Abstract
Objective(s)
Exosomal HER2 has been evidenced to interfere with antibody-induced anti-tumor effects. However, whether the blockade of HER2+ exosomes release would affect antibody-mediated tumor inhibition has yet to be investigated.
Methods
Exosomes derived from BT-474, SK-BR3 and SK-OV3 (HER2-overexpressing tumor cells) and MDA-MB-231 cells (HER2 negative) were purified and characterized by bicinchoninic acid (BCA) assay, western blotting and Transmission electron microscopy (TEM). Inhibition of exosome release was achieved by neutral sphingomyelinase-2 (nSMase-2) inhibitor, GW4869. The effects of exosome blockade on the anti-proliferative effects, apoptosis induction, and antibody-mediated cellular cytotoxicity (ADCC) activity of Trastuzumab were examined using MTT, flow cytometry, and LDH release assays. Also, the effects of exosome inhibition on the surface expression and endocytosis/internalization of HER2 were studied by flow cytometry.
Results
Purified exosomes derived from HER2 overexpressing cancer cells were positive for HER2 protein. Blockade of exosome release was able to significantly improve apoptosis induction, anti-proliferative and ADCC responses of Trastuzumab dose dependently. The pretreatment of Trastuzumab/purified NK cells, but not PBMCs, with HER2+ exosomes could also decrease the ADCC effects of Trastuzumab. Exosome inhibition also remarkably downregulated surface HER2 levels in a time-dependent manner, but does not affect its endocytosis/internalization.
Conclusion
Based on our findings, HER2+ exosomes may benefit tumor progression by dually suppressing Trastuzumab-induced tumor growth inhibition and cytotoxicity of NK cells. It seems that concomitant blocking of exosome release might be an effective approach for improving the therapeutic effects of Trastuzumab, and potentially other HER2-directed mAbs. In addition, the exosome secretion pathway possibly contributes to the HER2 trafficking to plasma membrane, since the blockade of exosome secretion decreased surface HER2 levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human epidermal growth factor receptor 2 (HER2) is a member of HER family receptor tyrosine kinases [1]. The overexpression of HER2 has been observed in about 25% of breast tumors, as well as in some types of ovarian, colorectal and etc. cancers that is correlated with the tumor aggression, proliferation, metastasis and resistance to the therapies [2]. Although the introduction of Trastuzumab (Herceptin) has transformed the treatment of HER2+ tumors, but acquired resistance to Trastuzumab, failure of the treatments and relapse of the disease are significant challenges in the treatment of tumors overexpressing HER2 [3, 4]. Previous observations from the addition of Lapatinib, a tyrosine kinase inhibitor, was a proof of the concept that single-agent treatments such as Trastuzumab could not lead to a durable outcome [5]. Therefore, better treatment regimens are still a medical need, and deep understandings of tumor biology to develop novel approaches in combination with currently available therapies, such as Trastuzumab, would further help us to overcome hurdles in treatment of HER2+ cancers [6].
Recently, it has been documented that extracellular vesicles, especially exosomes, derived from tumor cells might play a role in impaired responses to therapeutic antibodies [7, 8]. These particles are nano-sized membrane vesicles (30–130 nm) released by several cell types, particularly tumor cells and are formed by inward budding of late endosomes and accumulate in multivesicular buddies (MVB), where they finally are released to the surrounding microenvironment by fusing with the plasma membrane [9]. Exosomes were shown to carry mRNA, miRNA, lncRNA, DNA, lipids and proteins, and their content is a mirror of original cells [9]. Numerous studies have shown that exosomes derived from tumor cells are involved in several aspects of cancer biology, including tumor development, angiogenesis, and metastasis. These particles also could compromise both cellular and humoral anti-tumor immune responses and thereby favor tumor immune escape [9, 10].
There is growing evidence that exosomes derived from HER2-overexpressing tumor cells transfer bioactive substances that can result in impaired responses or confer resistance to Trastuzumab [11, 12]. Of particular note, exosomes from HER2+ cancer cells were shown to carry a full length of HER2 molecules that can be engaged with Trastuzumab and perturbs its binding to the cell surface HER2, decreasing its therapeutic effects [11, 13]. Moreover, exosomal HER2 levels have been shown to be consistent with its expression in tumor tissues [14, 15]. Accumulating findings show that targeting tumor exosomes or blocking their secretion could improve the efficacy of current therapies [16, 17]. However, whether the blockade of HER2+ exosomes release could improve anti-tumor responses by Trastuzumab has not been explored. Also, there is no evidence whether exosomes secretion pathway regulates/contributes to the trafficking of HER2 molecules to the plasma membrane. In the present study, we examined the effect of HER2+ exosomes release blockade on the anti-proliferative effects, apoptosis and ADCC responses-induced by Trastuzumab. We also examined the endocytosis/internalization and surface expression levels of HER2 molecules upon inhibiting exosome release. Taken together, our findings indicated that blocking exosome secretion could strengthen Trastuzumab-induced tumor growth inhibition and revealed a role for exosome secretion pathway in regulating HER2 trafficking to the cell surface. However, future studies are warranted to examine the importance of exosome secretion pathway in responses to HER2-dericted antibody–drug conjugate (TDM-1) and tyrosine kinase inhibitors, as well as its role in controlling signaling cascade downstream HER2.
Materials and methods
Cell culture
Human breast cancer SK-BR3 cells overexpressing HER2 and HER2 negative MDA-MB-231 cells were obtained from Iranian National Cell Bank (Pasteur Institute of Iran, Tehran). BT-474 cells and ovarian cancer SK-OV3 cell lines were also, respectively, purchased from the Iranian Biological Resource Center (IBRC, Iran, Tehran), and School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran. SK-BR3, MDA-MB-231 and SK-OV3 cells were cultured in 90% RPMI-1640 (Gibco, NY, USA) medium with 10% FBS in a humidified atmosphere plus 5% CO2 at 37 °C. BT-474 cells were maintained in DMEM (Gibco) supplemented with 10% FBS (Gibco) at 37 °C in a humidified atmosphere with 5% CO2.
