Abstract
Background
The significant clinical benefits of PD-1/PD-L1 immune checkpoint inhibitors (ICIP) in non-small cell lung cancer (NSCLC) have been widely recognized, emphasizing the urgent need for a reliable biomarker. In this study, we find the remarkable capacity of tumor mutational burden (TMB) to serve as an accessible and streamlined indicator.
Patients and methods
We designed a retrospective cohort study, consisting of 600 NSCLC patients treated with ICIP. Association between TMB and overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR) has been explored.
Results
A strong positive correlation between TMB levels and OS, PFS rates, clinical benefit has been found when TMB > = 16(TMB > = 16 mutations/megabase (mut/Mb)). However, when TMB < 16, increasing TMB values did not exhibit a gradual stepwise increase in OS and PFS rates. The median months of OS in the TMB > = 16 and < 16 are 35.58, and 10.71 months respectively with average 12.39 months (p < 0.0001). The median months of PFS in the TMB > = 16 and < 16 are not-obtained, and 2.79 months respectively with an average of 3.32 months (p < 0.0001). The DCR in the TMB > = 16 and < 16 are 71.4% and 44.2% respectively with an average of 47.7% (p < 0.0001). The ORR in the TMB > = 16 and < 16 are 49.4% and 20.8% respectively with an average of 24.5% (p < 0.0001).
Conclusion
The TMB > = 16 shows significantly associated with optimal ICIP treatment outcomes, including higher patient survival rates, delayed disease progression, and significant clinical benefits. These results present the potential of TMB as a promising biomarker candidate for NSCLC patients undergoing ICIP treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-small cell lung cancer (NSCLC) represents a complex and challenging disease that requires a multifaceted approach to treat [1]. The current treatment modes for NSCLC encompass a range of interventions, including surgery, chemotherapy, targeted therapy, radiotherapy, immunotherapy, anti-angiogenesis therapy, etc. [1,2,3,4,5,6]. In recent years, it has been remarkable progress in the field of immunotherapy, with advanced NSCLC patients achieving long-term survival and ushering in a new era of chronic disease management [7]. Nowadays, the emergence of programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1) immune checkpoint inhibitors has markedly altered the therapeutic outlook for NSCLC, offering tremendous hope and promise for patients worldwide [8]. However, the clinical benefit rates of immune checkpoint inhibitors remain relatively low with the objective response rate (ORR) of 20% [9]. The identification and validation of biomarkers will be critical in guiding personalized treatment decisions and ultimately improving outcomes for NSCLC patients.
Tumor mutational burden (TMB) is defined as the relative number of gene mutations in a specific tumor tissue, calculated as follows: TMB (Mut/Mb) = total number of mutations (including synonymous, non-synonymous point mutations, substitutions, insertions, and deletions)/target region coding region size [10, 11]. Recently, TMB has been identified as a biomarker for ICIP in melanoma. The FDA has also approved pembrolizumab in all cancers with a TMB > 10Mut/Mb, emphasizing the potential predictive power of this biomarker [12, 13]. TMB has been shown to be particularly valuable in predicting prognosis in advanced NSCLC patients receiving adjuvant immunotherapy [14]. Further investigation to validate and expand upon these findings will be critical in fully unlocking the potential of TMB as a powerful tool for prognostication and therapeutic guidance in cancer patients.
In this paper, the aim of this retrospective cohort study is to explore the correlation between TMB levels and survival and response in NSCLC patients treated with ICIP. Our results showed A strong positive correlation between TMB levels and OS, PFS rates, clinical benefit has been found when TMB > = 16. However, when TMB < 16, there is no significant correlation between the TMB and OS and PFS rates. Our results underscore the potential utility of TMB as a valuable prognostic marker for patients being treated with ICIP, providing a promising avenue for further research in identifying high-risk patient subgroups for targeted intervention.
Methods
Data source and patients selection
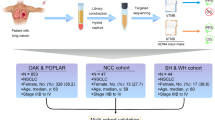
The clinical data of 600 NSCLC patients who received ICIP treatment were sourced from the Memorial Sloan Kettering Cancer Center [15]. TMB, derived from DNA in tumor tissue, was defined as the total number of somatic non-synonymous tumor mutations normalized to the exonic coverage of the respective MSK-IMPACT panel in megabases (mutations/megabase) [15]. TMB was obtained from the nearest blood test preceding the initial ICIP infusion. Patients selected for the study were all those who had been selected by Luc G.T. Morris and his colleagues [15]. We excluded patients with a history of SCLC, and those who had received combination immune checkpoint inhibitors of PD-1/PD-L1 and CTLA-4 (Fig. 1A, B).
