Abstract
Background
Geriatric nutritional risk index (GNRI) on the prognosis of patients with diffuse large B-cell lymphoma (DLBCL) remains unclear. The purpose of this meta-analysis was to discuss the value of the GNRI in evaluating long-term outcomes in DLBCL.
Methods
We systematically and roundly retrieved PubMed, Cochrane Library, Embase, Scopus and Web of Science electronic databases from inception of the databases to March 20, 2023. At the same time, we calculated the pool hazard ratios (HRs) with their 95% confidence interval (CI) for overall survival and progression-free survival to assess the effect of GNRI on the prognosis of DLBCL patients.
Results
In our primary meta-analysis, 7 trials with a total of 2448 patients were enrolled. Results showed that lower level of GNRI was related to poorer overall survival (HR = 1.78, 95% CI 1.27, 2.50, p < 0.01) and worse progression-free survival (HR = 2.31, 95% CI 1.71, 3.13, p < 0.01) in DLBCL patients.
Conclusion
The results of our meta-analysis indicate that a lower GNRI significantly associated with poorer prognosis for DLBCL. It is believed that GNRI was a promisingly predictive indicator of survival outcomes in DLBCL patients. However, large multicenter prospective studies are necessary to verify the results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lymphoma is classified into Hodgkin lymphoma and non-Hodgkin lymphoma, 90% of cases are non-Hodgkin lymphoma [1]. Approximately, 30 to 40% of non-Hodgkin lymphomas are diffuse large B-cell lymphomas (DLBCL), which is the most common non-Hodgkin lymphoma [2]. According to statistics, the number of newly diagnosed cases and mortalities from DLBCL in men globally was 3.03 million and 1.49 million, respectively [3]. At present, chemotherapy is a main treatment method for DLBCL patients. Although chemotherapy is effect on DLBCL patients, the survival rate is less than 40% and the 5-year survival rate is only 20 to 30% [4]. Researchers point out that the low survival rate was related to the poor nutritional status for DLCBL patients [5]. Thus, the indexes including International Prognostic Index (IPI), Grupo Español de Linfomas/Trasplante de Médula ósea International Prognostic Index (GELTAMO-IPI) and Comprehensive Cancer Network International Prognostic Index (NCCN-IPI) were used to evaluate the prognosis of patients with DLBCL. However, these indexes did no deeply considered deeply patient’s nutritional status [6,7,8]. Therefore, there is an urgent need to explore a prognosis marker for DLCBL patients that reflects their nutritional status.
Malnutrition is a common characteristic for all cancer patients. The data reported that malnutrition was implicated in 10–20% of deaths in cancer patients, with an incidence of about 80% [9, 10]. Previous studies illustrated that malnutrition had adverse effects on patients’ prognosis [11, 12]. Thus, medical staff should pay close attention to patients' nutritional status and be able to use nutritional risk assessment tools skillfully. Geriatric nutritional risk index (GNRI) is an effective tool used to assess nutritional status and is based on serum albumin and Body Mass Index (BMI). GNRI is calculated according to 14.89 × serum albumin (mg/dl) + 41.7 × (current body weight /ideal body weight) [13]. And GNRI has shown a good prognostic value in tumor field [14, 15]. Nevertheless, there is not been proven whether GNRI plays a vital role in the prognosis of DLBCL patients. Hence, the present study was intended to discuss the effect of GNRI on prognosis of DLBCL.
Materials and methods
Search strategy
This meta-analysis performed based on previous publications, so it did not require any approval by ethical committee. The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [16] (CRD42023413624). We systematically and comprehensively retrieved PubMed, Embase, Cochrane Library, Scopus and Web of Science databases from inception of the databases to March 20, 2023. Search terms were: (“geriatric nutritional risk index” OR “GNRI”) AND (“Lymphoma” OR “Lymphomas” OR “Lymphadenoma” OR “adenolymphoma” OR “Lymph-gland tumour” OR “Lymphoma, Malignant” OR “Lymphomas, Malignant” OR “Malignant Lymphoma” OR “Malignant Lymphomas”). Only human studies were considered for inclusion in this article. Meanwhile, there are no language restrictions on all studies. Furthermore, we carefully read the references and manually checked the citations of the original studies in order to maximize access to relevant reviews.