Exosome isolation from conditioned media
For collecting conditioned media (CM), the aforementioned cells were cultured at a density of 10 × 106 cells in T-75 tissue flasks. The cells were allowed to attach overnight in complete media, following which the cells were washed with sterile PBS1x and the media were changed to FBS-free media (10 ml) for 24–48 h, and then supernatants were collected for exosome isolation. The collected CM was centrifuged at 400 g for 10 min and 2000 g for 15 min to remove cells, debris and large particles, and was then filtered through a 0.22 μm membrane and concentrated using an ultra-filtration system (100 KD, Amicon Ultra-4, Millipore, USA). Subsequently, the obtained concentrate was loaded on qEV column (Izon Science, Cambridge, MA, US) to purify exosomes. The first 3 ml was discarded according to the manufacturer’s instruction, and the exosome-rich fractions of 7–9 were collected in 0.5 ml tubes and stored at − 80 °C for the future experiments.
Characterization of exosomes
The isolated exosomes were characterized according to the ISEV guidelines [18].
BCA assays
Samples from exosome-rich fractions were incubated with RIPA buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% NP40, 0.5% Na-deoxycholate, and 0.1% SDS) in the presence of protease/proteinase inhibitors (sigma). Then, the protein content of samples, representing exosomes, was assessed using a micro-BCA protein assay kit (Pierce Company).
Western blotting
After obtaining the exosome concentration, western blot analysis was carried out as described elsewhere [19]. In brief, the samples (5 µg/sample) were mixed with loading buffer (250 mM Tris–HCl, 8% SDS, 40% glycerol, 20% β-mercaptoethanol, 0.008% Bromophenol Blue, pH 6.8), boiled for 5 min at 95 °C and electrophoresed on 10% Bis–Tris gel (Invitrogen) under denaturing conditions. The gel was electroblotted onto a nitrocellulose membrane, blocked with Skim-milk 3%, and incubated with primary antibodies (Trastuzumab and anti-CD63), that was followed by incubation with secondary antibodies (anti-human and –mouse-HRP conjugated antibodies). Finally, the blots were revealed on autoradiography film by incubating with ECL detection reagent (Thermo Fisher Scientific, Waltham, USA).
Electron microscopy
Additional morphological characteristics of the exosomes were evaluated by negative staining using transmission electron microscope (TEM) (Model of the PHILIPS EM2085 100 kV based in Rastak Laboratory, Tehran, Iran). In details, carbon-coated copper grids were placed on the sample drops for 20 min and were fixed by 2% paraformaldehyde. The grids were then washed with distilled water several times for 5 min, stained with uranyl acetate for 15 min and washed again with distilled water. The stained grids were left to air-dry and finally were examined using an FEI/Philips TEM 208S microscope (Eindhoven, Netherlands) operating at an accelerating voltage of 100 kV.
Proliferation assay
Cell viability was measured by the MTT (methylthiazolyldiphenyl-tetrazolium bromide) (Kia-Zist Life Sciences, Iran) assay according to the protocol described elsewhere [20]. Briefly, 3 × 103 SK-BR3 and MDA-MB-231 cells, 2 × 103 SK-OV-3 cells, 6 × 103 BT-474 cells were seeded in flat-bottom 96-well culture plates and allowed to adhere overnight. The cells were then washed several times and treated with the desired concentrations of Trastuzumab (40, 20, 10, 5, 1, 0.1 µg/ml) in triplicate wells with or without 10 µM GW4869 (standard exosome inhibitor) (Gibco) for 72 h. Controls of individual GW4869, 1% DMSO (excipient), untreated (negative control) and TritonX100 (maximum death, positive control)-treated cells were also included in the experiments. After 3 days of incubation, the cell supernatant was removed and replaced with serum-free media containing MTT solution (5 mg/ml) and incubated for 3–4 h at 37 °C. Next, DMSO was added to the wells to solubilize the formazan crystals, and the optical density (O.D.) was measured at 545 nm using a Hiperion microplate reader system (Miami, FL, USA). In each individual experiment, changes in cell survival following treatments are expressed as percent of untreated control. Data for the combination group (Tra + GW4869) were also adjusted to the individual GW4869, before statistical analysis and interpretation.
Flow cytometry experiments
Analysis of surface expression
The effects of exosome blockade on HER2 surface expression was assessed by flow cytometry. For this purpose, 1 × 106 SK-BR3, BT-474 and SK-OV3 cells were seeded in T25 tissue flasks and incubated for overnight attachment. In the next day, culture media was discarded and replaced with 1%FBS-supplemented media with or without 10 µM GW4869. The surface expression of HER2 was assessed as described elsewhere [20, 21] after 24, 48 and 72 h of treating with exosome inhibitor. In this regard, the cells were trypsinized, washed several times and incubated with Trastuzumab (2 µg/ml) for 30 min at 4 °C. Then, they were washed again and incubated with anti-human FITC-conjugated secondary antibody (BioLegend) for additional 30 min at 4 °C. Finally, the cells were washed again and at least 10,000 events were analyzed by flow cytometry.