Clinical model for predicting clinical outcomes of ICIP treatment using pre-treatment blood tests (A) and Flow diagram for clinical data filter (B). TMB values were gathered from the nearest blood test preceding the initial ICIP infusion. We present a precision medicine-based approach utilizing the TMB as a critical prognostic marker in ICIP treatment. Through a systematic and data-driven analysis of NSCLC patients (n = 600), we have demonstrated the powerful predictive value of TMB in guiding treatment decisions and improving clinical outcomes, including OS, PFS, DCR, and ORR. ICIs, Immune checkpoint inhibitors; NSCLC, Non-small cell lung cancer; SCLC, Small cell lung cancer; Combo, Combination of anti-PD-1/PD-L1 and anti-CTLA-4; CTLA-4, Cytotoxic T lymphocyte-associated antigen-4
Survival analysis
The Kaplan–Meier method was utilized for survival analyses of OS and PFS of NSCLC patients treated with ICIP. The Cox proportional hazards regression model was subsequently applied to adjust for potential confounding factors, including sex, age, tumor stage, drug class of ICIP, and ICIP as the first or subsequent line of treatment. The response was classified using the Response Evaluation Criteria in Solid Tumors (RECIST) V.1.1 criteria [16].
Validation cohort
To verify the above results, the analysis of 400 randomly resampled cohorts was conducted by the bootstrap resampling method for validation of the TMB thresholds analyzed in the original cohort by SPSS. We reevaluated the correlation between TMB and OS, PFS, DCR, and ORR, then investigated the differences between the validation cohort and original cohort with median months of OS and PFS, as well as ORR and DCR.
Statistical analysis
Pearson’s χ2-test was utilized to compare response and clinical outcomes between groups. Kaplan–Meier analysis was employed to calculate the corresponding survival rates. The log-rank test compares survival rates between groups. Cox proportional hazard regression model was thereafter utilized to calculate hazard ratios (HRs). All statistical analyses were conducted by SPSS 24.0.
Results
The characteristics of NSCLC patients after ICIP treatment
To evaluate the predictive value of pre-treatment TMB, we analyzed data of 600 NSCLC patients with response and survival outcomes after immune checkpoint inhibitors of ICIP treatment. There are a total of 287 patients under 65 years old, and 313 patients over 65 years old. 300 patients (50%) were male (Table 1). The median follow-up duration was recorded at 18 months. The objective response rate was calculated at 24.5%, along with a disease control rate (DCR) of 47.7%.
TMB is associated with the clinical outcomes of ICIP treatment
To understand the relationship between TMB and the clinical outcomes of ICIP treatment for NSCLC patients, we analyzed a cohort of 600 NSCLC patients. Kaplan–Meier curves showed the OS (Fig. 2A–I) and PFS (Fig. 2J–R) in NSCLC patients of ICIP treatment according to TMB percentile using as cutoff top 10th, top 20th, top 30th, top 40th, top 50th, top 60th, top 70th, top 80th, and top 90th percentile. We found a strong positive correlation between TMB levels and OS, PFS rates when TMB percentile > = 50%.
The OS and PFS in ICIP-treated NSCLC patients according to TMB percentile. The Kaplan–Meier curves show the OS (A–I) and PFS (J–R) in ICIP-treated NSCLC patients correlated with respective TMB percentiles, with cutoff top 10th, top 20th, top 30th, top 40th, top 50th, top 60th, top 70th, top 80th, and top 90th percentile. P values are calculated by log-rank test. OS overall survival, PFS progression-free survival, TMB tumor mutational burden, ICIP immune checkpoint inhibitors of PD-1/PD-L1
To further explore the correlation between TMB levels and clinical outcomes of ICIP treatment, the TMB levels ranging from 0 to 77 patients were separated into 0–1.99, 2–3.99, 3–4.99, 4–5.99, 6–7.99, 8–9.99, 10–11.99, 12–15.99, 16–19.99, 20–77. Next, the univariate and multivariate analysis using the Cox regression model showed that a strong positive correlation was revealed between TMB levels and OS and PFS, when TMB > = 16. However, when TMB < 16, any increase in TMB did not exhibit the same trend associations in either OS or PFS rates (p > 0.05; Fig. 3A–D), taking the TMB levels of 6–7.99 as the reference.
