Abstract
The prevalence of non-small-cell lung cancer (NSCLC) is rising every year all around the world. The interaction between cancer cells and the tumor microenvironment (TME) is a crucial factor in determining the development of human neoplasms. Organellar and cellular stress are induced during immunogenic cell death (ICD), a particularly functional response pattern. ICD is a separate but poorly characterized entity caused by various cancer treatments. The induction of ICD has the potential to change TME and the recruitment of tumor-infiltrating lymphocytes (TILs), and the coupling of ICD-inducers and other therapeutic approaches can have a synergistic role in boosting anticancer impacts. The purpose of this study is to review the studies in the field of NSCLC using ICD-inducers as a treatment strategy or as a combination therapy. This review provide for researches a better view of what has been done so far and the challenges they face in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many different genetic and epigenetic abnormalities are linked to the highly varied and complicated group of diseases characterized as cancer. The primary cause of cancer-related mortality is lung cancer. Small cell lung carcinoma (SCLC) and non-small-cell lung carcinoma (NSCLC), which account for 15% and 85% of all instances of lung cancer, respectively, are the two primary subtypes of lung cancer distinguished by their histological characteristics. Squamous cell carcinoma (SCC), adenocarcinoma (ADC), and large-cell carcinoma (LCC) are the subtypes that fall under the NSCLC. Among all cases of pulmonary cancer, 25–30% are SCC, and 5–10% are LCC (undifferentiated carcinoma). The ADC subtype of pulmonary cancer is by far the most prevalent, accounting for around 40 percent of all cases of pulmonary cancer [1].
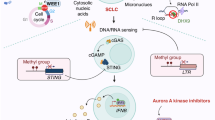
A group of cellular characteristics required for malignant transformation and tumor maintenance makes up the characteristics of cancer. Among them are the ability to resist apoptosis, persistent proliferative signaling, stimulated neovascularization, activation of invasion and metastasis, and evade immunological surveillance. All constitute the immunological system to identify and eradicate many damaged cells. Immunological surveillance is considered a regulator of normal cell differentiation, cancerous cell multiplication, and apoptosis processes during the multiphase development of cancer. Malignant cells evolve several tactics to control the immunological system and produce an environment that encourages their multiplication to avoid immunological surveillance. It is possible that the incidence and recurrence rates of cancer will rise if the functionality of the immune system is impaired over an extended length of time [84]. To describe a functionally unusual type of apoptosis that, in immunocompetent hosts, can elicit an immunological response against dead cell-associated antigens in the absence of any adjuvant, Kepp et al. coined the term “immunogenic cell death” (ICD) in 2005 [2, 3]. In fact, syngeneic mice were sufficiently protected against a subsequent challenge with malignant cells of the same kind by subcutaneous injection of cancerous cells susceptible to doxorubicin (an anthracycline recognized by regulatory bodies for the treatment of numerous malignancies), but not by cancerous cells from a different origin [3]. The potential of a particular stimulus to cause ICD as opposed to a non-immunogenic instance of apoptosis, as well as the host's capacity to recognize ICD and then develop a therapeutically relevant immunological reaction to dying cells, have all been explained by a variety of research [4,5,6]. ICD was recently proposed to be defined by Legrand et al. based on the communication between dying cells and immunological cells as a successful interaction between a dying cell and an immune system that is correctly disposed of [7]. ICD depends on the coordinated discharge of several damage-associated molecular patterns (DAMPs) [8,9,10]. These DAMPs include the accessibility of endoplasmic reticulum (ER) chaperones on the cell surface, the efflux of ATP, and the discharge of the non-histone chromatin-binding protein high mobility group box 1 (HMGB1) [11,12,13,14], as well as immunostimulatory cytokines, like type I interferon [15]. These DAMPs, once discharged in the appropriate spatiotemporal pattern [16], engage antigen-presenting cells (APC), such as dendritic cells (DC), to the site of ICD and stimulate them to engulf killed cell-associated antigens, process and introduce them to CD4+ and CD8+ T lymphocytes in the context of co signals. This results in the induction of a powerful, antigen-specific immunological reaction [17,18,19] (Fig. 1). In this study, we will review the use of ICD-inducers in the treatment of NSCLC to have a complete view of what has been done so far.