Internalization studies
To explore the effects of exosome inhibition on antibody-mediated receptor internalization, the flow cytometric assays were performed as described elsewhere [22, 23]. Briefly, the cells were cultured in the presence or absence of GW4869 (10 µM) for 48 h. Afterward, the cells were trypsinized, washed and stained with primary antibody for 30 at 4 °C, similar to the surface expression experiments. After then, the cells were washed to remove excess antibodies and were incubated at 37 °C for 1 h or placed on ice. This was followed by washing and incubating the cells with secondary FITC-conjugated antibody at 4 °C for 30 min. The cells were analyzed by flow cytometry and the percentage of endocytosis/internalization was measured using the following formula:
Endocytosis/internalization (%) = Residual MFI at 4 °C–Residual MFI at 37 °C/Residual MFI at 4 °C × 100.
Apoptosis assay
The Annexin V-FITC/PI Kit (BD Bioscience, 556,547) was used to detect the apoptotic cells. In details, cells were treated with Trastuzumab (20 µg/ml), GW4869 (10 µM), or their combination (Tra + GW4869) for 48 h along with non-treated cells (vehicle). Then, the flow cytometric-based Annexin V/PI assays were employed to obtain the percentage of apoptotic cells.
Antibody-dependent cellular cytotoxicity (ADCC) assay
The lactate dehydrogenase (LDH) release assay was employed to analyze ADCC. In details, BT-474, SK-BR3 and SKOV-3 cells (target cells) treated with or without GW4869 for 48 h, were trypsinized and seeded at density of 10 × 103 cells/well in round bottom 96-well plates and were incubated with 20 µg/ml Trastuzumab for 30 min at 37 °C. Peripheral blood mononuclear cells (PBMCs) isolated by Ficoll gradient centrifugation (Histopaque, Sigma-Aldrich, UK) or magnetically purified NK cells (Miltenyi Biotech, Somerville, MA) from healthy donors were added on top of cells at different effector: target ratios (60:1, 40:1, 20:1, 10:1 E:T for PBMCs or 10:1 E:T for NK cells) and incubated in a humidified atmosphere containing 5% CO2 at 37 °C for an additional 6 h. Similar experiments were also done by Trastuzumab, PBMCs or NK cells pre-incubated with purified exosomes (50 µg/ml). Triton-X 100-treated and untreated cells were also considered as maximum and spontaneous release of the LDH from target cells. It should be noted that for PBMCs isolation, a signed consent letter was taken from healthy donors (n = 3), and all the protocols of this study were approved by the Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.MED.REC.1399.569). Finally, the specific cell lysis was assessed by measuring LDH release (Promega, Madison, WI, USA), and the percentage of cytotoxicity was calculated by the following formula:
Specific lysis or cytotoxicity % = (Experimental release − Effector cells spontaneous release − Target cells spontaneous release)/(Target cells maximum release − Target cells spontaneous release) × 100.
Statistical analysis
All data were analyzed with SPSS 13.0 software and were illustrated via GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA). Data normality was assessed using Kolmogorov–Smirnov test and the statistical analysis was performed by Students’ t test, One-Way ANOVA Tukey test or Kruskal–Wallis H test. Due to the baseline differences, the data for combinational treatment were adjusted for the difference between GW4869 and isotype IgG results prior to statistical analysis. The mean ± standard deviations (SD) of at least three independent experiments was used to represent results. P < 0.05 was considered as statistically significant.
Results
HER2 overexpressing tumor cells release exosomes with HER2 cargo
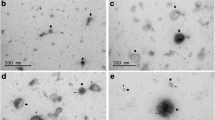
The purified exosomes from breast cancer cell lines SKBR3 and BT474 and ovarian cancer SK-OV3 cells overexpressing HER2 along with those obtained from HER2 negative MDA-MB-231 cells were visualized by TEM (at 100 and 200 nm), and then were examined for CD63 and HER2 expression by immunoblotting. The results showed that all the exosome preparations were positive for exosomal marker CD63, however, only exosomes isolated from the BT-474, SK-BR3 and SK-OV3 cells had HER2 cargo (Fig. 1). Although the amount of exosomal HER2 seems to differ between different HER2 + tumor cells, but it is obvious that HER2 overexpressing cancer cells release a considerable amount of HER2 in an exosomal manner (Fig. 1c).
Characteristics of exosomes derived from HER2 overexpressing tumor cells. a, b Images of exosomes by TEM (100 and 200 nm, respectively). c Western blotting assays of HER2 and CD63 (exosomal marker) in exosomes derived from BT-474, SK-BR3 and SK-OV3 (HER2 overexpressing cells), as well as HER2 low/negative MDA-MB-231 cells
Blocking exosome release from HER2 + cancer cells enhances anti-proliferative effects of Trastuzumab
Having demonstrated that exosomes derived from HER2 overexpressing cells carry considerable amount of HER2 protein, we investigated the effects of exosome inhibition on the anti-proliferative effects of Trastuzumab. As shown in Fig. 2a, b, c, although exosome inhibition itself does not induce obvious changes in viability of SKBR-3, BT-474, and SK-OV3 cells, but remarkable anti-proliferative effects are induced when blockade of exosome release was combined with Trastuzumab. More potent anti-proliferative effects were observed for BT-474 and SK-BR3 cells compared to SK-OV3 cells, as well as for Trastuzumab at higher doses when exosome inhibitor was added (Fig. 2a,b,c). However, the viability of HER2 negative MDA-MB-231 cells did not alter by Trastuzumab, GW4869, or their combination (Fig. 2d).