The OS and PFS in ICIP-treated NSCLC patients based on different TMB levels. The TMB levels ranging from 0.3 to 77 patients were divided into 0–1.99, 2–3.99, 4–5.99, 6–7.99, 8–9.99, 10–11.99, 12–15.99, 16–19.99, 20–77. The forest plot showed the association of TMB levels with OS (A, B) and PFS (C, D) following ICIP therapy. HR and 95% CI were calculated by Cox proportional hazards regression analysis, using the TMB level of 6–7.99 as the reference. Multivariate analysis was performed by using the covariates of sex, age, tumor stage, ICI drug class, ICI line of treatment and ECOG score. ICIP immune checkpoint inhibitors of PD-1/PD-L1, OS overall survival, PFS progression-free survival, TMB tumor mutational burden
The correlation between TMB levels and survival in NSCLC patients treated with ICIP
Based on the results, NSCLC patients were classified into TMB > = 16 and TMB < 16. There are a total of 77 patients TMB > = 16, and 523 patients TMB < 16 (Table 2). Next, the Kaplan–Meier analysis showed that the OS and PFS rates of TMB > = 16 were significantly higher than those of TMB < 16 (p < 0.0001; Fig. 4A, E). The median months of OS in the TMB levels of > = 16 and < 16 are 35.58, and 10.71 months respectively with average 12.39 months (p < 0.0001; Fig. 4B). The median months of PFS in the TMB levels of > = 16 and < 16 are not-obtained, and 2.79 months respectively with an average of 3.32 months (p < 0.0001).
The OS and PFS in ICIP-treated patients based on reclassified TMB levels. The Kaplan–Meier curves exhibited the OS (A) and PFS (E) in ICIP-treated patients with reclassified TMB levels. p values are calculated by log-rank test. The median survival times (B) has been analyzed by the χ2 test. TMB levels were divided into > = 16 and < 16. The forest plot shows the association of TMB with OS (C, D) and PFS (F, G) following ICIP therapy. HR and 95% CI were calculated by Cox proportional hazards regression analysis, using the TMB level of > = 16 as the reference. Multivariate analysis was performed using the covariates of sex, age, tumor stage, ICI drug class, ICI line of treatment and ECOG score. ICIP Immune checkpoint inhibitors of PD-1/PD-L1, TMB tumor mutational burden, OS overall survival, PFS progression-free survival
Furthermore, the univariate and multivariate analysis utilizing the Cox regression model exhibited the associations of TMB with OS (> = 16: univariate, HR = 0.425; 95% CI 0.295–0.613; p < 0.0001; multivariate, HR = 0.412; 95% CI 0.283–0.598; p < 0.0001) (Fig. 4C, D) and PFS (> = 16: univariate, HR = 0.424; 95% CI 0.294–0.610; p < 0.0001; multivariate, HR = 0.402; 95% CI 0.277–0.583; p < 0.0001) (Fig. 4F, G) respectively (Table 3).
The correlation between TMB levels and the best response to ICIP treatment
Subsequently, the optimal treatment responses to ICIP therapy were classified as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). To assess the efficacy of ICIP therapy, the objective response rate (ORR) was employed, reflecting the proportion of patients who achieved a CR or PR, which was calculated at 24.5%. Furthermore, DCR was established to evaluate the effectiveness of the treatment, encompassing the percentage of patients who underwent a CR, PR, or SD, with a total of 47.7% of NSCLC patients demonstrating a positive outcome (Fig. 5A, B). Patients with TMB > = 16 exhibited the ICIP response with an ORR of 49.4% and a DCR of 71.4%, higher than TMB < 16 with an ORR of 20.8% and a DCR of 44.2%.
The correlation between TMB levels and best response to ICIP treatment. The stacked bar plot exhibited the percentage of DCR and ORR in different TMB levels. TMB levels were divided into > = 16 and < 16. p values are calculated by the χ2 test. The blue dashed line represented the average DCR (CR + PR + SD) of 47.7% and ORR (CR + PR) of 24.5% in all NSCLC patients. ICIP immune checkpoint inhibitors of PD-1/PD-L1, TMB tumor mutational burden, DCR disease control rate, ORR objective response rate, CR complete response, PR partial response, SD stable disease
The variability and reproducibility between TMB and ICIP treatment clinical outcomes
To validate the correlation between TMB and ICIP treatment clinical outcomes, we conduct an internal verification using 400 randomly bootstrap-resampled cohorts (Table 4). There are a total of 77 patients TMB > = 16, and 523 patients TMB < 16. The statistical evaluation involved measurement of clinical outcomes including the median survival times of OS and PFS, and the ORR and DCR (Fig. 6A–C). Patients with TMB > = 16 exhibited the ICIP response with an ORR of 44.4% and a DCR of 68.9%, higher than TMB < 16 with an ORR of 19.4% and a DCR of 41.7% with same trends as the original group (Table 5).