Non-small cell lung cancer (NSCLC)
Globally, there were an anticipated 18.1 million new cancer cases in 2018, according to the GLOBOCAN 2018 study [20]. Pulmonary cancers accounted for 2,093,876 new cases (11.6%) across all ages and sexes (cumulative risk: 2.75) and were the most prevalent malignancy, followed by breast, prostate, and colorectal tumors. The most frequent cause of cancer-related death worldwide, pulmonary cancer accounted for 18.4% of all cancer-related deaths, followed by colorectal, stomach, and hepatic cancers [20]. NSCLC accounts for the vast majority of pulmonary cancer diagnoses. The three subtypes of NSCLC are referred to above: ADC, SCC, and LCC. In the airway epithelial cells of the bronchial tubes in the middle of the lungs, SCC develops from initial forms of squamous cells. Small airway epithelial type II alveolar cells that release mucus and other compounds give rise to ADCs. Due to the fact that LCC does not exhibit any signs of squamous or glandular growth, it is frequently identified by default after all other possibilities have been ruled out [21].
Over the past two decades, thanks to increased knowledge of cancer biology, the landscape of therapy options for metastatic NSCLC has improved significantly [22]. In advanced or metastatic disease, the steady increase in overall survival and quality of life has been attributed to introducing various novel and more efficient molecules into therapeutic management [23]. Despite this, the five-year survival rates for total resected stage I cancer vary from 50 to 70%, while stage IIIA NSCLC survival rates range from 10 to 30% [24]. A moderate survival advantage has been demonstrated by adjuvant chemotherapy (with an absolute enhancement in the survival of 4% at five years); however, based on the stage [25, 26], more than half of patients will still relapse [27]. In a similar manner, a neoadjuvant strategy only results in a five percent absolute gain in 5 year survival [28], providing potential for further development.
Immunogenic cell death (ICD)
A growing body of evidence suggests that dying cells dynamically control immunological reactions by releasing or exposing compounds that act as danger signals to activate the innate immunological system. Despite this, the interplay between the apoptosis trigger and the downstream molecular mechanisms, which define the immunogenicity of apoptosis, is extremely complex. In an immunocompetent environment, ICD, a type of regulated cell death (RCD), is adequate to elicit an adaptive immunological reaction [81]. It ends with apoptosis, followed by the exposure, active secretion, or passive release of multiple DAMPs [9, 29,30,31]. Different pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) and NOD-like receptors (NLRs), can be used by both the innate and adaptive immunological systems to identify DAMPs. These PRRs are responsible for chemoattraction, homing, stimulation, and/or maturation, which in turn leads to the cross-presentation of tumor antigens to CD8+ CTLs in the context of powerful immune stimulation [32,33,34]. In support of this idea, mounting preclinical and clinical evidence suggests that a number of DAMPs and DAMP-associated mechanisms may be useful in predicting the prognosis of individuals experiencing a range of cancers [35, 86]. In addition, there is a substantial body of research indicating that treatment-driven ICD has the potential to trigger antitumor immunological reactions that augment the curative impacts of traditional cancer treatments such as chemotherapy and radiotherapy [36,37,38].
ICD can be triggered by a relatively small number of stimuli, including both chemical and physical substances at this point [39]. ICD can be triggered by various stressors, such as (1) intracellular pathogens [40, 41]; (2) traditional chemotherapeutic, including doxorubicin [42], epirubicin [42], idarubicin [43], mitoxantrone [42], bleomycin [44], bortezomib [45, 46], cyclophosphamide [47], oxaliplatin [48], and patupilone [49, 50]; (3) targeted antitumor compounds, like tyrosine kinase inhibitor, crizotinib, the epidermal growth factor receptor (EGFR)-specific monoclonal antibody (7A7) [51, 52], cetuximab and poly-ADP-ribose polymerase (PARP) inhibitors [52, 53]; and (4) several physical approaches, including hypericin- and redaporfin-based photodynamic treatment [80], extracorporeal photochemotherapy [80], different types of ionizing radiation, high hydrostatic pressure, and severe heat shock [13, 54, 55]. It is possible that these medicines will be especially useful in the creation of combinatorial chemotherapy strategies that will effectively stimulate the host immunological system in combating cancerous cells.
