Abstract
Purpose
Cutaneous melanoma is an aggressive and deadly cancer resulting from malignant transformation of cells involved in skin pigmentation. Glycolysis is widely implicated in cancer progression, but its precise role in melanoma has not been extensively studied. Here, we investigated the role of the glycolysis regulator phosphofructokinase 1 platelet isoform (PFKP) in melanoma progression.
Methods
PFKP expression in human melanoma tissues was analyzed by immunohistochemistry. Knockdown of PFKP by siRNA and overexpression of PFKP were performed to evaluate its functions in vitro. CCK-8 assay was used to assess cell proliferation. Glycolytic activity was determined via measurement of extracellular acidification rate (ECAR), lactic acid level, and ATP content. A tumor xenograft model was used to test the function of PFKP in vivo.
Results
PFKP upregulation was observed in human melanoma tissues and correlated with poor patient survival. Knockdown of PFKP in human melanoma cells suppressed cell proliferation and reduced ECAR, ATP levels, and lactic acid levels, while overexpression of PFKP displayed the opposite effects. In vivo, knockdown of PFKP in melanoma cells markedly reduced tumorigenesis. Inhibitory effects on cell proliferation, glycolysis, and tumorigenesis due to PFKP knockdown were further augmented upon treatment with the glycolysis inhibitor 2-deoxy-D-glucose (2-DG).
Conclusion
Collectively, these results indicate that PFKP expression in melanoma cells increases proliferation and glycolytic activity in vitro and promotes tumorigenesis in vivo, suggesting that suppression of PKFP and inhibition of glycolysis may potently suppress melanoma progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanoma is a cancer that arises due to genetic alterations in pigment-producing cells called melanocytes [1], and occurs in tissues such as the skin, leptomeninges, eye, and inner ear [2, 3]. Cutaneous melanoma represents approximately 1% of all skin malignancies, yet it is responsible for 80% of skin cancer-related mortality, making it the deadliest type of skin cancer [4]. Current treatment strategies for melanoma include surgery, targeted therapy, chemotherapy, and immunotherapy [1]. Surgery can often effectively treat early-stage melanoma [5]. However, late-stage, metastatic melanoma is resistant to majority of current therapies and is associated with extremely poor prognosis [6, 7]. Based on recent studies, patients with metastatic melanoma have a 1-year survival rate of ~ 25% and a 2-year survival rate of 10%, with 6-month median overall survival [8]. Thus, the identification of molecular factors that influence melanoma metastasis is crucial in developing effective therapy for this cancer.
Several signaling pathways have been identified as major drivers in the pathogenesis of melanoma, including MAPK, WNT, and PI3K, all of which play central roles in cancer cell proliferation and growth [9]. N-RAS and BRAF activating mutations are also observed in ~ 13–25% [10,11,12] and ~ 50% [13, 14] of cutaneous melanoma, respectively. More recently, it is increasingly recognized that pathways involved in energy metabolism, including PI3K, c-MYC, TP53, and HIF-1α, affect initiation and progression of skin cancers and determine tumor responses to targeted therapies [15]. Glucose metabolism provides energy source for cellular activities and is critical for the normal operation of the body [16,17,18]. However, in tumor cells, glucose metabolism is altered, shifting from oxidized glucose to aerobic glycolysis, a phenomenon called the Warburg effect [18, 19]. Past studies have uncovered a large number of metabolic profiles in cancer cells, in which high glycolytic activity was consistently observed in fast-growing cells [20, 21]. The reliance of cancer cells on aerobic glycolysis—which is an ineffective metabolic pathway for energy metabolism—amidst availability of sufficient oxygen results in alterations of energy metabolism that favor cancer cell growth and proliferation.
