, p = 0.642) (Supplementary Fig. 2 C and D). The above results indicate the need for further investigation into novel and effective treatments for L858R mutation patients with MET expression positivity.
Discussion
Dysregulation of MET signalling, whether as a primary driver or secondary to EGFR–TKI resistance, has been widely demonstrated in oncogenic processes in NSCLC, including MET exon 14 skipping mutation, MET high-level amplification, and rearrangement and protein overexpression [14, 15]. Compared to MET exon 14 skipping mutation and MET amplification, which have been widely applied in clinical trials as predicting biomarkers for therapeutic efficacy of MET inhibitors, the role of MET expression is facing considerable challenges because of conflicting results regarding its use as a prognostic and predictive biomarker in several previous trials [19, 20]. Numerous previous studies supported a close interaction between the EGFR and MET signalling pathways, which may cooperate in driving tumorigenesis [6,7,8,9]. Most current clinical trials proposed MET IHC as a predictive biomarker of response to MET inhibitors in EGFR-mutant NSCLC patients who acquired resistance to prior EGFR–TKI therapy [20,21,22,23]. However, few studies demonstrated the prognostic and predictive value of MET expression in untreated EGFR-mutant LUAD [16]. Our research, based on real-world retrospective data, demonstrates the difference of MET expression on prognosis predictive effects according to EGFR-sensitive mutation subtypes (19_del and L858R mutations) and first-line EGFR–TKI-based therapy.
The present study observed 81.7% MET positivity (2 + or 3 +) in EGFR-mutant LUAD using the METmab scoring algorithm for the assessment of IHC staining, which was significantly higher than previous reports using the H-score algorithm [13, 24]. Notably, the positive rate of MET expression differs according to the study subpopulation, disease stage, positivity threshold, and scoring algorithm for IHC staining. Giorgio et al. showed a MET positivity of 59.6% (MET expression of at least 2 + in > 60% of tumour cells) in stage IV EGFR-mutant NSCLC [16]. There were no statistically significant correlations of MET expression with baseline clinicopathological features or EGFR mutation subtypes. Our results demonstrated that the impact of MET-positive expression in predicting prognosis differed by EGFR-sensitive mutation subtypes and first-line treatment regimens. MET positivity assessed was an independent factor predicting unfavourable outcomes in untreated advanced LUAD patients harbouring the L858R mutation but not the 19_del mutation, according to METmab analysis method of MET immunoreactivity. The result was similar to our previous research using H-score scoring, which suggests that the conclusions are robust. In comparison to H-score analysis, METmab scoring was considered to be more convenient, popular, consistent, and widely used in clinical practice. Previous research demonstrated that the 19_del mutation subtype and L858R mutation subtype separately contributed to different structural conformation domains of EGFR–tyrosine kinase [25]. The MET/HGF signalling pathway was more correlated with L858R mutations than 19_del in EGFR-mutated NSCLC [9]. Based on these results, we speculate that EGFR L858R synergizes with MET overexpression to generate stronger activation of downstream signalling pathways, which led to shortened patient survival. Notably, the underlying mechanisms of the interaction between L858R mutations and MET overexpression should be clarified using cell function experiments in vitro and animal experiments in the future. For patients with the L858R mutation, the presence of MET positivity is associated with a poor prognosis when treated with EGFR–TKI monotherapy. However, this association is not observed in patients receiving a combination of EGFR–TKI and chemotherapy. Nevertheless, in patients with concurrent MET positivity and L858R mutation, the effectiveness of EGFR–TKI plus chemotherapy is comparable to that of EGFR–TKI monotherapy. These findings highlight the need for the development of novel and more effective therapeutic strategies for patients who have both the L858R mutation and MET positivity.