It is valuable to note that several apoptosis inducers, but not all of them, are competent to induce ICD [56], and structural or functional factors cannot predict this characteristic [57, 58]. Oxaliplatin is the sole drug that causes ICD because it causes a pre-mortem ER stress response, despite the fact that both cisplatin and oxaliplatin cause inter-and intra-strand DNA adducts [59,60,61], which have both cytostatic/cytotoxic effects [62, 63]. Consequently, the standard gold method for establishing whether a cytotoxic intervention induces ICD is still vaccination trials employing murine cancer cells and syngeneic, immunocompetent mice [4, 85], despite the availability of tests for the identification of alternative ICD markers [64]. The ER chaperones calreticulin and heat-shock proteins (HSPs) presented on the cell surface, the non-histone chromatin-binding protein HMGB1, the cytoplasmic protein annexin A1 (ANXA1), and the small metabolite ATP released from dying cells into the extracellular space, as well as type I interferons (IFNs) discharged upon de novo synthesis are primary ICD hallmarks [65, 66, 83]. Phosphorylation of eukaryotic translation initiation factor 2 subunit-α (EIF2S1, also recognized as eIF2α) and autophagy stimulation are two further markers of ICD [67, 68].
ICD-inducers in NSCLC
ICD opens up a fresh door of possibility to enhance the efficacy of cancer treatment and bring some measure of relief to sufferers. ICD comprises the destruction of cells generated not only by ICD inducers but also dying cancerous cells, which operate as a tumor vaccine by eliciting a tumor-specific immunological reaction that attacks surviving cancer cells and remaining tumor tissue. According to Fucikova et al., the level of eIF2 phosphorylation and calreticulin expression on tumor cells had a beneficial impact on the clinical outcome of NSCLC. A larger density of invading mature DC and effector memory T cell subsets and increased calreticulin expression on tumor cells were both related to the activation of adaptive immune responses in the TME. Therefore, individuals who showed intratumoral infiltration of DC or CD8+ T cells and increased calreticulin expression due to the ICD had better clinical outcomes [69].
According to research by Liu et al. cisplatin, a non-ICD-inducing medication, combined with high-dose crizotinib, an ICD-inducing tyrosine kinase inhibitor, successfully suppressed the development of several orthotopic NSCLC models that are transplantable, carcinogen- or oncogene-induced. These anticancer effects are associated with an increase in T lymphocyte recruitment and are reversed by IFN‐γ neutralization or T cell depletion. The combination of crizotinib and cisplatin increased the expression of programmed death-ligand 1 (PD-L1) and PD-1 in NSCLC and strongly sensitized NSCLC to immune checkpoint inhibitors [70]. In Liu et al. study, concurrent administration of cisplatin, crizotinib, and PD-1 blocking antibodies to mice results in acute hepatotoxicity, which can be prevented by administering these drugs in a sequential manner by first administering cisplatin plus crizotinib, then PD-1 blockade one week later [71]. Yu et al. study showed that pemetrexed plus cisplatin therapy prevented the growth of A549 cell-driven tumors in nude mice and controlled the expression of genes involved in apoptosis and the cell cycle. Using pemetrexed and cisplatin in combination, the STING pathway and ICD were further stimulated. With this form of sequential treatment, TNF-α, IFN-β, and IL-12 levels, Cytotoxic T lymphocytes (CTL) infiltration, and PD-L1 expression were elevated [72].