The correlation between TMB and clinical outcomes to ICIP therapy in the validation cohort (n = 400). The median survival times of OS (A), and DCR (B), and ORR (C) in different TMB levels has been provided. The blue dashed line represented the average DCR of 44.8% and ORR of 22.3% in the validation cohort. p values are calculated by the χ2 test. ICIP immune checkpoint inhibitors of PD-1/PD-L1, TMB tumor mutational burden, DCR disease control rate, ORR objective response rate, CR complete response, PR partial response, SD stable disease
Discussion
Immunotherapy has high therapeutic efficacy and long-term survival potential in selected patient populations, and its emergence has completely changed the pattern of cancer treatment, making it one of the most promising pillars of modern oncology [17, 18]. Recent approvals of PD-1 and PD-L1 inhibitors, such as nivolumab, pembrolizumab, atezolizumab, and durvalumab for NSCLC patients, underscore the potential of immunotherapy with their ability to deliver safety and significant improvements [19,20,21,22]. In routine clinical practice, the expression rate of PD-L1 is often used to guide the selection of appropriate treatments, such as pembrolizumab showing high efficacy in advanced NSCLC patients with PD-L1 ≥ 50% [23, 24]. However, Lung cancer cells have a very high mutation frequency, and clear driving genes can be found in most non-smoking adenocarcinoma, while smoking squamous cell carcinoma mostly has high-frequency mutations [25, 26]. Moreover, the inherent complexity of the tumor microenvironment, including heterogeneity within the tumor, genetic processes controlling PD-L1 expression, and temporal variability of PD-L1 expression between tumors, still have limitations [27]. In immunotherapy, the expression of PD-L1 can guide clinical medication, but it is not yet a perfect biomarker [28]. Given this reality, identifying effective biomarkers that can provide accurate prognostic indicators remains a top priority for optimizing patient prognosis.
High tumor TMB leads to higher tumor immunogenicity and stronger induced T cell responses. There is a significant correlation between high tumor TMB and clinical response to ICIP [12]. Therefore, TMB, as a predictive biomarker for immunotherapy, can help screen out populations more likely to benefit from immunotherapy. In this study, TMB has emerged as a promising biomarker, with its levels potentially indicative of cancer progression and efficacy of immunotherapy. Notably, we found that higher TMB was linked to higher survival outcomes and a greater probability of response to ICIP treatment. However, we also noted an interesting finding: when TMB < 16, there was no significant correlation between TMB and OS and PFS. In summary, our findings add to the growing body of evidence supporting the prognostic significance of TMB in the context of immunotherapeutic checkpoint inhibitors and underscore the potential of TMB as a valuable tool in guiding personalized treatment decisions.
There are several limitations in our study. First, at present, expression of PD-L1 determined by IHC remains the only validated biomarker that has demonstrated strong correlation with ICI response. We have not taken the ability of PD-L1 expression to co-predict with TMB. Previous studies on the relationship between PD-L1 and TMB in NSCLC produced inconsistent results, with some studies failing to find any correlation and others finding a favorable relationship [29, 30]. Yarchoan et al. examined 9887 individual specimens and discovered a small but significant relationship between PD-L1 expression and TMB [31]. Furthermore, Lamberti et al. proposed a significant relationship between high PD-L1 expression and elevated TMB in 421 NSCLC specimens, especially noteworthy in cases with PD-L1 tumor proportion score (TPS) ≥ 90% (N = 133) or < 1% (N = 288) [32]. The ORR to PD-1/PD-L1 inhibition was as high as 57% in patients with high TMB and PD-L1 expression 50% or higher and as low as 8.7% in patients with low TMB and PD-L1 expression less than 1% [33]. Therefore, TMB-High and PD-L1-High can be considered independent populations in NSCLC.