Inclusion and exclusion criteria
The inclusion criteria were set base on the principle of PICOS (P: Participants; I: Intervention; C: Control; O: Outcomes; S: Study design). The special contents were listed below: (1) participants of studies were DLBCL patients; (2) GNRI formula was as follows: 14.89 × albumin(g/L) + 41.7 × (current body weight/ideal body weight). And 22 × [height(m)]2 was defined as ideal weight; (3) the patient was classified as exposed groups according to low GNRI; (4) in the original sources, there had accurate cut off value of GNRI; (5) the complete data of hazard ratios (HRs) and 95% confidence interval (CI) were acquired; (6) overall survival (OS), cancer special survival (CSS), progression-free survival (PFS) and recurrence-free survival (RFS) were reported; (7) only cohort studies were eligible. Publications meeting the following criteria were excluded: studies had repeated publications or could not be extracted the survival outcomes; animal studies, reviews, conference, letters, case reports and so on.
Data extraction and quality assessment
Two researchers dependently extract and select literatures in accordance with the eligible criteria. When it comes to disagreements, they carefully discussed and resolved until agreement the reach. If disagreements could not be reasonable solved, consensus was achieved by a third investigator. Basic information primarily involved the last name of first author, publishing time, study design, study center, simple size, age, gender, cancer stage, treatment, follow-up period, cut-off value and analysis methods. Outcome variables of this literature review includes OS and PFS. Using HRs and CI to analyze the relationship between GNRI and OS or PFS. Quality of the eligible studies was assessed via Newcastle Ottawa Scale (NOS) [17]. The score of scale aggregate 9, ≥ 7 scores were considered high-quality literature. Conversely, < 7 scores were considered low-quality literature.
Statistical analysis
Statistical analyses was done with Stata (version 17.0). The perspective value of the GNRI in patients with DLBCL was estimated by integrating HRs and 95%CI for outcome indicators. Heterogeneity of the included researches was confirmed using I2 statistics and Cochran’s Q test. Pooled effect size was combined with the random-effects model (REM) or fix-effects model (FEM) according to heterogeneous outcome. If I2 > 50% and (or) PQ < 0.1, we chose the random-effects model (REM) for significant heterogeneity. Otherwise, we used fixed-effects model (FEM) to combine effect size. Subgroup analysis were conducted to evaluate the effect of the confounding factors in the meta-analysis. We also performed sensitivity analysis to verify the reliability of results. The risk of publication bias was evaluated by Egger’s and Begg’s tests. p < 0.05 was considered statistically significant.
Results
Literature search
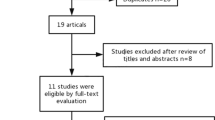
Figure 1 shows process of studies screening. In conclusion, 178 publications in total were retrieved from all kinds of databases based on the search strategy. First, 117 initial studies remained after filtering out duplicates. Then, after reading tittles and abstracts of the studies, including no relevant studies with the topic of this study and reviews and conferences, 102 studies were removed. Finally, by reading full-text articles, 15 studies were left for the meta-analysis. 8 articles were excluded from the review due to the following reasons: (1) author cannot provide survival outcomes (n = 2); (2) there were not cut-off value (n = 2); (3) GNRI not consider as exposure (n = 3); (4) OS data were not reported (n = 1). Finally, this meta-analysis included 7 studies involving 2448 participants [18,19,20,21,22,23,24].
Characteristics of the included studies
Table 1 shows the main information of the seven cohort studies. Two of them were multi-center studies [23, 24], while the remaining studies were single-center studies [18,19,20,21,22], with simple size ranging from 205 to 615. These studies were published during 2018–2023. And two studies were from China, three studies were from Japan and remaining studies were from Korea or Turkey. Treatment of patients with DLBCL mainly involved two methods, including immunochemotherapy and chemotherapy, and the cancer stage varied from I to IV. All the studies were retrospective cohorts, with using multivariate analysis. In addition, seven studies reported OS and three studies reported PFS [18,19,20]. The seven studies had a NOS scores ranged from 7 to 9.
GNRI and OS in patients with DLBCL
Seven cohort studies, which included a total of 2, 448 patients [18,19,20,21,22,23,24], reported association between the GNRI and OS. As displayed in Fig. 2, the forest plot showed that a low GNRI was significant associated with poor OS in DLBCL patients (HR 1.78, 95% CI 1.27–2.50, p < 0.01). As subgroup analysis showed that the GNRI was an independent prognostic factor influencing the OS of patients with DLBCL regardless of publishing time, GNRI of cut-off value and study design (Table 2).