Furukawa et al. study determined the efficacy of seven chemotherapy drugs (cisplatin, carboplatin, pemetrexed, gemcitabine, docetaxel, paclitaxel, and vinorelbine) and the third-generation EGFR-tyrosine kinase inhibitors (TKI) osimertinib to induce ICD in NSCLC cell lines. In A549, H1299, EBC1, PC9, H1650, and L858R NSCLC cell lines, antimetabolites and microtubule inhibitors increased the phosphorylation of eIF2, which increased calreticulin expression at the cell surface. In NSCLC cells treated with various chemotherapeutic drugs, calreticulin expression was strongly linked with the induction of apoptosis. The pan-caspase inhibitor Z-VAD-FMK inhibited the drug-induced up-regulation of calreticulin in NSCLC cells. In five NSCLC cell lines with EGFR mutations, osimertinib also boosted calreticulin expression and apoptosis [73]. Moreover, according to Flieswasser et al., in three of the four NSCLC cell lines, docetaxel and its combinations with carboplatin or cisplatin induced high levels of calreticulin, HMGB1, and ATP. These regimens also caused the phagocytosis of NSCLC cells and the maturation of DCs. Besides, all C57BL/6 J mice vaccinated with docetaxel and cisplatin-treated NSCLC cells remained a tumor-free following challenge. Data from both in vitro and in vivo experiments, however, indicated that oxaliplatin was unable to cause ICD in NSCLC cells [74].
According to research by Zhou et al. blocking Polo Like Kinase (PLK)-1 might change the TME by enhancing T cell recruitment and maturation of DCs. As ICD inducers, PLK1 inhibitors effectively activated DCs by increasing phagocytosis and surface expression of costimulatory molecules in DCs. Additionally, when PLK1 was targeted, ICD-treated tumor cells were transformed into an endogenous vaccine that induced immunological memory responses and shielded the mice from tumor development [75]. To control the TME and enhance the ICD-induced immune response against lung cancer, Wan et al. designed a cell membrane vehicle (CV) to co-deliver doxorubicin and sorafenib. Sorafenib was able to modify the TME, suppress Treg, activate T lymphocytes, and reduce PD-1 expression, and doxorubicin could significantly increase ICD [76].
According to research by Wang et al., the iridium (III) complex (Ir1) can trigger ER-localized ICD in NSCLC. In A549 lung cancer cells, Ir1 caused the release of DAMPs, which included calreticulin cell surface exposure, extracellular exclusion of HMGB1, and ATP, along with an increase in ER stress and ROS. Immunocompetent mice were vaccinated with dying cells treated with Ir1 to induce an antitumor CTL response and Treg depletion, which ultimately led to the activation of ICD in lung cancer cells to provide antitumor immunity [77]. Lotsberg et al. study showed that bemcentinib, a selective AXL kinase inhibitor, treatment-induced ICD in drug-resistant NSCLC in vitro, as well as the transcription of genes related to autophagy in vivo. Autophagic flux was reported to be inhibited by bemcentinib or siRNA-mediated AXL gene silencing [78]. Moreover, the effectiveness of PT-112, a pyrophosphate-platinum ICD-inducer, in the treatment of NSCLC patients was examined by Karp et al. NSCLC patients treated with PT-112 experienced persistent immune responses in this phase 1 clinical study (NCT02266745) [79].
Conclusion
ICD-inducers have garnered a lot of attention from researchers ever since it was discovered that ICD has a special role that allows it to eradicate cancer cells. Scientists have been investing more time and money in recent years to find new ICD-inducers that may prove to be useful instruments and aid in the indirect long-term anticancer properties. Almost all in vivo and in vitro showed the effectiveness of this therapeutic approach in the treatment of NSCLC. ICD-inducers in combination with chemotherapy, immunotherapy, and irradiation appeared to have far more significant clinical and therapeutic consequences than ICD induction via chemotherapy alone. However, to make a final judgment about the effectiveness of ICD-inducers in NSCLC, more clinical trials are needed, as well as predicting the consequences and side effects of different combinations on the overall health of patients.
References
Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer. 2017;17(11):637–58.
Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72.
Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–701.
Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3(9): e955691.
Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30(1):61–9.