Phosphofructokinase (PFK) is an enzyme that converts fructose-6-phosphate to fructose-1,6-diphosphate in an irreversible manner [18, 19] and is a major rate-limiting enzyme in glycolysis. There are three isoforms of PFK, one of which is the platelet-specific isoform, or PFKP, which plays a crucial role in several cancers, including breast cancer [22], glioblastoma [23], renal clear cell carcinoma [24], pancreatic cancer [25], and oral squamous cell carcinoma [26]. However, the precise function of PFKP in melanoma, has not been explored. In this study, we uncover a notable role of PFKP and glycolysis in promoting melanoma cell proliferation and tumorigenicity.
Materials and methods
Bioinformatics
Survival analysis was performed on UALCAN website (http://ualcan.path.uab.edu/analysis.html) using The Cancer Genome Atlas (TCGA) dataset. First, selecting the TCGA analysis on the main page of UALCAN website; then, typing PFKL, PFKM, and PFKP genes and choosing Skin cutaneous melanoma; after click the explore button, the related analysis links were exhibited including survival analysis.
Human samples
101 Human melanoma tissues were collected from ZhongShan Hospital subjected to immunohistochemistry (IHC) to analyze PFKP protein expression. All patients provided signed, informed consent.
Cell culture
Human melanoma cells, including A2058, A375, SKMEL1, and SKMEL-28, and normal melanocytes HEMn-MP and HEMn-DP were provided by the cell bank of Shanghai Biology Institute (Shanghai, P.R. China) and maintained in DMEM media (Trueline, Kaukauna, WI, USA) containing 10% FBS (Thermo Fisher Scientific), 2 mM 1-glutamine, and 1% penicillin/streptomycin (Solarbio, Beijing, P.R. China). Cells were incubated under a 5% CO2 at 37 ºC. A2058 and A375 were characterized as more aggressive phenotype with high proliferation, migration and invasion ability, while SKMEL-28 was characterized as less aggressive phenotype.
Quantitative polymerase chain reaction (qRT-PCR)
TRIzol Reagent (Invitrogen, Waltham, MA, USA) was used to extract total RNA. A cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to reverse transcribe RNA into cDNA per instructions of the manufacturer, and real-time PCR was carried out with the following conditions: 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 45 s. Samples were normalized to GAPDH, and the 2−∆∆Ct method was used to calculate relative gene expression. Data represent the average of three replicates. The following primers were used: PFKP, F: 5′-TCACACCGCTTCCCTTGTC-3′, R: 5′-CTTCTGCACGGCCTCCTTATC-3′. GAPDH, F: 5′-AATCCCATCACCATCTTC-3′, R: 5′-AGGCTGTTGTCATACTTC-3′.
Western blot
Western blot was performed as previously described. The following primary antibodies were used: PFKP (Ab204131, Abcam, UK), PCNA (Ab92552, Abcam, UK), and β-actin (#4970, CST, USA), followed by secondary anti-mouse IgG antibody (1:1000, Beyotime, Shanghai, P.R. China). An enhanced chemiluminescence system (Tanon, Shanghai, P.R. China) was used to detect protein expression.
Knockdown and overexpression
For knockdown studies, short hairpin interfering RNAs (shRNAs) targeting human PFKP (shPFKP-1 (site: 212–230): 5′-CCAAGGTGTACTTCATCTA-3′; siPFKP-2 (site 795–815), 5′-GGAGCAGATGTGTGTCAAA-3′; and siPFKP-3 (1280–1298) 5′-GCAACGTAGCTGTCATCAA-3′), along with a negative control siRNA (siNC, 5′-UUCUCCGAACGUGUCACGUTT-3′) (Major Industrial Co., Ltd, Shanghai, China), were designed and constructed into pLKO.1 lentiviral plasmids. For overexpression studies, PFKP (NM_002627.5) cDNA was cloned into pLVX-puro lentiviral plasmid, and a mock plasmid was used as a negative control. Plasmid transfection into prostate epithelial cells was carried out using Lipofectamine 2000 per manufacturer’s instruction. Cells were cultured for 48 h prior to analysis.