Concomitant inhibition of exosome release with Trastuzumab halts proliferation of HER2 + tumor cells. a BT474 cells, b SK-BR3 cells, c SK-OV3 cells were incubated with GW4869 (10 µM) and increasing concentration of Trastuzumab for 72 h. d MDA-MB-231 cells were also employed as HER2 low/negative cells. Cellular viability was measured by MTT tests and the resultant data were expressed as Mean ± SD of at least two independent triplicate experiments. Due to the baseline differences, the data for combinational treatment were adjusted for the difference between GW4869 and isotype IgG results prior to statistical analysis. *P < 0.05 **P < 0.01; ***P < 0.001. NS: non-significant
Exosome inhibition improves apoptosis induced by Trastuzumab
Next, we examined whether exosome inhibition alters apoptosis induction by Trastuzumab. Based on our findings individual DMSO or exosome inhibition for 48 h induces 2–5% apoptosis in treated cells. However, Trastuzumab or its combination with exosome inhibitor could significantly enhance apoptosis of HER2+ cancer cells quantified by Annexin V binding assay. As shown in Fig. 3, there is also a significant difference in the apoptosis percentage between Trastuzumab alone and its combination with GW4869 for BT-474, SK-BR3 and SK-OV3 cells. Also, the differences were more obvious for the breast tumor SK-BR3 and BT-474 cells compared to ovarian cancer SK-OV3 cells. This shows that blocking exosome release could prone HER2+ cancers to cell death, therefore, the addition of exosome inhibitor to Trastuzumab was more potent than individual Trastuzumab in inducing apoptosis. Although, the apoptosis induction via the exosomes inhibition can be different between various tumor cells.
Blocking exosome release signifies apoptosis induction by Trastuzumab. a BT474 cells, b SK-BR3 cells, c SK-OV3 cells are exposed to Trastuzumab (20 µg/ml) with or without GW4869 (10 µM) for 48 h. Proportion of Annexin V + and Annexin V/PI + cells from triplicate experiments were analyzed by flow cytometry and then presented in corresponding bar charts
Exosome inhibition downregulates HER2 levels on tumor cell surfaces
Demonstrating that exosome inhibitor could enhance both anti-proliferative and apoptosis-inducing activities of Trastuzumab, we sought the effects of exosome inhibition on the cell surface expression of HER2. We found that blocking exosome release robustly downregulates the expression of HER2 molecules from the cell surface as indicated by decreased mean fluorescent intensity (MFI) (Fig. 4). The pattern of decreased surface expression for HER2 molecules upon exosome blocking was almost similar for the BT-474, SK-BR3 and SK-OV3 cells (Fig. 4d). These findings support an idea about possible exosome-dependent trafficking pathway of HER2 molecules to the plasma membrane. Furthermore, as it is shown in Fig. 4a,b,c, the decrease in surface HER2 levels was observed to be time dependent, where less HER2 molecules were determined on the surface of HER2 overexpressing cells by increasing the incubation with exosome inhibitor from 24 to 48 and 72 h.
Exosome release inhibition downmodulates the surface expression of HER2. Representative graphs show the expression of HER2 on the surface of a BT474 cells, b SK-BR3 cells, c SK-OV3 cells after being exposed to GW4869 (10 µM) for 24, 48 and 72 h. A time-dependent decrease of the mean fluorescence intensity (MFI) was observed for the cells treated with GW4869 (10 µM) compared to non-treated cells (control). T24, T48 and T72 corresponds to 24, 48 and 72 h treatments, respectively
Blockade of exosome release barely affects HER2 endocytosis/internalization
Since exosome inhibition was found to downregulate the surface expression of HER2 molecules, we have speculated that exosome inhibition might have induced endocytosis (higher internalization) of HER2 molecules, hence has decreased their surface expression. To evaluate this speculation, we have examined the internalization percentage of HER2 in the cells incubated with or without GW4869 for 48 h. In accordance with the previous experiments, we observed reduced HER2 molecules on the surface of GW4869-treated cells relative to controls. However, the internalization percentage did not change in the GW4869-treated compared to untreated cells as measured by flow cytometry at 4 °C and 37 °C with an antibody recognizing the extracellular part of HER2 (Fig. 5). These findings imply that the lower surface expression of HER2 upon blocking exosome secretion might not be due to the higher endocytosis or internalization rate.
Blockade of exosome secretion does not favor HER2 endocytosis/internalization. a BT474 cells, b SK-BR3 cells, c SK-OV3 cells were cultured in the presence or absence of GW4869 (10 µM) for 48 h and then examined for the HER2 expression at 4 °C and 37 °C by flow cytometry. The percentage of receptor endocytosis/internalization were examined between treated (presence of 10 µM GW4869) and non-treated (absence of 10 µM GW4869) groups
Exosomal inhibition strengthens Trastuzumab-mediated ADCC
In spite of its direct effects on tumor cells, Trastuzumab can also induce anti-tumor effects by engaging with FcγRIIIa on NK cells, the phenomenon called antibody-dependent cellular cytotoxicity (ADCC) [3]. Since blocking exosome release decreased HER2 surface expression, thus we expected that treatment with exosome inhibitor may hamper Trastuzumab-mediated ADCC responses. On contrast, we observed that ADCC activity of Trastuzumab was significantly improved for BT-474 and SK-BR3 cells, but no obvious changes were found for SK-OV3 cells (Fig. 6). Furthermore, the difference between ADCC responses induced by Trastuzumab and its combination with GW4869 was more remarkable at higher E: T ratios (Fig. 6). The results indicated that, although the HER2 antigen was reduced from the tumor cells surface, but exosome inhibition still could improve ADCC activity of Trastuzumab.