Cirone M, Di Renzo L, Lotti LV, Conte V, Trivedi P, Santarelli R, et al. Activation of dendritic cells by tumor cell death. Oncoimmunology. 2012;1(7):1218–9.
Legrand AJ, Konstantinou M, Goode EF, Meier P. The diversification of cell death and immunity: memento mori. Mol Cell. 2019;76(2):232–42.
Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59(3):583–94.
Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochimica et Biophysica Acta-Rev Cancer. 2010;1805(1):53–71.
Garg A, Martin S, Golab J, Agostinis P. Danger signalling during cancer cell death: origins, plasticity and regulation. Cell Death Differ. 2014;21(1):26–38.
Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334(6062):1573–7.
Garg AD, Dudek AM, Ferreira GB, Verfaillie T, Vandenabeele P, Krysko DV, et al. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy. 2013;9(9):1292–307.
Fucikova J, Moserova I, Truxova I, Hermanova I, Vancurova I, Partlova S, et al. High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int J Cancer. 2014;135(5):1165–77.
Rezaiemanesh A, Mahmoudi M, Amirzargar AA, Vojdanian M, Babaie F, Mahdavi J, et al. Upregulation of Unfolded Protein Response and ER Stress–Related IL-23 Production in M1 Macrophages from Ankylosing Spondylitis Patients. Inflammation. 2022;45(2):665-76.
Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20(11):1301–9.
Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140(6):798–804.
Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–6.
Ma Y, Adjemian S, Galluzzi L, Zitvogel L, Kroemer G. Chemokines and chemokine receptors required for optimal responses to anticancer chemotherapy. Oncoimmunology. 2014;3(2): e27663.
Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38(4):729–41.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2018;68(6):394–424.
Massafra M, Passalacqua MI, Gebbia V, Macrì P, Lazzari C, Gregorc V, et al. Immunotherapeutic Advances for NSCLC. Biologics. 2021;15:399.
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–54.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA, editors. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo clinic proceedings; 2008: Elsevier.
Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the surveillance, epidemiology, and end results (SEER) program. Oncologist. 2003;8(6):541–52.
Pisters KM, Le Chevalier T. Adjuvant chemotherapy in completely resected non–small-cell lung cancer. J Clin Oncol. 2005;23(14):3270–8.
Group NM-aC. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375(9722):1267–77.
Pignon J-P, Tribodet H, Scagliotti GV, Douillard J-Y, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. Database of Abstracts of Reviews of Effects (DARE): Quality-Assessed Reviews [Internet]: Centre for Reviews and Dissemination (UK); 2008.
Group NM-aC. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383(9928):1561–71.
Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. Journal for immunotherapy of cancer. 2020;8(1):e00037
Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat Rev Immunol. 2017;17(4):262–75.
Rajabinejad M, Asadi G, Ranjbar S, Varmaziar FR, Karimi M, Salari F, et al. The MALAT1-H19/miR-19b-3p axis can be a fingerprint for diabetic neuropathy. Immunol Lett. 2022;245:69–78.
Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95–112.
Vanpouille-Box C, Hoffmann JA, Galluzzi L. Pharmacological modulation of nucleic acid sensors—therapeutic potential and persisting obstacles. Nat Rev Drug Discovery. 2019;18(11):845–67.
Lotfi R, Nasiri Kalmarzi R, Rajabinejad M, Hasani S, Zamani F. The role of immune semaphorins in the pathogenesis of multiple sclerosis: Potential therapeutic targets. Int Immunopharmacol. 2021;95: 107556.
Fucikova J, Moserova I, Urbanova L, Bezu L, Kepp O, Cremer I, et al. Prognostic and predictive value of DAMPs and DAMP-associated processes in cancer. Front Immunol. 2015;6:402.
Ye W, Gunti S, Allen CT, Hong Y, Clavijo PE, Van Waes C, et al. ASTX660, an antagonist of cIAP1/2 and XIAP, increases antigen processing machinery and can enhance radiation-induced immunogenic cell death in preclinical models of head and neck cancer. Oncoimmunology. 2020;9(1):1710398.