Cell proliferation
Cell Counting Kit-8 (CCK-8) (Signalway Antibody, Maryland, USA) was used to quantify proliferation. Following 0, 12, 24, and 48 h in culture, cells were given CCK-8 solution-containing media (1:10) and incubated for 1 h. Cell proliferation was determined by measuring optical densities (ODs) at 450 nm wavelength on a microplate reader (Pulangxin, Beijing, P.R. China). Each experiment was conducted in triplicates.
ATP and lactic acid analysis
The concentrations of ATP and lactic acid were analyzed using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following instructions from the manufacturers. Briefly, treated cells were collected and diluted accordingly. After setting the blank control and standard control, the samples were incubated with the reagent provided in the kit accordingly. Finally, the ATP and lactic acid level were determined by measuring ODs at 636 nm and 530 nm, respectively.
Glycolytic assay
A2058, A375, and SKMEL-28 cells were plated into 24-well plates, washed, then cultured in XF Base Medium containing L-glutamine (2 mM) and glucose (25 mM). An XFe24 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA) was used to measure extracellular acidification rate (ECAR).
Immunohistochemistry (IHC)
Freshly obtained tissue blocks (Normally 1.0 cm × 1.0 cm × 0.2 cm) were fixed with 10% formalin and embedded in a mold, then sliced, and conventionally de-waxed into water. Immunohistochemistry was performed as previously described and images were obtained using a microscope. Positive areas of PFKP (ab110283, Abcam, UK) were calculated.
Xenograft model
Nude mice (4–6 weeks old) from the Shanghai Laboratory Animal Company were randomly divided into two groups: shNC and shPFKP. SKMEL-1 cells transfected with 100 µL shNC or shPFKP (5 × 106 cells) were injected into nude mice (n = 6 in each group) subcutaneously. After tumor formation on day 12, tumor growth in both groups was monitored until day 33. On day 33, tumor samples were collected and tumor weight (g) recorded. PCNA expression was evaluated by IHC using antibody against Rb PCNA (10205-2-AP, Proteintech, USA). Care of animal and experimentation were approved by the ethics committee of ZhongShan Hospital. All procedures were performed according to internationally accepted ethical guidelines.
Statistical analysis
GraphPad Prism Software Version 7.0 (La Jolla CA, USA) was used for data statistics and analysis. Data were shown as mean ± SD and represented three replicates. t test analysis was used to compare data between two groups. A one-way analysis with Tukey’s post hoc test was used to compare data among multiple groups. A p value < 0.05 was deemed statistically significant.
Results
PFKP is upregulated in human melanoma tissues
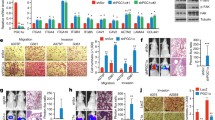
Using the TCGA database, we analyzed different phosphofructokinase (PFK) enzymes, including PFKL, PFKM, and PFKP, in melanoma tissues and their correlation with patient survival. As shown in Fig. 1A, PFKL expression did not show significant correlation with melanoma patient survival. Similarly, no significant correlation was noted between PFKM expression and melanoma patient survival (Fig. 1B). On the other hand, increased PFKP expression strongly correlated with reduced survival of melanoma patients (Fig. 1C). Immunohistochemistry confirmed upregulation of PFKP in melanoma tissues compared with corresponding para-cancerous tissues (Fig. 1D), suggesting a potential role in melanoma progression.
PFKP expression is elevated in human melanoma tissues. A–C The relationship between PFKL/PFKM/PFKP gene expression and SKCM patient overall survival was analyzed. Data were collected from TCGA database (http://ualcan.path.uab.edu/analysis.html). D IHC staining was performed to examine PFKP expression in human melanoma tissues. ***p < 0.001 vs para-cancerous tissues
PFKP silencing inhibited human melanoma cell proliferation and glycolytic activity
We examined PFKP mRNA expression in different human melanoma cell lines, including A2058, A375, SKMEL-1, and SKMEL-28, compared to HEMn-MP and HEMN-DP normal melanocytes. As indicated in Figure S1A, upregulation of PFKP mRNA was noted in all 4 melanoma cell lines compared to HEMn-MP and HEMn-DP normal melanocytes (Figure S1A–B), with A375 cells displaying the highest mRNA expression of PFKP, followed by A2058 (Figure S1A). We, therefore, used these two cell lines to knockdown PFKP in vitro to further evaluate the functions of PFKP. As shown in Figure S1C–D, knockdown of PFKP using three different short interfering RNAs (shRNAs) dramatically decreased PFKP mRNA and protein levels (p < 0.001), indicating efficiency of knockdown.