GNRI and PFS in patients with DLBCL
Three cohort studies involved 639 patients [18,19,20] described HRs and 95%CIs for PFS. The forest plot for combined results showed that the lower GNRI associate with worse of PFS (HR: 2.31, 95%CI: 1.71–3.13, p < 0.01). As shown in Fig. 3, the heterogeneity was not significant in three studies (I2 = 14.1%, p = 0.312). The subgroup analysis indicated that a low GNRI was correlated with poor PFS regardless of publishing time, GNRI cut-off value and treatment method (Table 3).
Sensitivity analysis
In addition, we also conducted a sensitivity analysis to explore the potential source of heterogeneity by removing each included study each time. The results demonstrated that regardless of eliminating any of eligible studies did not significant alter the effect of the GNRI on the combine meta-analysis for OS (Fig. 4). In brief, the present findings were reliable and robust. At the same time, the pooled HRs showed that the results of PFS also was reliable and robust (Fig. 5).
Publication bias
Assessment of publication bias was assessed base on Egger’s test. The results of Egger’s test (p = 0.206) showed that no significant publication bias for OS (Fig. 6). Then, we also conducted a bias test analysis for PFS (Fig. 7). The results showed that no significant publication bias for PFS (p = 0.955). Due to the small number of literatures included in this study, Begg’s test was not conducted.
Discussion
In this study, we intended to explore the effect of GNRI on prognosis of patients with DLBCL. Seven studies involving 2448 patients in total were included. According to the research results, GNRI was related to prognosis of DLBCL patients. Namely, the lower the GNRI, the worse the OS and PFS of patients (p < 0.01). Meanwhile, publication bias test suggested that the possibility of publication bias was small. In addition, in order to control the effect of potential confounding factors on the main outcome indicators, sensitivity analysis also confirmed further that the results of this study were reliable and stable. But, not every subgroup analysis had statistically significant results. Reasons as follow: first, there were few literatures that met the criteria, and the sample size was relatively insufficient; second, all studies were published by Asian countries; finally, meta-analysis included were retrospective studies.
Malnutrition is well known to increase the risk of postoperative complications in cancer patients, extend their stay in hospital and augment the cost of treatment [25]. However, sufficiently nutritional support can avoid these disadvantages. As a consequence, it is important to accurately assess the nutritional status in cancer patients for their better prognosis. GNRI is a nutritional index based on serum albumin and weight, with the simple, convenient and economic features, which has been proven to be effective in many diseases [26,27,28,29,30]. The effect of serum albumin and weight in cancer may provide a new perspective on the association between GNRI and prognosis in DLBCL patients. It is well known that serum albumin level is an important indicator of nutritional status. There was a correlation between low albumin levels with high cancer risk, increased systemic inflammatory response, dyssynthesis of liver function, and shorter overall survival time [31, 32]. Thus, serum albumin was deemed to play a significant role in prognosis of cancer patients. In other words, the lower albumin level of tumor patients, the more obvious the malnutrition status and the worse the prognosis. A growing of evidence reveals that hypoalbuminemia has been recognized as a strong predictor for survival outcomes of DLBCL patients [33, 34]. At the same time, weight loss is another important indicator for the nutritional status of cancer patients [25]. The previous study found that lower BMI was highly associated with poorer cancer prognosis [35]. In conclusion, a combination of serum albumin and body weight may have a significant prognostic value in the DLBCL patients.
It is worth nothing that this is the first meta-analysis to explore and analyze the prognostic effect of GNRI in DLBCL patients. Based on the results of published articles, a previous study found that regardless of the age of patients, GNRI was an independently predictive factor of OS in patients with non-small cell lung cancer, low GNRI was associated with a higher risk of OS [36]. Similarly, Tamuro et al. investigated the predictive value of preoperative GNRI in patients with colorectal cancer, and showed that low GNRI was associated with poor OS and RFS [37]. Besides that, a meta-analysis of GNRI in predicting for human malignancy yielded similar results in OS, PFS, disease-free survival, and cancer-specific survival [14]. This is consistent with the results of our meta-analysis. These findings have demonstrated that assessment of malnutrition using the lower GNRI may improve OS, PFS, DFS and CSS in cancer disease. Our study integrates existing evidence and found that GNRI is of good value in predicting the outcome of DLBCL patients.