Tumor exosome inhibition augments Trastuzumab-mediated cellular cytotoxicity. ADCC experiments with the a BT474 cells, b SK-BR3 cells, c SK-OV3 cells were performed with PBMCs (E:T ratios at 10:1, 20:1, 40:1 and 60:1) and Trastuzumab (20 µg/ml) for 5–6 h at 37 °C, and the released LDH was measured in the supernatants. For combination experiments, the tumor cells (target cells) were cultured in the presence of GW4869 (10 µM) for 48 h before the ADCC experiments. The percentage of specific lysis was calculated in the treatment conditions (Trastuzumab alone or in combination with GW4869) and compared to untreated conditions (individual GW4869 and irrelevant isotype IgG). *P < 0.05 **P < 0.01; ***P < 0.001. NS non-significant
HER2-carrying exosomes abrogate responses to Trastuzumab dually by serving as decoy receptors and contributing directly to NK cells dysfunction
Having shown that inhibition of exosome release from HER2 over expressing cells improve Trastuzumab-induced tumor growth inhibition and its related ADCC activity, we wonder whether the HER2+ exosomes act as decoy targets for Trastuzumab or directly affect the NK cells function. In this regard, Trastuzumab/PBMCs and both pre-treated with exosomes isolated from SK-OV3, SK-BR3 and BT-474 cells were used in ADCC experiments. Our findings showed that pretreatment of Trastuzumab, but not PBMCs could reduce ADCC responses, demonstrating a decoy role for exosomal HER2 (Fig. 6a,c,e). In PBMCs experiments, the significant improvement in ADCC responses was only observed for BT-474 and SK-BR3 cells, however, ADCC effects for Trastuzumab in SK-OV3 cells was not improved by blocking exosome secretion. On the other hand, similar experiments with purified NK cells showed more pronounced effect of exosome inhibition on the Trastuzumab-mediated ADCC responses and revealed that the pretreatment of NK cells with HER2+ exosomes have negative impacts on the cytotoxicity of NK cells. As it is shown in Fig. 7d, f, the pre-incubation of NK cells with exosomes from SK-BR3 and SK-OV3 cells could significantly negate the ADCC activity of NK cells. Although, exosomes derived from SK-OV3 cells exhibited more negative effects on the cytotoxic activity of NK cells. These findings suggest that HER2-positive exosomes could demolish Trastuzumab responses by acting either as decoy receptors as well as by inhibiting cytolytic activity of NK cells. Comparing the results, it can also be inferred that the ADCC responses to exosomes blocked differ for various cells, and might be dependent to their exosomal cargo rather than HER2 proteins.
Discussion
Tumor exosomes have been proven to negate anti-tumor effects of chemo- and immune-therapies [24, 25]. It has recently been shown that exosomes derived from tumor cells carry functional proteins like PD-L1 molecules on their surface that can trap anti-PD-L1 antibodies, thereby decreasing their clinical efficacy. However, blocking exosome release was able to improve the therapeutic effects of anti-PDL1 antibodies in preclinical studies [26]. Likewise, exosomes derived from HER2-overexpressing cancer cells were shown to carry a significant amount of HER2 molecules that can play as a decoy receptors for ani-HER2 antibodies (e.g. Trastuzumab) and decrease their tumor killing and ADCC activities [11, 13]. The present study was designed to examine whether blocking the exosome release from HER2-overexpressing cancers could affect Trastuzumab-mediated tumor growth inhibition.
Here, we showed that GW4869, used as exosome inhibitor, in combination with anti-HER2 antibody (Trastuzumab) could robustly decrease the proliferation of tumor cells overexpressing HER2 (Fig. 2). Shreds of evidence indicates that compounds blocking receptor endocytosis could improve target availability, thereby would increase the efficacy of clinical antibodies [27]. Since HER2 molecules are shredded from cells surface, in part by exosomes release, hence we postulated that inhibiting exosome secretion might have increased HER2 levels on the surface of tumor cells probably by decreasing its endocytosis/shedding [28]. On the other hand, one of the mechanisms has been proposed for therapeutic activity of Trastuzumab, is its ability in induction of Ab-mediated receptor internalization [29]. Hence, we examined whether the improvement in anti-proliferative effects of Trastuzumab was due to the higher availability of HER2 molecules (inhibition of endocytosis) or enhancement in the Ab-mediated receptor internalization. As shown in Fig. 4, blocking exosome release remarkably decreased HER2 levels from the cell surface. The decrease in the HER2 levels was markedly enhanced by increasing the incubation time from 24 to 72 h (Fig. 4d). However, further experiments revealed no changes in the percentage of receptor internalization upon blocking exosome release compared to non-treated groups (Fig. 5). These findings support that the enhanced anti-proliferative effects of Trastuzumab combined with exosome blockade are not due to higher HER2 availability (endocytosis inhibition) or enhanced Ab-mediated receptor internalization. Indeed, although target availability is of particular importance in inducing anti-tumor effects by therapeutic antibodies, but there are several studies showing that decreased levels of the surface HER2 could be associated with increased efficacy of Trastuzumab [30]. Consistent with our results, a previous study showed that D609, an inhibitor of phosphatidylcholine-specific phospholipase C, downregulates HER2 expression while could markedly impede proliferation of BT-474 and SK-BR3 cells in combination with Trastuzumab [30]. Retinoids and melatonin were also shown to decrease surface HER2 protein levels, however, enhances the cytotoxic effects of Trastuzumab and Neratinib, a HER2 targeting tyrosine kinase inhibitor [31, 32]. In addition, since the survival of the HER2+ tumor cells strongly depended on the signaling cascades downstream of these molecules, therefore, the decreased levels of cell surface HER2 is more likely to be associated with reduced signaling capacity, resulting in less proliferative phenotype [33]. Moreover, as it is obvious in Fig. 4, it can also be claimed that although the exosomes blockade reduces HER2 expression, but there is still significant amount of HER2 molecules on the cell surface that can be engaged with Trastuzumab and leads to tumor growth inhibition. These findings also provide the evidence that exosomal pathway play a pivotal role in the trafficking of HER2 (possibly other surface expressing antigens) to the plasma membrane. More recent findings also indicated that nSMase-2, the target for GW4869, acts as an important regulator of autophagy [34, 35]. It was shown that blocking autophagy could also increase the therapeutic efficacy of Trastuzumab while decreases surface HER2 expression by increasing its accumulation in the early and late endosomes [36, 37]. Therefore, it can be assumed that blocking exosome release might have induced accumulation and degradation of HER2 molecules in endosomal compartments in a same scenario, thereby has decreased its surface expression, however, further experiments are warranted to shed light on the fate of HER2 molecules upon exosome blockade as well as the possible connection between exosome secretion pathway and autophagy.