Second, this study is an observational investigation that was conducted at a solitary center in a relatively small cohort of patients. To this end, we recommend conducting multi-center prospective cohort studies to further validate and expand upon our initial observations. Such studies will be instrumental in enhancing external validity and ultimately maximize their impact on clinical practice. We have attempted to address this concern by performing internal validation, but lack of external verification. In addition, we have carefully stratified patients according to TMB levels and identified that different TMB levels are associated with divergent survival and response outcomes, we recognize that these findings require further validation. The clinical benefits vary among different groups of the ECOG and 15% of patients have not-obtained which is also a disruptive factor. The FDA approved pembrolizumab on June 16, 2020, for the treatment of adult and pediatric patients with unresectable or metastatic tumor mutational burden-high [TMB > = 10 (mut/Mb)] solid tumors. In our study, when 10 < TMB < 16, any increase in TMB did not exhibit the trend associations in either OS or PFS rates. These doubts require more clinical cases to verify.
Lastly, although our study highlights the potential of TMB as an inflammatory marker for predicting therapeutic efficacy, further research is still required to unravel the exact mechanisms driving this association.
Conclusion
In this study of 600 NSCLC patients treated with ICIP, the prognostic value of TMB has been confirmed. A strong positive correlation between TMB levels and OS, PFS rates, clinical benefit has been found when TMB > = 16. However, there is no significant difference in this trend when TMB < 16. In summary, TMB can serve as predictive indicators of treatment response and prognosis in NSCLC patients receiving ICIP treatment. These results exhibit great promise for guiding the selection and optimization of ICIP treatment programs for NSCLC patients, providing actionable advice for doctors, and improving clinical decision-making.
References
Bryan DS, Donington JS. The role of surgery in management of locally advanced non-small cell lung cancer. Curr Treat Options Oncol. 2019;20(4):27.
Nagasaka M, Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther. 2018;18(1):63–70.
Brown S, Banfill K, Aznar MC, Whitehurst P, Faivre FC. The evolving role of radiotherapy in non-small cell lung cancer. Br J Radiol. 2019;92(1104):20190524.
Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17(1):38.
Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol. 2022;40(6):586–97.
Tan AC, Pavlakis N. Anti-angiogenic therapy in ALK rearranged non-small cell lung cancer (NSCLC). Int J Mol Sci. 2022;23(16):8863.
Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22(1):40.
Patel SA, Weiss J. Advances in the treatment of non-small cell lung cancer: immunotherapy. Clin Chest Med. 2020;41(2):237–47.
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30.
Addeo A, Friedlaender A, Banna GL, Weiss GJ. TMB or not TMB as a biomarker: that is the question. Crit Rev Oncol Hematol. 2021;163: 103374.
Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30(1):44–56.
Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016;15(4):857–65.
Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65.
Kim ES, Velcheti V, Mekhail T, et al. Blood-based tumor mutational burden as a biomarker for atezolizumab in non-small cell lung cancer: the phase 2 B-F1RST trial. Nat Med. 2022;28(5):939–45.
Valero C, Lee M, Hoen D, Weiss K, Kelly DW, Adusumilli PS, et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun. 2021;12(1):729.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Zhang M, Fan Y, Nie L, Wang G, Sun K, Cheng Y. Clinical outcomes of immune checkpoint inhibitor therapy in patients with advanced non-small cell lung cancer and preexisting interstitial lung diseases: a systematic review and meta-analysis. Chest. 2022;161(6):1675–86.
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–61.
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus Ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–31.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344–57.
Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301–11.
Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50.
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–8.
Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150(6):1121–34.
Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer. 2020;126(2):260–70.
Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–56.
Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–41.
Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018;33(5):843–52.
Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight. 2019;4(6): e126908.
Lamberti G, Spurr LF, Li Y, Ricciuti B, Recondo G, Umeton R, et al. Clinicopathological and genomic correlates of programmed cell death ligand 1 (PD-L1) expression in nonsquamous non-small-cell lung cancer. Ann Oncol. 2020;31(6):807–14.
Ricciuti B, Wang X, Alessi JV, Rizvi H, Mahadevan NR, Li YY, et al. Association of high tumor mutation burden in non-small cell lung cancers with increased immune infiltration and improved clinical outcomes of PD-L1 blockade across PD-L1 expression levels. JAMA Oncol. 2022;8(8):1160–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval (Research involving human participants and/or animals), Informed consent
No human subjects were directly involved in this study. All the data used in this study were derived from existing biological samples from prior studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shao, Mm., Xu, Yp., Zhang, Jj. et al. Tumor mutational burden as a predictive biomarker for non-small cell lung cancer treated with immune checkpoint inhibitors of PD-1/PD-L1. Clin Transl Oncol 26, 1446–1458 (2024). https://doi.org/10.1007/s12094-023-03370-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03370-8