Nonetheless, there were a few limitations of this study. Firstly, only seven studies were enrolled in this study, all of which were retrospective studies, and five of which were single-center studies. The results need to be validated by more large-sample multi-center prospective studies. Secondly, due to the small number of literatures, Egger’s test was only carried out. The Begg’s method was not used to test for publication bias. Thirdly, all included studies were from Asia. The results were not necessarily applicable to other ethnic groups. Finally, there was high heterogeneity among studies, which may be related to the included studies were all retrospective studies.
Conclusions
To summarize, our meta-analysis to explore prognostic significance of the GNRI in DLBCL.
Results revealed that GNRI can act as a predictor of DLBCL. In other words, low GNRI serves as an indicator of poor survival outcomes in patients with DLBCL. Therefore, the GNRI could be a potential biomarker for the evaluation of cancer prognosis in DLBCL. Nevertheless, further large multi-center prospective cohorts are still needed to validate our results in the future.
Availability of data and materials
Please contact author for data requests.
References
Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012;380(9844):848–57.
Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018;50(1):74–87.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz N, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(5):1187–96.
Takahara T, Nakamura S, Tsuzuki T, Satou A. (2023) The Immunology of DLBCL. Cancers (Basel) 15(3):835
A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993; 329(14): 987–94.
Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837–42.
Montalbán C, Díaz-López A, Dlouhy I, Rovira J, Lopez-Guillermo A, Alonso S, et al. Validation of the NCCN-IPI for diffuse large B-cell lymphoma (DLBCL): the addition of β(2) -microglobulin yields a more accurate GELTAMO-IPI. Br J Haematol. 2017;176(6):918–28.
Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin Nutr. 2021;40(5):2898–913.
Sonmez O, Tezcanli E, Bas D, Kazanci HB, Altinok A, Demir A, et al. Identifying knowledge and practices regarding cancer patient malnutrition: a survey study among oncologists. Nutr Cancer. 2022;74(7):2392–9.
Kose E, Wakabayashi H,Yasuno N. Polypharmacy and Malnutrition Management of Elderly Perioperative Patients with Cancer: A Systematic Review. Nutrients. 2021; 13(6):1961.
Salas S, Cottet V, Dossus L, Fassier P, Ginhac J, Latino-Martel P, et al. Nutritional factors during and after cancer: impacts on survival and quality of life. Nutrients. 2022; 14(14):2958.
Kobayashi I, Ishimura E, Kato Y, Okuno S, Yamamoto T, Yamakawa T, et al. Geriatric nutritional risk index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol Dial Transplant. 2010;25(10):3361–5.
Lv GY, An L, Sun DW. Geriatric nutritional risk index predicts adverse outcomes in human malignancy: a meta-analysis. Dis Markers. 2019;2019:4796598.
Wang H, Li C, Yang R, Jin J, Liu D, Li W. Prognostic value of the geriatric nutritional risk index in non-small cell lung cancer patients: a systematic review and meta-analysis. Front Oncol. 2021;11: 794862.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372: n160.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Atas U, Sozel H, Iltar U, Yucel OK, Salim O, Undar L. The prognostic impact of pretreatment geriatric nutritional risk index in patients with diffuse large b-cell lymphoma. Nutr Cancer. 2023;75(2):591–8.
Chuang TM, Liu YC, Hsiao HH, Wang HC, Du JS, Yeh TJ, et al. Low geriatric nutritional risk index is associated with poorer prognosis in elderly diffuse large b-cell lymphoma patients unfit for intensive anthracycline-containing therapy: a real-world study. Nutrients. 2021; 13(9):3243.
Go SI, Kim HG, Kang MH, Park S, Lee GW. Prognostic model based on the geriatric nutritional risk index and sarcopenia in patients with diffuse large B-cell lymphoma. BMC Cancer. 2020;20(1):439.
Kanemasa Y, Shimoyama T, Sasaki Y, Hishima T, Omuro Y. Geriatric nutritional risk index as a prognostic factor in patients with diffuse large B cell lymphoma. Ann Hematol. 2018;97(6):999–1007.
Li Z, Guo Q, Wei J, Jin J, Wang J. Geriatric nutritional risk index is not an independent predictor in patients with diffuse large B-cell lymphoma. Cancer Biomark. 2018;21(4):813–20.
Lee S, Fujita K, Morishita T, Negoro E, Oiwa K, Tsukasaki H, et al. Prognostic utility of a geriatric nutritional risk index in combination with a comorbidity index in elderly patients with diffuse large B cell lymphoma. Br J Haematol. 2021;192(1):100–9.