Our results also showed that, although exosome blockade decrease the HER2 expression, but could potentiate apoptosis induced by Trastuzumab in three cell lines used in this study (Fig. 3). These findings are in accordance with the less proliferative phenotype of HER2+ cells after exosome inhibition, and it can be inferred that the increase in anti-proliferative effects of Trastuzumab in combination with exosome inhibition, might be partially mediated through increased induction of apoptosis. Others have also shown that the downregulation of HER2 receptor is associated with enhanced TRAIL-mediated apoptosis by Trastuzumab [38].
Exosomal HER2 was also shown to be engaged with Trastuzumab and decreases its ADCC activity [13]. Likewise, we have found that pretreatment of Trastuzumab with exosome derived from BT-474 an SK-BR3 cells could decrease its ADCC effects, while no significant changes were observed for SK-OV3 cells and when PBMCs pre-treated with HER2+ exosomes (Fig. 6). These findings potentially indicated that exosomal HER2 can serve as decoy for Trastuzumab, perturbing its engagement with cell surface HER2 molecules, hence decreasing its ADCC activity. However, in terms of the SK-OV3 cells, it is probable that the HER2 content of exosomes derived from these cells is much lower compared to those exosomes derived from BT-474 and SK-BR3 cells. Previous findings have also proven a decoy role for exosomal HER2 in counteracting anti-tumor effects of Trastuzumab [11]. Similarly, the expression of CD20 on B-cell lymphoma-derived exosomes were also shown to abolish the anti-tumor effects of the anti-CD20 antibodies (rituximab) by serving as decoy receptors and consuming complement, which protects target cells from antibody attack [39]. However, pharmacological blockade of exosome release from B-cell lymphoma cells was found to sensitize the target cells to anti-CD20-mediated lysis [39]. There is also evidence supporting our speculation that total HER2 (including exosomal HER2) shed from SK-OV3 is not comparable to that amount from BT-474 and SK-BR3 cells [28]. Strikingly, we observed that blocking exosome secretion could significantly improve Trastuzumab-mediated ADCC compared to individual Trastuzumab or Trastuzumab pre-treated with HER2 carrying exosomes. Due to the decreased levels of HER2 expression upon exosome blockade, we expected a decline in the ADCC activity of Trastuzumab in combination with exosome inhibitor, however, as it is shown in Fig. 6, we observed a significant improvement in the Trastuzumab-mediated cellular cytotoxicity. In the same line, there are several studies indicating that even cancers expressing low levels of HER2 are capable of binding significant fraction of Trastuzumab to induce ADCC responses [40]. Lapatinib and Levatinib were also, respectively, shown to upregulate and downregulate the cell surface HER2 expression, but more potent ADCC responses are induced when Levatinib is added to Trastuzumab [41, 42]. Considering these findings, it can be concluded that the decreased surface HER2 levels would not predict the final outcome of therapeutic regimens. It seems that there is still higher amount of HER2 on the cell surface after blocking exosome release that can bind to Trastuzumab to induce ADCC.
As we have reviewed elsewhere, exosomes are rich in several mediators that can compromise the cytotoxic activity of NK cells, therefore, the expression of HER2 on tumor exosomes may not be considered as the sole factor for decreased ADCC-mediated by Trastuzumab [43]. Regarding this aspect, we investigated whether HER2-carrying exosomes abolish the responses to Trastuzumab by only serving as decoy receptors or also by contributing to the dysfunction of NK cells. According to Fig. 7, different patterns were observed when ADCC experiments were carried out with NK cells instead of PBMCs. In this setting, more robust increase in the ADCC activity of Trastuzumab was observed in combination with exosome inhibitor. Particularly, different results were obtained where NK cells were pre-treated with exosomes derived BT-474, SK-BR3 and SK-OV3 cells (Fig. 7), representing differences in their exosomal content. The findings showed that pretreating NK cells with exosomes derived from BT-474 cells could decrease the ADCC responses compared to individual Trastuzumab, although the differences were not significant. However, strikingly, NK cells pre-treated with exosomes derived from SK-BR3 and SK-OV3 cells had significantly compromised ADCC activity. These findings indicated that exosomes derived from HER2 overexpressing cancer cells could hamper anti-tumor responses of therapeutic antibodies by dually serving as decoy receptors and directly compromising cytolytic function of NK cells with their specific cargo irrespective of HER2 molecules.
HER2 carrying exosomes have a dual inhibitory effect on ADCC responses. ADCC experiments with the a BT474 cells, b SK-BR3 cells, c SK-OV3 cells were performed with PBMCs (E:T ratio 60:1) or NK cells (10:1) and Trastuzumab (20 µg/ml) in the absence or presence of prior treatment with the GW4869 for 48 h. The percentage of specific lysis was calculated and compared between the treated and untreated conditions. Data were indicating of at least three independent experiments, of which one representative example is shown; bars represent mean ± SD; *P < 0.05 **P < 0.01; ***P < 0.001. NS non-significant
Concluding remarks and perspectives
Our findings demonstrate that blocking exosome release from HER2-overexpressing cancers would add to the benefits of anti-HER2 monoclonal antibodies, such as Trastuzumab, by inducing apoptosis, decreasing cellular proliferation and potentiating ADCC responses without affecting antigen internalization. Furthermore, we revealed that HER2 trafficking to plasma membrane was disrupted by inhibiting exosome secretion in a time-dependent manner. This result support a role for exosome release pathway in regulating surface expression of HER2. However, further studies are needed to examine the effects of exosome blocking on the signaling cascade downstream HER2 molecules and with other HER2-directed antibodies, including TDM-1 (Trastuzumab-emtansine). Examining the combination of exosome blockade and tyrosine kinase inhibitors (Lapatinib, etc.) might also provide deeper insights into the benefits of exosome inhibition. Since a link has been postulated between autophagy and exosome release compartments (nSMase-2), hence future studies would be of interest to explore the potential mechanisms behind this connection and its relation with the changes observed in our experiments [35]. There is also other data indicating a role for molecules like hyaluronic acid (HA) in the internalization of exosomes into the recipient cells [44,45,46]. Therefore, further research on the interaction of extracellular matrix (including HA molecules) with tumor exosomes may help to the development of novel therapeutics in tackling challenges related to tumor-derived exosomes. Finally, the effects of other clinically available exosome inhibitors, such as Ketoconazole, can also be investigated in similar experimental settings for potential use in the future [47, 48].