Xiao R, Allen CT, Tran L, Patel P, Park S-J, Chen Z, et al. Antagonist of cIAP1/2 and XIAP enhances anti-tumor immunity when combined with radiation and PD-1 blockade in a syngeneic model of head and neck cancer. Oncoimmunology. 2018;7(9): e1471440.
Deutsch E, Chargari C, Galluzzi L, Kroemer G. Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol. 2019;20(8):e452–63.
Dudek AM, Garg AD, Krysko DV, De Ruysscher D, Agostinis P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev. 2013;24(4):319–33.
Sun C, Wang H, Mao S, Liu J, Li S, Wang J. Reactive oxygen species involved in CT26 immunogenic cell death induced by Clostridium difficile toxin B. Immunol Lett. 2015;164(2):65–71.
Donnelly OG, Errington-Mais F, Steele L, Hadac E, Jennings V, Scott K, et al. Measles virus causes immunogenic cell death in human melanoma. Gene Ther. 2013;20(1):7–15.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini J-L, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61.
Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, et al. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Can Res. 2011;71(14):4821–33.
Bugaut H, Bruchard M, Berger H, Derangère V, Odoul L, Euvrard R, et al. Bleomycin exerts ambivalent antitumor immune effect by triggering both immunogenic cell death and proliferation of regulatory T cells. PLoS ONE. 2013;8(6): e65181.
Cirone M, Di Renzo L, Lotti LV, Conte V, Trivedi P, Santarelli R, et al. Primary effusion lymphoma cell death induced by bortezomib and AG 490 activates dendritic cells through CD91. PLoS ONE. 2012;7(3): e31732.
Demaria S, Santori FR, Ng B, Liebes L, Formenti SC, Vukmanovic S. Select forms of tumor cell apoptosis induce dendritic cell maturation. J Leukoc Biol. 2005;77(3):361–8.
Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor ApoptosisCTX and IFN-I synergism in DC-driven antitumor response. Can Res. 2011;71(3):768–78.
Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–91.
Hoffmann J, Vitale I, Buchmann B, Galluzzi L, Schwede W, Senovilla L, et al. Improved cellular pharmacokinetics and pharmacodynamics underlie the wide anticancer activity of sagopilone. Can Res. 2008;68(13):5301–8.
Pellicciotta I, Yang C-PH, Goldberg GL, Shahabi S. Epothilone B enhances Class I HLA and HLA-A2 surface molecule expression in ovarian cancer cells. Gynecologic Oncology. 2011;122(3):625–631.
de La Motte RT, Galluzzi L, Olaussen KA, Zermati Y, Tasdemir E, Robert T, et al. A novel epidermal growth factor receptor inhibitor promotes apoptosis in non-small cell lung cancer cells resistant to Erlotinib. Can Res. 2007;67(13):6253–62.
Garrido G, Rabasa A, Sánchez B, López MV, Blanco R, López A, et al. Induction of immunogenic apoptosis by blockade of epidermal growth factor receptor activation with a specific antibody. J Immunol. 2011;187(10):4954–66.
Pozzi C, Cuomo A, Spadoni I, Magni E, Silvola A, Conte A, et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat Med. 2016;22(6):624–31.
Krombach J, Hennel R, Brix N, Orth M, Schoetz U, Ernst A, et al. Priming anti-tumor immunity by radiotherapy: dying tumor cell-derived DAMPs trigger endothelial cell activation and recruitment of myeloid cells. Oncoimmunology. 2019;8(1): e1523097.
Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. Embo J. 2012;31(5):1062–79.
Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22(1):58–73.
Cirone M, Garufi A, Di Renzo L, Granato M, Faggioni A, D’Orazi G. Zinc supplementation is required for the cytotoxic and immunogenic effects of chemotherapy in chemoresistant p53-functionally deficient cells. Oncoimmunology. 2013;2(9): e26198.
Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25.
Galluzzi L, Vitale I, Michels J, Brenner C, Szabadkai G, Harel-Bellan A, et al. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis. 2014;5(5): e1257.
Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–83.