MET-targeted therapy has been introduced into clinical practice for patients harbouring MET exon 14 skipping mutations or high-level amplification [26, 27]. MET IHC is emerging as a clinically relevant biomarker for predicting the treatment efficacy of MET inhibitors. A phase II trial in 2013 indicated that patients with MET overexpression (≥ 2 +) significantly benefited from the combination of onartuzumab (anti-MET monoclonal antibody) and erlotinib (the first-generation EGFR–TKI) compared to MET-negative populations in the OAM4558g trial [28]. An increasing number of studies of MET IHC were designed for EGFR-mutant patients who acquired resistance to prior EGFR–TKIs. The INSIGHT study was a multicentre randomised trial comparing the efficacy of tepotinib (a highly selective MET–TKI) plus gefitinib (the first-generation EGFR–TKI) versus standard chemotherapy to overcome acquired resistance to EGFR–TKIs in patients with MET-overexpressing EGFR-mutant NSCLC. The results suggested that patients with MET overexpression (3 +) had better outcomes (improved PFS, OS, and objective response) with tepotinib plus gefitinib than chemotherapy [23]. The ORR was 75% in MET-overexpressing patients (MET protein + 3 in ≥ 50% of tumour cells) with no prior treatment with third-generation EGFR–TKIs after receiving osimertinib (a third-generation EGFR–TKI) plus savolitinib (a highly selective MET–TKI) in the TATTON Part B cohort [29]. Amivantamab, an EGFR–MET bispecific antibody, showed an ORR of 90% and a median PFS of 12.5 months in osimertinib-relapsed EGFR-mutant patients with EGFR/MET-positive expression (combined EGFR + MET H-score ≥ 400) when used in combination with Lazertinib (the third-generation EGFR–TKI) [21]. These results provide clinical evidence of the efficacy of a combinatorial regimen of EGFR–TKIs and MET–TKIs or an EGFR–MET bispecific antibody after acquired EGFR–TKI resistance in patients with concurrent MET-positive expression and EGFR mutations. For treatment-naïve EGFR-mutant NSCLC, combination treatment with emibetuzumab (a MET inhibitor) and erlotinib (the first-generation EGFR–TKI) provided a clinically meaningful benefit for stage IV patients with MET overexpression (MET 3 + in ≥ 90% of tumour cells) [16]. These results reveal that combination therapy with EGFR–TKIs and MET–TKIs may yield substantial efficacy in untreated NSCLC with concomitant EGFR mutations and MET positivity. Our study suggested a limited efficacy of EGFR–TKI monotherapy in untreated advanced LUAD patients with MET-overexpressing EGFR L858R mutation. Further investigations are necessary to determine whether MET positivity in conjunction with the L858R mutation would better define subpopulations that are most likely to benefit from EGFR–TKIs plus MET–TKIs.
There are several limitations in the present study, including a small sample size and the retrospective, single-centre study design. Therefore, a prospective trial with a large sample size and multi-centre data is necessary to confirm our findings, and the underlying mechanism of interactions between the L858R mutations and MET overexpression should be clarified in the future. Second, other methods (fluorescence in situ hybridization and NGS) to identify MET dysregulation were not performed in the present study. Therefore, a combination of approaches should be used in the future to confirm MET status.
In conclusion, our research provides clinical evidence of the different prognosis predictive value of MET expression in treatment-naïve advanced LUAD patients with differing EGFR-sensitive mutation subtypes and first-line EGFR–TKI-based therapy. Our results suggest that MET-positive expression was an independent predictor of poor prognosis in untreated EGFR L858R mutation patients treated with EGFR–TKI monotherapy. Combinatorial therapy with EGFR–TKIs and MET–TKIs or EGFR–MET bispecific antibodies may be a potential therapeutic strategy for the concomitant alteration of L858R mutation and MET overexpression.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(5):497–530.
Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022. https://doi.org/10.3322/caac.21731.
Liu Q, Yu S, Zhao W, Qin S, Chu Q, Wu K. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol Cancer. 2018;17(1):53. https://doi.org/10.1186/s12943-018-0793-1.
Nagano T, Tachihara M, Nishimura Y. Mechanism of Resistance to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors and a Potential Treatment Strategy. Cells. 2018. https://doi.org/10.3390/cells7110212.