We then evaluated how PFKP knockdown affected cell proliferation in vitro. As shown by cell-counting kit (CCK)-8 assay, silencing of PFKP strongly inhibited proliferation of A375 and A2058 cells (Fig. 2A–B) after 48 and 72 h (p < 0.001). In addition, PFKP knockdown suppressed the growth of melanoma cells as indicated by colony-forming assay (Fig. 2C). Consistent with its role in glycolysis, knockdown of PFKP significantly reduced the levels of lactic acid and ATP (Fig. 2D) and extracellular acidification rate (ECAR, p < 0.001). Together, these results demonstrate that PFKP increases proliferation and glycolytic activity of human melanoma cells.
PFKP silencing inhibited human melanoma cell proliferation and glycolytic activity. A–B Proliferation of human A375 and A2058 cells was suppressed upon knockdown of PFKP using shPRKP-1 and shPFKP-2. ***p < 0.001 vs shNC. C. Knockdown of PFKP blocked colony-forming capacity of human A375 and A2058 cells in vitro. ***p < 0.001 vs shNC. D–E Lactic acid and ATP levels were significantly decreased in shPFKP-1- and shPFKP-2-transfected human A375 and A2058 cells. ***p < 0.01 vs shNC. F–G Knockdown of PFKP decreased glycolytic activity in human A375 and A2058 cells
Knockdown of PFKP suppressed tumorigenic properties of melanocytes in vivo
To probe the function of PFKP in vivo, stable SKMEL-1 cells with or without PFKP knockdown were generated by expressing shPFKP or shNC, respectively. Cells were then injected into nude mice (n = 6 in each group) subcutaneously. After formation of tumors on day 12, tumor growth in both groups was monitored until day 33, on which day mice were sacrificed and tumors were harvested. Interestingly, knockdown of PFKP in SKMEL-1 cells slowed down tumor growth (p < 0.001) in vivo (Fig. 3A), resulting in decreased size of tumors (Fig. 3B) and reduced tumor weight (Fig. 3C, p < 0.001). Moreover, PFKP knockdown resulted in decreased expression of the proliferating cell nuclear antigen (PCNA), as shown by immunostaining and Western blot (Fig. 3D–E). These data demonstrate that PFKP induces tumorigenicity of melanoma cells in vivo.
Knockdown of PFKP suppressed tumorigenic properties of melanoma cells in vivo. A PFKP silencing in vivo decreased tumor volume. *p < 0.05 vs shNC, ***p < 0.001 vs shNC. B Tumor size comparison between shNC and shPFKP group. C Knockdown of PFKP reduced tumor weight in vivo. ***p < 0.001 vs shNC. D Immunofluorescence staining was performed to analyze PFKP and PCNA protein expression in shNC and shPFKP tumors
PFKP overexpression stimulated proliferation and glycolysis of human SKMEL-28 cells
To further establish the functions of PFKP in regulating cell proliferation and glycolysis, we used the human melanoma cell line SKMEL-28, which displayed the lowest mRNA and protein levels of PFKP among melanoma cell lines examined (Figure S1A-B), to generate stable cells with PFKP overexpression or control vector. Increased mRNA and protein levels of PFKP in PFKP-overexpressing SKMEL-28 cells confirmed efficiency of overexpression (Figure S1E–F).
PFKP overexpression markedly enhanced proliferation of SKMEL-28 cells after 72 h (Fig. 4A, p < 0.001), and resulted in increased colony formation (Fig. 4B). Moreover, overexpression of PFKP in SKMEL-28 cells elevated the levels of lactic acid and ATP (Fig. 4C–D, p < 0.001), and increased ECAR (Fig. 4E), indicating regulation of glycolysis by PFKP.