Matsukawa T, Suto K, Kanaya M, Izumiyama K, Minauchi K, Yoshida S, et al. Validation and comparison of prognostic values of GNRI, PNI, and CONUT in newly diagnosed diffuse large B cell lymphoma. Ann Hematol. 2020;99(12):2859–68.
Liu C, Lu Z, Chen L, Yang X, Xu J, Cui H, et al. Predictive value of geriatric nutritional risk index in older adult cancer patients. J Nutr Health Aging. 2022;26(2):153–6.
Sakamoto T, Makinoya M, Sunaguchi T, Goto K, Morimoto M, Murakami Y, et al. Geriatric nutritional risk index as a prognostic factor in patients with recurrent pancreatic cancer. PLoS ONE. 2022;17(7): e0271073.
Xiong J, Wang M, Zhang Y, Nie L, He T, Wang Y, et al. Association of geriatric nutritional risk index with mortality in hemodialysis patients: a meta-analysis of cohort studies. Kidney Blood Press Res. 2018;43(6):1878–89.
Li H, Cen K, Sun W, Feng B. Prognostic value of geriatric nutritional risk index in elderly patients with heart failure: a meta-analysis. Aging Clin Exp Res. 2021;33(6):1477–86.
Zhao Y, Lin T, Hou L, Zhang M, Peng X, Xie D, et al. Association between geriatric nutritional risk index and frailty in older hospitalized patients. Clin Interv Aging. 2021;16:1241–9.
Lee M, Lim JS, Kim Y, Lee JH, Kim CH, Lee SH, et al. Association between Geriatric Nutritional Risk Index and Post-Stroke Cognitive Outcomes. Nutrients. 2021; 13(6):1776.
Yang Z, Zheng Y, Wu Z, Wen Y, Wang G, Chen S, et al. Association between pre-diagnostic serum albumin and cancer risk: results from a prospective population-based study. Cancer Med. 2021;10(12):4054–65.
Spinella R, Sawhney R, Jalan R. Albumin in chronic liver disease: structure, functions and therapeutic implications. Hepatol Int. 2016;10(1):124–32.
Ochi Y, Kazuma Y, Hiramoto N, Ono Y, Yoshioka S, Yonetani N, et al. Utility of a simple prognostic stratification based on platelet counts and serum albumin levels in elderly patients with diffuse large B cell lymphoma. Ann Hematol. 2017;96(1):1–8.
Bairey O, Shacham-Abulafia A, Shpilberg O, Gurion R. Serum albumin level at diagnosis of diffuse large B-cell lymphoma: an important simple prognostic factor. Hematol Oncol. 2016;34(4):184–92.
Cui L, Yu H, Sun Q, Miao Y, Jiang K, Fang X. Effects of body mass index and serum albumin on overall survival in patients with cancer undergoing pancreaticoduodenectomy: a single-center retrospective cohort study. World J Surg Oncol. 2022;20(1):221.
Peng SM, Yu N, Ren JJ, Xu JY, Chen GC, Yang JR, et al. The geriatric nutritional risk index as a prognostic factor in patients with advanced non-small-cell lung cancer. Nutr Cancer. 2021;73(11–12):2832–41.
Hayama T, Hashiguchi Y, Ozawa T, Watanabe M, Fukushima Y, Shimada R, et al. The preoperative geriatric nutritional risk index (GNRI) is an independent prognostic factor in elderly patients underwent curative resection for colorectal cancer. Sci Rep. 2022;12(1):3682.
Acknowledgements
We thank all researchers and study participants for their contributions.
Funding
Key R&D Projects, Jiangxi (20181BBG70022).
Author information
Authors and Affiliations
Contributions
The final manuscript was read, reviewed, and approved by all authors. CY and YX: study concept and design; literature search and acquisition of data; data analysis; preparation of manuscript. YH and SL: literature search; critical revision before submission. HF and ZC: revision of the manuscript. JW: study concept and design; critical revision of the manuscript; final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval and consent to participates
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, C., Xie, Y., Hua, Y. et al. Prognostic value of geriatric nutritional risk index in patients with diffuse large B-cell lymphoma: a meta-analysis. Clin Transl Oncol 26, 515–523 (2024). https://doi.org/10.1007/s12094-023-03271-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03271-w