Passaro A, Janne PA, Mok T, Peters S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat Cancer. 2021;2(4):377–91. https://doi.org/10.1038/s43018-021-00195-8.
Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43. https://doi.org/10.1126/science.1141478.
Guo A, Villen J, Kornhauser J, Lee KA, Stokes MP, Rikova K, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci U S A. 2008;105(2):692–7. https://doi.org/10.1073/pnas.0707270105.
Hong S, Gao F, Fu S, Wang Y, Fang W, Huang Y, et al. Concomitant Genetic Alterations With Response to Treatment and Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With EGFR-Mutant Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018;4(5):739–42. https://doi.org/10.1001/jamaoncol.2018.0049.
Liang H, Li C, Zhao Y, Zhao S, Huang J, Cai X, et al. Concomitant Mutations in EGFR 19Del/L858R Mutation and Their Association with Response to EGFR-TKIs in NSCLC Patients. Cancer Manag Res. 2020;12:8653–62. https://doi.org/10.2147/CMAR.S255967.
Guo R, Berry LD, Aisner DL, Sheren J, Boyle T, Bunn PA Jr, et al. MET IHC Is a Poor Screen for MET Amplification or MET Exon 14 Mutations in Lung Adenocarcinomas: Data from a Tri-Institutional Cohort of the Lung Cancer Mutation Consortium. J Thorac Oncol. 2019;14(9):1666–71. https://doi.org/10.1016/j.jtho.2019.06.009.
Li A, Niu FY, Han JF, Lou NN, Yang JJ, Zhang XC, et al. Predictive and prognostic value of de novo MET expression in patients with advanced non-small-cell lung cancer. Lung Cancer. 2015;90(3):375–80. https://doi.org/10.1016/j.lungcan.2015.10.021.
Tsakonas G, Botling J, Micke P, Rivard C, LaFleur L, Mattsson J, et al. c-MET as a biomarker in patients with surgically resected non-small cell lung cancer. Lung Cancer. 2019;133:69–74. https://doi.org/10.1016/j.lungcan.2019.04.028.
Huang L, An SJ, Chen ZH, Su J, Yan HH, Wu YL. MET expression plays differing roles in non-small-cell lung cancer patients with or without EGFR mutation. J Thorac Oncol. 2014;9(5):725–8. https://doi.org/10.1097/JTO.0000000000000105.
Raghav K, Bailey AM, Loree JM, Kopetz S, Holla V, Yap TA, et al. Untying the gordion knot of targeting MET in cancer. Cancer Treat Rev. 2018;66:95–103. https://doi.org/10.1016/j.ctrv.2018.04.008.
Drilon A, Cappuzzo F, Ou SI, Camidge DR. Targeting MET in Lung Cancer: Will Expectations Finally Be MET? J Thorac Oncol. 2017;12(1):15–26. https://doi.org/10.1016/j.jtho.2016.10.014.
Scagliotti G, Moro-Sibilot D, Kollmeier J, Favaretto A, Cho EK, Grosch H, et al. A Randomized-Controlled Phase 2 Study of the MET Antibody Emibetuzumab in Combination with Erlotinib as First-Line Treatment for EGFR Mutation-Positive NSCLC Patients. J Thorac Oncol. 2020;15(1):80–90. https://doi.org/10.1016/j.jtho.2019.10.003.
Koeppen H, Yu W, Zha J, Pandita A, Penuel E, Rangell L, et al. Biomarker analyses from a placebo-controlled phase II study evaluating erlotinib+/-onartuzumab in advanced non-small cell lung cancer: MET expression levels are predictive of patient benefit. Clin Cancer Res. 2014;20(17):4488–98. https://doi.org/10.1158/1078-0432.CCR-13-1836.
Kang SJ, Cho YR, Park GM, Ahn JM, Han SB, Lee JY, et al. Predictors for functionally significant in-stent restenosis: an integrated analysis using coronary angiography, IVUS, and myocardial perfusion imaging. JACC Cardiovasc Imaging. 2013;6(11):1183–90. https://doi.org/10.1016/j.jcmg.2013.09.006.