PFKP overexpression stimulated proliferation and glycolysis of human SKMEL-2B cells. A Overexpression of PFKP induced proliferation of SKMEL-2B cells. PFKP overexpression increased colony-forming capacity of SKMEL-2B cells. C–D PFKP overexpression elevated the levels of lactic acid and ATP in SKMEL-2B cells. E PFKP overexpression increased glycolytic activity in SKMEL-2B cells
Inhibition of glycolysis augmented the effects of PFKP knockdown in vitro
To determine how inhibition of glycolysis affected PFKP-mediated functions, human A375 cells were treated with the glycolysis inhibitor 2-deoxy-D-glucose (2-DG). As shown in Fig. 5A, treatment with 2-DG inhibited proliferation of A375 cells at 48 h and 72 h compared to vehicle-treated cells, and further decreased proliferation in A375 cells with PFKP knockdown (p < 0.001). Likewise, treatment with 2-DG reduced ATP and lactic acid levels in A375 cells (Fig. 5B–C), and further reduced these levels in A375 cells with PFKP knockdown (p < 0.001). Furthermore, 2-DG treatment markedly diminished ECAR in A375 cells and even more dramatically in A375 cells with PFKP knockdown (Fig. 5D), indicating that inhibition of glycolysis and knockdown of PFKP can potently inhibit melanoma cell proliferation and glycolysis.
Inhibition of glycolysis augmented the effects of PFKP knockdown in vitro. A Proliferation of shNC- or shPFLP-expressing A375 cells ± 2-DG treatment was evaluated by CCK-8 assay. *p < 0.05, ***p < 0.001. B–C Treatment with 2-DG decreased ATP and lactic acid levels in shPFKP-transfected A375 cells. ***p < 0.001 vs shNC + vehicle. !p < 0.05 vs shNC + 2-DG, !!p < 0.01 vs shNC + 2-DG, !!!p < 0.001 vs shNC + 2-DG. D Treatment with 2-DG decreased glycolysis in shPFKP-transfected A375 cells
2-DG treatment further reduced tumorigenicity of PFKP-silenced melanoma cells in vivo
Finally, we examined how 2-DG treatment affected tumorigenicity of melanoma cells in vivo. Stable SKMEL-1 cells with or without PFKP knockdown that were subjected to either vehicle or 2-DG treatment were injected subcutaneously into nude mice, then tumor growth was monitored for 33 days. 2-DG treatment strongly suppressed tumor cell proliferation (Fig. 6A, p < 0.001), resulting in significantly reduced tumor size (Fig. 6B). The effect on melanoma cell proliferation was observed even more dramatically in combination with PFKP knockdown (Fig. 6C), indicating that inhibition of glycolysis and suppression of PFKP expression have a potent combined effect in blocking tumorigenicity.
2-DG treatment further reduced tumorigenicity of PFKP-silenced melanoma cells in vivo. A–B 2-DG-treated shPFKP tumors displayed reduced tumor volume and weight. ***p < 0.001. C PCNA protein expression in shNC or shPFKP tumors with or without 2-DG treatment was analyzed by immunofluorescence staining
Discussion
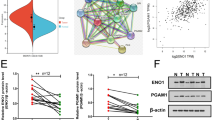
Previous studies have emerged demonstrating a key role of the glycolysis regulator PFKP in cancer development, as observed in studies on breast cancer, bladder cancer, glioma, pancreatic cancer, and renal cell carcinoma [22,23,24,25,26]. Our study uncovered that FPKP also plays a central role in melanoma progression. Specifically, we found that PFKP expression was elevated in human melanoma tissues, and higher expression level of PFKP was observed in A375 cell line which was characterized as more aggressive phenotype according to Melanoma Aggressiveness Score, while lower PFKP was observed in SKMEL-28 cell line which was characterized as less aggressive phenotype, indicating the oncogene role of PFKP. Moreover, inhibition of PFKP strongly inhibited melanoma cell proliferation and glycolysis in vitro, while overexpression of PFKP displayed the converse effects. In vivo, knockdown of PFKP reduced tumorigenicity, and this effect was further augmented upon 2-DG treatment, indicating that inhibition of PFKP and glycolysis potently suppressed melanoma progression. Consistently, PFKP has been found to be highly evaluated in breast cancer and lung cancer, associated with poor survival. Hence, our results add to the growing body of evidence supporting tumor-promoting function of PFKP.