References
Hsu JL, Hung M-C. The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 2016;35(4):575–88.
Gradishar WJ. HER2 therapy—an abundance of riches. N Engl J Med. 2012;366(2):176–8.
Petricevic B, Laengle J, Singer J, Sachet M, Fazekas J, Steger G, et al. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J Transl Med. 2013;11(1):1–11.
Hoeferlin LA, Chalfant CE, Park MA. Challenges in the treatment of triple negative and HER2-overexpressing breast cancer. J Surg Sci. 2013;1(1):3.
Xu Z-q, Zhang Y, Li N, Liu P-j, Gao L, Gao X, et al. Efficacy and safety of lapatinib and trastuzumab for HER2-positive breast cancer: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2017;7(3):e013053.
Oh D-Y, Bang Y-J. HER2-targeted therapies—a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17(1):33–48.
Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–6.
Shimada Y, Matsubayashi J, Kudo Y, Maehara S, Takeuchi S, Hagiwara M, et al. Serum-derived exosomal PD-L1 expression to predict anti-PD-1 response and in patients with non-small cell lung cancer. Sci Rep. 2021;11(1):7830.
Hosseini R, Asef-Kabiri L, Yousefi H, Sarvnaz H, Salehi M, Akbari ME, et al. The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells. Mol Cancer. 2021;20(1):83.
Marar C, Starich B, Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22(5):560–70.
Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227(2):658–67.
Martinez VG, O’Neill S, Salimu J, Breslin S, Clayton A, Crown J, et al. Resistance to HER2-targeted anti-cancer drugs is associated with immune evasion in cancer cells and their derived extracellular vesicles. Oncoimmunology. 2017;6(12): e1362530.
Battke C, Ruiss R, Welsch U, Wimberger P, Lang S, Jochum S, et al. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol Immunother. 2011;60(5):639–48.
Tian F, Zhang S, Liu C, Han Z, Liu Y, Deng J, et al. Protein analysis of extracellular vesicles to monitor and predict therapeutic response in metastatic breast cancer. Nat Commun. 2021;12(1):1–13.
Liu C, Yang Y, Wu Y. Recent advances in exosomal protein detection via liquid biopsy biosensors for cancer screening, diagnosis, and prognosis. AAPS J. 2018;20(2):1–13.
Hekmatirad S, Moloudizargari M, Moghadamnia AA, Kazemi S, Mohammadnia-Afrouzi M, Baeeri M, et al. Inhibition of exosome release sensitizes U937 cells to PEGylated liposomal doxorubicin. Front Immunol. 2021;12:2008.
Poggio M, Hu T, Pai C-C, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414-27. e13.
Schneider E, Winzer R, Rissiek A, Ricklefs I, Meyer-Schwesinger C, Ricklefs FL, et al. CD73-mediated adenosine production by CD8 T cell-derived extracellular vesicles constitutes an intrinsic mechanism of immune suppression. Nat Commun. 2021;12(1):1–14.
Hosseini-Ghatar R, Soltantoyeh T, Bahadori M, Golara M, Hassannia H, Khosravi-Eghbal R, et al. Epitope mapping of human HER2 specific mouse monoclonal antibodies using recombinant extracellular subdomains of HER2. Asian Pac J Cancer Prev. 2017;18(11):3103–10.
Hosseini-Ghatar R, Soltantoyeh T, Bahadori M, Khoshnoodi J, Golsaz-Shirazi F, Jeddi-Tehrani M, et al. Polyclonal antibody against different extracellular subdomains of HER2 induces tumor growth inhibition in vitro. Iran J Immunol. 2017;14(3):200–14.
Hassannia H, Amiri MM, Jadidi-Niaragh F, Hosseini-Ghatar R, Khoshnoodi J, Sharifian R-A, et al. Inhibition of tumor growth by mouse ROR1 specific antibody in a syngeneic mouse tumor model. Immunol Lett. 2018;193:35–41.
Yu S-F, Zheng B, Go M, Lau J, Spencer S, Raab H, et al. A novel anti-CD22 anthracycline-based antibody–drug conjugate (ADC) that overcomes resistance to auristatin-based ADCs. Clin Cancer Res. 2015;21(14):3298–306.
Nejadmoghaddam M-R, Zarnani A-H, Ghahremanzadeh R, Ghods R, Mahmoudian J, Yousefi M, et al. Placenta-specific1 (PLAC1) is a potential target for antibody-drug conjugate-based prostate cancer immunotherapy. Sci Rep. 2017;7(1):1–13.
Steinbichler TB, Dudás J, Skvortsov S, Ganswindt U, Riechelmann H, Skvortsova I-I. Therapy resistance mediated by exosomes. Mol Cancer. 2019;18(1):58.