Spigel DR, Edelman MJ, O’Byrne K, Paz-Ares L, Mocci S, Phan S, et al. Results From the Phase III Randomized Trial of Onartuzumab Plus Erlotinib Versus Erlotinib in Previously Treated Stage IIIB or IV Non-Small-Cell Lung Cancer: METLung. J Clin Oncol. 2017;35(4):412–20. https://doi.org/10.1200/JCO.2016.69.2160.
Wu YL, Zhang L, Kim DW, Liu X, Lee DH, Yang JC, et al. Phase Ib/II Study of Capmatinib (INC280) Plus Gefitinib After Failure of Epidermal Growth Factor Receptor (EGFR) Inhibitor Therapy in Patients With EGFR-Mutated, MET Factor-Dysregulated Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36(31):3101–9. https://doi.org/10.1200/JCO.2018.77.7326.
Bauml J, Cho BC, Park K, Lee KH, Cho EK, Kim DW, et al. Amivantamab in combination with lazertinib for the treatment of osimertinib-relapsed, chemotherapy-naive EGFR mutant (EGFRm) non-small cell lung cancer (NSCLC) and potential biomarkers for response. J Clin Oncol. 2021. https://doi.org/10.1200/JCO.2021.39.15_suppl.9006.
Oxnard GR, Yang JC, Yu H, Kim SW, Saka H, Horn L, et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol. 2020;31(4):507–16. https://doi.org/10.1016/j.annonc.2020.01.013.
Wu YL, Cheng Y, Zhou J, Lu S, Zhang Y, Zhao J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med. 2020;8(11):1132–43. https://doi.org/10.1016/S2213-2600(20)30154-5.
Wang N, Zhu Y, Wu Y, Huang B, Wu J, Zhang R, et al. MET overexpression in EGFR L858R mutant treatment-naive advanced lung adenocarcinoma correlated with poor prognosis: a real-world retrospective study. J Cancer Res Clin Oncol. 2022. https://doi.org/10.1007/s00432-022-04225-5.
Kumar A, Petri ET, Halmos B, Boggon TJ. Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol. 2008;26(10):1742–51. https://doi.org/10.1200/JCO.2007.12.1178.
Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med. 2020;383(10):931–43. https://doi.org/10.1056/NEJMoa2004407.
Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383(10):944–57. https://doi.org/10.1056/NEJMoa2002787.
Spigel DR, Ervin TJ, Ramlau RA, Daniel DB, Goldschmidt JH Jr, Blumenschein GR Jr, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2013;31(32):4105–14. https://doi.org/10.1200/JCO.2012.47.4189.
Hartmaier R, Han JY, Cho BC, Markovets A, Kurian N, Cantarini M, et al. Tumor response and MET-detection methods exploratory biomarker analysis of Part B of the Ph 1b TATTON study. Cancer Res. 2021;81(13):2.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China [Grant number 82072333]; and the Natural Science Foundation of Hubei Province [Grant number 2023AFB986]; and the National Key Research and Development Program of China [Grant number 2022YFF1203300].
Author information
Authors and Affiliations
Contributions
NW: methodology, writing—original draft, formal analysis. YZ: methodology, conceptualization. JW: formal analysis, resources. YZ: investigation, data curation. YW: investigation. BH: resources, project administration. RZ: resources. JF: conceptualization, supervision, project administration. XN: conceptualization, supervision, funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethics approval and consent to participate
Approval was obtained from the ethics committee of Tongji Medical College of Huazhong University of Science and Technology, and all patients signed informed consent forms. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, N., Zhang, Y., Wu, J. et al. MET overexpression correlated with prognosis of EGFR-mutant treatment‑naïve advanced lung adenocarcinoma: a real‑world retrospective study. Clin Transl Oncol 26, 1696–1707 (2024). https://doi.org/10.1007/s12094-024-03391-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-024-03391-x