Melanoma is an extremely aggressive tumor that displays metabolic plasticity [27]. During cancer progression, malignant melanocytes can generate ATP levels via cytosolic and mitochondrial compartments, but mostly rely on glycolysis for energy to sustain tumor development and immune escape [27]. Evidence supports that metabolism is a critical player in the emergence of drug-resistant cells [15], so combining targeted therapies with drugs that target factors involved in energy metabolism may yield promising results for melanoma treatment. Our results demonstrate that PFKP increases proliferation and glycolytic activity of melanoma cells in vitro, as well as promotes melanoma cell tumorigenicity in vivo. Thus, our data add to the body of evidence indicating tumor-promoting function of PFKP likely via regulation of glucose metabolism [28,29,30]. Moreover, our findings underscore the potential of PFKP as a target for cancer therapy.
Our findings raise important questions for future study. For instance, the upstream regulator or mechanism responsible for the upregulation of PFKP in melanoma tissues requires investigation. In breast cancer, it has been noted that BRCA1/ZBRK1 transcriptionally represses PFKP [31] while in glioblastoma, it has been demonstrated that protein kinase B (AKT) stabilizes PFKP to promote tumorigenesis [23]. Whether these proteins are involved in regulating PFKP expression in melanoma remains to be investigated. The identification of proteins and molecular factors that interact with PFKP will be a critical step toward understanding how this protein can be targeted for cancer treatment.
Data Availability
The data generated or analysed during this study are included in this article (and its supplementary materials).
References
Domingues B, Lopes JM, Soares P, Pópulo H. Melanoma treatment in review. Immunotargets Ther. 2018;7:35–49.
Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445(7130):851–7.
Tolleson WH. Human melanocyte biology, toxicology, and pathology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2005;23(2):105–61.
Eddy K, Chen S. Overcoming immune evasion in melanoma. Int J Mol Sci. 2020;21(23):8984.
Liu J, Fukunaga-Kalabis M, Li L, Herlyn M. Developmental pathways activated in melanocytes and melanoma. Arch Biochem Biophys. 2014;563:13–21.
Jiang BP, Zhang L, Guo XL, Shen XC, Wang Y, Zhu Y, et al. Poly(N-phenylglycine)-based nanoparticles as highly effective and targeted near-infrared photothermal therapy/photodynamic therapeutic agents for malignant melanoma. Small. 2017;13(8):1602496.
Bombelli FB, Webster CA, Moncrieff M, Sherwood V. The scope of nanoparticle therapies for future metastatic melanoma treatment. Lancet Oncol. 2014;15(1):e22-32.
Maio M. Melanoma as a model tumour for immuno-oncology. Ann Oncol. 2012;23(Suppl 8):viii10–4.
Paluncic J, Kovacevic Z, Jansson PJ, Kalinowski D, Merlot AM, Huang ML, et al. Roads to melanoma: key pathways and emerging players in melanoma progression and oncogenic signaling. Biochim Biophys Acta. 2016;1863(4):770–84.
Ball NJ, Yohn JJ, Morelli JG, Norris DA, Golitz LE, Hoeffler JP. Ras mutations in human melanoma: a marker of malignant progression. J Invest Dermatol. 1994;102(3):285–90.
Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–47.
van’t Veer LJ, Burgering BM, Versteeg R, Boot AJ, Ruiter DJ, Osanto S, et al. N-ras mutations in human cutaneous melanoma from sun-exposed body sites. Mol Cell Biol. 1989;9(7):3114–6.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54.
Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95(24):1878–90.
Hosseini M, Kasraian Z, Rezvani HR. Energy metabolism in skin cancers: a therapeutic perspective. Biochim Biophys Acta Bioenerg. 2017;1858(8):712–22.
Mosley AL, Ozcan S. Glucose regulates insulin gene transcription by hyperacetylation of histone h4. J Biol Chem. 2003;278(22):19660–6.
Keating ST, El-Osta A. Epigenetics and metabolism. Circ Res. 2015;116(4):715–36.
Mor I, Cheung EC, Vousden KH. Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol. 2011;76:211–6.
Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol. 2009;86(3):174–9.
Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21(3):297–308.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33.
Wang G, Xu Z, Wang C, Yao F, Li J, Chen C, et al. Differential phosphofructokinase-1 isoenzyme patterns associated with glycolytic efficiency in human breast cancer and paracancer tissues. Oncol Lett. 2013;6(6):1701–6.
Lee JH, Liu R, Li J, Zhang C, Wang Y, Cai Q, et al. Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat Commun. 2017;8(1):949.
Wang J, Zhang P, Zhong J, Tan M, Ge J, Tao L, et al. The platelet isoform of phosphofructokinase contributes to metabolic reprogramming and maintains cell proliferation in clear cell renal cell carcinoma. Oncotarget. 2016;7(19):27142–57.
Cheng L, Qin T, Ma J, Duan W, Xu Q, Li X, et al. Hypoxia-inducible factor-1α mediates hyperglycemia-induced pancreatic cancer glycolysis. Anticancer Agents Med Chem. 2019;19(12):1503–12.
Chen G, Liu H, Zhang Y, Liang J, Zhu Y, Zhang M, et al. Silencing PFKP inhibits starvation-induced autophagy, glycolysis, and epithelial mesenchymal transition in oral squamous cell carcinoma. Exp Cell Res. 2018;370(1):46–57.
Avagliano A, Fiume G, Pelagalli A, Sanità G, Ruocco MR, Montagnani S, et al. Metabolic plasticity of melanoma cells and their crosstalk with tumor microenvironment. Front Oncol. 2020;10:722.
Umar SM, Kashyap A, Kahol S, Mathur SR, Gogia A, Deo SVS, et al. Prognostic and therapeutic relevance of phosphofructokinase platelet-type (PFKP) in breast cancer. Exp Cell Res. 2020;396(1): 112282.
Kim NH, Cha YH, Lee J, Lee SH, Yang JH, Yun JS, et al. Snail reprograms glucose metabolism by repressing phosphofructokinase PFKP allowing cancer cell survival under metabolic stress. Nat Commun. 2017;8:14374.
Zhou K, Yao YL, He ZC, Chen C, Zhang XN, Yang KD, et al. VDAC2 interacts with PFKP to regulate glucose metabolism and phenotypic reprogramming of glioma stem cells. Cell Death Dis. 2018;9(10):988.
Yeerken D, Hong R, Wang Y, Gong Y, Liu R, Yang D, et al. PFKP is transcriptionally repressed by BRCA1/ZBRK1 and predicts prognosis in breast cancer. PLoS ONE. 2020;15(5): e0233750.
Acknowledgements
We sincerely acknowledged the support given by Department of Plastic Surgery, ZhongShan Hospital, FuDan University, Fenglin Rd No.180, Shanghai 200032, China for present research.
Author information
Authors and Affiliations
Contributions
CC conceived and designed the study, and drafted the manuscript. XZ collected, analyzed and interpreted the experimental data. CC and XZ revised the manuscript for important intellectual content. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by Ethical Committee of ZhongShan Hospital, FuDan University and conducted in accordance with the ethical standards.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, C., Zhang, X. Glycolysis regulator PFKP induces human melanoma cell proliferation and tumor growth. Clin Transl Oncol 25, 2183–2191 (2023). https://doi.org/10.1007/s12094-023-03096-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03096-7