Hayatudin R, Fong Z, Ming LC, Goh B-H, Lee W-L, Kifli N. Overcoming chemoresistance via extracellular vesicle inhibition. Front Mol Biosci. 2021;8:158.
Yang Y, Li C-W, Chan L-C, Wei Y, Hsu J-M, Xia W, et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28(8):862–4.
Chew HY, De Lima PO, Cruz JLG, Banushi B, Echejoh G, Hu L, et al. Endocytosis inhibition in humans to improve responses to ADCC-mediating antibodies. Cell. 2020;180(5):895-914. e27.
Gall VA, Philips AV, Qiao N, Clise-Dwyer K, Perakis AA, Zhang M, et al. Trastuzumab increases HER2 uptake and cross-presentation by dendritic cells. Can Res. 2017;77(19):5374–83.
Wymant JM, Sayers EJ, Muir D, Jones AT. Strategic trastuzumab mediated crosslinking driving concomitant HER2 and HER3 endocytosis and degradation in breast cancer. J Cancer. 2020;11(11):3288.
Paris L, Cecchetti S, Spadaro F, Abalsamo L, Lugini L, Pisanu ME, et al. Inhibition of phosphatidylcholine-specific phospholipase C downregulates HER2 overexpression on plasma membrane of breast cancer cells. Breast Cancer Res. 2010;12(3):1–16.
Koay DC, Zerillo C, Narayan M, Harris LN, DiGiovanna MP. Anti-tumor effects of retinoids combined with trastuzumab or tamoxifen in breast cancer cells: induction of apoptosis by retinoid/trastuzumab combinations. Breast Cancer Res. 2010;12(4):1–19.
Liu Z, Sang X, Wang M, Liu Y, Liu J, Wang X, et al. Melatonin potentiates the cytotoxic effect of Neratinib in HER2+ breast cancer through promoting endocytosis and lysosomal degradation of HER2. Oncogene. 2021;40(44):6273–83.
She Q-B, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE. 2008;3(8): e3065.
Menck K, Sönmezer C, Worst TS, Schulz M, Dihazi GH, Streit F, et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J Extracell Vesicles. 2017;6(1):1378056.
Back MJ, Ha HC, Fu Z, Choi JM, Piao Y, Won JH, et al. Activation of neutral sphingomyelinase 2 by starvation induces cell-protective autophagy via an increase in Golgi-localized ceramide. Cell Death Dis. 2018;9(6):1–18.
Hao M, Yeo SK, Turner K, Harold A, Yang Y, Zhang X, et al. Autophagy blockade limits HER2+ breast cancer tumorigenesis by perturbing HER2 trafficking and promoting release via small extracellular vesicles. Dev Cell. 2021;56(3):341-55. e5.
Zhang J, Fan J, Zeng X, Nie M, Chen W, Wang Y, et al. Targeting the autophagy promoted antitumor effect of T-DM1 on HER2-positive gastric cancer. Cell Death Dis. 2021;12(4):1–14.
Cuello M, Ettenberg SA, Clark AS, Keane MM, Posner RH, Nau MM, et al. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Can Res. 2001;61(12):4892–900.
Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci. 2011;108(37):15336–41.
Collins D, O’donovan N, McGowan P, O’sullivan F, Duffy M, Crown J. Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in HER-2-non-amplified breast cancer cell lines. Ann Oncol. 2012;23(7):1788–95.
Collins DM, Madden SF, Gaynor N, AlSultan D, Le Gal M, Eustace AJ, et al. Effects of HER family–targeting tyrosine kinase inhibitors on antibody-dependent cell-mediated cytotoxicity in HER2-expressing breast cancer. Clin Cancer Res. 2021;27(3):807–18.
Collins DM, Gately K, Hughes C, Edwards C, Davies A, Madden SF, et al. Tyrosine kinase inhibitors as modulators of trastuzumab-mediated antibody-dependent cell-mediated cytotoxicity in breast cancer cell lines. Cell Immunol. 2017;319:35–42.
Hosseini R, Sarvnaz H, Arabpour M, Ramshe SM, Asef-Kabiri L, Yousefi H, et al. Cancer exosomes and natural killer cells dysfunction: biological roles, clinical significance and implications for immunotherapy. Mol Cancer. 2022;21(1):1–18.
Ganau M. Tackling gliomas with nanoformulated antineoplastic drugs: suitability of hyaluronic acid nanoparticles. Clin Transl Oncol. 2014;16(2):220–3.
Ragni E, PeruccaOrfei C, De Luca P, Lugano G, Viganò M, Colombini A, et al. Interaction with hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoarthritic synoviocytes. Stem Cell Res Ther. 2019;10(1):1–17.
Mu W, Rana S, Zöller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15(8):875-IN4.
Greenberg JW, Kim H, Moustafa AA, Datta A, Barata PC, Boulares AH, et al. Repurposing ketoconazole as an exosome directed adjunct to sunitinib in treating renal cell carcinoma. Sci Rep. 2021;11(1):1–12.
Datta A, Kim H, McGee L, Johnson AE, Talwar S, Marugan J, et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: a drug repurposing strategy for advanced cancer. Sci Rep. 2018;8(1):1–13.
Acknowledgements
This study was supported by grants from Isfahan University of Medical Sciences, Isfahan, Iran (grant number#51435); and Cancer Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and Informed consent
It should be noted that for PBMCs isolation, a signed consent letter was taken from healthy donors (n = 3), and all the protocols of this study were approved by the Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.MED.REC.1399.569).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosseini, R., Asef-Kabiri, L., Sarvnaz, H. et al. Blockade of exosome release alters HER2 trafficking to the plasma membrane and gives a boost to Trastuzumab. Clin Transl Oncol 25, 185–198 (2023). https://doi.org/10.1007/s12094-022-02925-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02925-5