Galluzzi L, Vitale I, Senovilla L, Olaussen KA, Pinna G, Eisenberg T, et al. Prognostic impact of vitamin B6 metabolism in lung cancer. Cell Rep. 2012;2(2):257–69.
Kepp O, Menger L, Vacchelli E, Locher C, Adjemian S, Yamazaki T, et al. Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev. 2013;24(4):311–8.
Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30(10):1147–58.
Sukkurwala AQ, Adjemian S, Senovilla L, Michaud M, Spaggiari S, Vacchelli E, et al. Screening of novel immunogenic cell death inducers within the NCI mechanistic diversity set. Oncoimmunology. 2014;3: e28473.
Vanpouille-Box C, Demaria S, Formenti SC, Galluzzi L. Cytosolic DNA sensing in organismal tumor control. Cancer Cell. 2018;34(3):361–78.
Rosenzweig R, Nillegoda NB, Mayer MP, Bukau B. The Hsp70 chaperone network. Nat Rev Mol Cell Biol. 2019;20(11):665–80.
Bezu L, Sauvat A, Humeau J, Leduc M, Kepp O, Kroemer G. eIF2α phosphorylation: a hallmark of immunogenic cell death. Oncoimmunology. 2018;7(6): e1431089.
Rybstein MD, Bravo-San Pedro JM, Kroemer G, Galluzzi L. The autophagic network and cancer. Nat Cell Biol. 2018;20(3):243–51.
Fucikova J, Becht E, Iribarren K, Goc J, Remark R, Damotte D, et al. Calreticulin expression in human non-small cell lung cancers correlates with increased accumulation of antitumor immune cells and favorable prognosis. Cancer Res. 2016;76(7):1746–56.
Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10(1):1486.
Liu P, Zhao L, Kepp O, Kroemer G. Crizotinib—a tyrosine kinase inhibitor that stimulates immunogenic cell death. Oncoimmunology. 2019;8(7):1596652.
Yu J, Zhang Q, Li J, Si Z, Guo Y, Xu X, et al. Sequential administration of pemetrexed and cisplatin reprograms tumor immune microenvironment and potentiates PD-1/PD-L1 treatment in a lung cancer model. J Investig Med. 2022;70(3):792–9.
Furukawa R, Inoue H, Yoneshima Y, Tsutsumi H, Iwama E, Ikematsu Y, et al. Cytotoxic chemotherapeutic agents and the EGFR-TKI osimertinib induce calreticulin exposure in non-small cell lung cancer. Lung Cancer. 2021;155:144–50.
Flieswasser T, Van Loenhout J, Freire Boullosa L, Van den Eynde A, De Waele J, Van Audenaerde J, et al. Clinically relevant chemotherapeutics have the ability to induce immunogenic cell death in non-small cell lung cancer. Cells. 2020;9(6):1474.
Zhou J, Yang Q, Lu L, Tuo Z, Shou Z, Cheng J. PLK1 Inhibition induces immunogenic cell death and enhances immunity against NSCLC. Int J Med Sci. 2021;18(15):3516–25.
Wan J, Wang J, Zhou M, Rao Z, Ling X. A cell membrane vehicle co-delivering sorafenib and doxorubicin remodel the tumor microenvironment and enhance immunotherapy by inducing immunogenic cell death in lung cancer cells. J Mater Chem B. 2020;8:7755.
Wang L, Guan R, Xie L, Liao X, Xiong K, Rees TW, et al. An ER-targeting iridium(III) complex that induces immunogenic cell death in non-small-cell lung cancer. Angew Chem Int Ed. 2021;60(9):4657–65.
Lotsberg ML, Wnuk-Lipinska K, Terry S, Tan TZ, Lu N, Trachsel-Moncho L, et al. AXL targeting abrogates autophagic flux and induces immunogenic cell death in drug-resistant cancer cells. J Thorac Oncol. 2020;15(6):973–99.
Karp DD, Camidge DR, Infante JR, Ames TD, Price MR, Jimeno J, et al. Phase I study of PT-112, a novel pyrophosphate-platinum immunogenic cell death inducer, in advanced solid tumours. EClinicalMedicine. 2022;49: 101430.
Jin Y, Yu Y, Siqi Y, Ruqing G, Wuyuan L, Wangxiao H, et al. (2020) Chiral Protein Supraparticles for Tumor Suppression and Synergistic Immunotherapy: An Enabling Strategy for Bioactive Supramolecular Chirality Construction. Nano Letters 20(8) 5844-5852. https://doi.org/10.1021/acs.nanolett.0c01757
Yuan-Y, Qu R, Zhao H-L, Zhang Q, Zhou F-J, Xu X, Zhang W-H, Xu N, Shao S-X, Zhou B, Dai Y, Zhu G-H, Shi Y-J, Shen Y-P, Zhu C-T, H K, Chang Y, Lin W-D, Zang W, Xu D-W, Ye S-M, Zhao J-Y, Zhao et al. (2020) Inactivation of the AMPK–GATA3–ECHS1 Pathway Induces Fatty Acid Synthesis That Promotes Clear Cell Renal Cell Carcinoma Growth. Cancer Research 80(2) 319-333. https://doi.org/10.1158/0008-5472.CAN-19-1023
Yang, Li Cui-Fang, Yao Fu-Jiang, Xu Yuan-Yuan, Qu Jia-Tao, Li Yan, Lin Zhong-Lian, Cao Peng-Cheng, Lin Wei, Xu Shi-Min, Zhao Jian-Yuan, Zhao Zhao et al. (2019) APC/CCDH1 synchronizes ribose-5-phosphate levels and DNA synthesis to cell cycle progression. Nature Communications 10(1) 2502. https://doi.org/10.1038/s41467-019-10375-x
Dan, W Rui, Zhao Y-Y, Qu X-Y, Mei X, Zhang Q, Zhou Y, Li S-B, Yang Z-G, Zuo Y-M, Chen Y, Lin W, Xu C, Chen S-M, Zhao J-Y, Zhao Zhao et al. (2018) Colonic Lysine Homocysteinylation Induced by High-Fat Diet Suppresses DNA Damage Repair. Cell Reports 25(2) 398-412.e6 S2211124718314529. https://doi.org/10.1016/j.celrep.2018.09.022
Yun, Feng F, Li J, Yan X, Guo F, Wang H, Shi J, Du H, Zhang Y, Gao D, Li Y, Yao W, Hu J, Han M, Zhang R, Ding X, Wang C, Huang J, Zhang Zhao et al. (2021) Pan-cancer analysis and experiments with cell lines reveal that the slightly elevated expression of DLGAP5 is involved in clear cell renal cell carcinoma progression. Life Sciences 287120056-S0024320521010432 120056. https://doi.org/10.1016/j.lfs.2021.120056
Xiaolie, He Y, Zhu L, Yang Z, Wang Z, Wang J, Feng X, Wen L, Cheng R, Zhu Z et al. (2021) MgFe‐LDH Nanoparticles: A Promising Leukemia Inhibitory Factor Replacement for Self‐Renewal and Pluripotency Maintenance in Cultured Mouse Embryonic Stem Cells. Advanced Science 8(9) 2003535-10.1002/advs.202003535
Chunping, Liu Y, Wang L, Li D, He J, Chi Q, Li Y, Wu Y, Zhao S, Zhang L, Wang Z, Fan Y, Liao Z et al. (2022) Engineered extracellular vesicles and their mimetics for cancer immunotherapy. Journal of Controlled Release 349679-698 S0168365922004333. https://doi.org/10.1016/j.jconrel.2022.05.062
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
HK, contributed to the idea design, literature search, writing the manuscript. AK, drafted the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
It is not applicable.
Informed consent
It is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kashefizadeh, A., Kazemizadeh, H. Immunogenic cell death (ICD)-inducers in non-small-cell lung carcinoma (NSCLC): current knowledge and future perspective. Clin Transl Oncol 25, 316–322 (2023). https://doi.org/10.1007/s12094-022-02949-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02949-x