Abstract
Colorectal cancer (CRC) is one of the leading causes of mortality among cancers. Many aspects of this cancer are under investigation to find established markers of diagnosis, prognosis, and also potential drug targets. In this review article, we are going to discuss the possible solution to all these aims by investigating the literature about cancer-associated fibroblasts (CAFs) involved in CRC. Moreover, we are going to review their interaction with the tumor microenvironment (TME) and vitamin D and their role in tumorigenesis and metastasis. Moreover, we are going to expand more on some markers produced by them or related to them including FAP, a-SMA, CXCL12, TGF- β, POSTN, and β1-Integrin. Some signaling pathways related to CAFs are as follows: FAK, AKT, activin A, and YAP/TAZ. Some genes related to the CAFs which are found to be possible therapeutic targets include COL3A1, JAM3, AEBP1 and, CAF-derived TGFB3, WNT2, and WNT54.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
-
a.
Colorectal cancer (CRC) and its treatment
As the new era has brought about unhealthy lifestyles, it has also bequeathed us related diseases such as soared incidence of colorectal cancer (CRC) [1]. Reportedly, CRC is the third most prevalent cancer in the US [2].
Currently, chemotherapy with 5-fluorouracil (5-FU) combined with oxaliplatin is the first-line treatment for CRC; however, due to its adverse effects, there is an investigation to find safer and also more efficient alternatives [3, 4]. Despite the progress in detecting appropriate biomarkers for screening, there is still room for investigations to predict biomarkers of metastasis, tumor staging, and to track the effectiveness of the treatments [5]. Studies suggest the benefit of cancer-associated fibroblasts (CAFs) and their products to serve this purpose.
-
b.
Cancer-associated fibroblasts (CAFs)
CAFs are known to be involved in different cancer types through their excess production of fibrosis, chemokines, and different factors such as hepatocyte growth factor (HGF), and fibroblast growth factors (FGFs), which also mediate their role in tumor metastasis and angiogenesis [6]. It is suggested that they are originated from bone marrow-derived mesenchymal cells, which are kindled by C-X-C motif chemokine ligand 12 (CXCL12) signaling and transforming growth factor beta1 (TGF-β) [7]. Moreover, they are heterogeneous with different effects on tumors [8]. They are an important component of the tumor microenvironment (TME) and are affected by the operative agents of TME such as vitamin D [9].
-
c.
CAF-related molecules
CAFs have vital roles in determining the characteristics of colorectal tumors. They can secrete factors that contribute to angiogenesis, tumor invasion, and therefore, markers for detecting colorectal tumor progression. Moreover, β1-Integrin activation in fibroblasts which is induced by TNIIIA2 may lead to malignancy [10]. The protein kinase Cζ- sex-determining region Y-box 2 (PKCζ-SOX2) axis is considered to be an essential component in controlling CAF tumorigenic properties in colorectal cancer [11]. Platelet-derived growth factor (PDGF) activates CAFs via snail through the FAK (focal adhesion kinase) pathway which in turn promotes tumor progression and angiogenesis [12]. For instance, they express wnt family member 2 (wnt2) which can be a potential target in colorectal cancer immunotherapy [13]. Wnt2 can increase pro-angiogenic signaling by increasing the expression of angiopoietin (ANG-2), interleukin-6 (IL-6), placental growth factor (PGF) and, granulocyte colony-stimulating factor (G-CSF) [14]. Moreover, fibroblast activation protein-α (FAPs) secreted by CAFs have a role in colorectal tumor angiogenesis [15]. Their role in colorectal tumor metastasis is also significant. It is observed that CAFs may act differently and their tendency for venous invasion varies [16]. Data suggest that CAF markers like fibroblast activation protein-α (FAP-α) and epithelial-mesenchymal transition (EMT) related agents can be beneficial for tracking colorectal tumor development [17]. Additionally, FAP is a factor that may cause resistance to immune-checkpoint blockade [18]. CAFs express higher levels of alpha-smooth muscle actin (a-SMA), HGF, fibroblast specific protein 1 (FSP1), cluster of differentiation 163 (CD163), periostin (POSTN/PN) gene and, dendritic cell-specific intercellular adhesion molecule-3-Grabbing non-integrin (DC-SIGN) which are involved in colorectal cancer invasion and can be suitable biomarkers for colorectal cancer progression [19,20,21]. A disintegrin and metalloproteinases (ADAMs) expressed by CAFs play a role in desmoplastic reaction (DR) which signifies their role in colorectal tumor metastasis [22]. Also, RAB31 (member RAS oncogene family) secreted by CAFs is indirectly involved in colorectal cancer metastasis via promoting HGF secretion [23]. CircSLC7A6 (a circRNA derived from the SLC7A6 gene) released from CAFs enhances colorectal tumor metastasis by acting on C-X-C chemokine receptor type 5 (CXCR5) receptors and a potential antagonist is matrine [24]. Periostin derived from fibroblasts promotes colorectal tumor progression and simultaneously, IL-6 secreted from colorectal tumor cells induces periostin expression [25]. Besides, it helps cell migration via the AKT pathway [26].
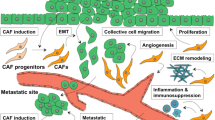
As the CAF’s role in carcinogenesis is so significant, many efforts have been done to discover CAF-related signaling pathways to control them. As a good example, some factors are known to contribute to the transformation of normal fibroblasts into CAFs like TGFβ1 [27]. Calcium/calmodulin-dependent protein kinase II (CaMKII) is proved to be a mediator in the TGFβ1 signaling pathway and it might be a potential treatment for CRC [27]. CAFs receive signals via their receptor, C–C motif chemokine receptor 5 (CCR5). Therefore, it is an essential contributor to their signaling pathway [28]. It is suggested that targeting CCR5 might be a potential anti-CRC therapy [28]. Cellular calcium availability plays an important role in CAF mobility and therapeutic approaches against it can be of benefit [29]. This pathway is induced by 12-hydroxyeicosatetraenoic acid (12(S)-HETE) which is secreted by colon cancer cells [29]. Small non-coding RNAs (Snc-RNAs) should also be taken into consideration as diagnostic and prognostic markers [30]. These molecules are CAF-derived and they act as a mediator between different extra-cellular matrix (ECM) components and colon cancer cells [30]. Exosomal micro-RNAs are known as mediators in ECM-tumor crosstalk [31]. In this review, we will investigate the factors secreted by CAFs and their tumorigenic, metastatic and, prognostic roles. Figure 1 provides a summary of CAF-related pathways involved in CRC development and pathogenesis. Figure 2 reviews some molecular pathways that regulate the function of CAFs. We also will discuss CAF-associated therapeutic targets.
An overview of CAF-related pathways associated with CRC angiogenesis, resistance to immune checkpoint blockade, metastasis, and tumorigenesis. Wnt2, wnt family member 2; ANG-2, angiopoietin; IL-6, interleukin-6; PDGF, platelet- derived growth factor; G-CSF, granulocyte colony-stimulating factor; FAP, fibroblast activation protein-α; CAF, cancer-associated fibroblast; CRC, colorectal cancer; α-SMA, alpha-smooth muscle actin; HGF, hepatocyte growth factor; FSP1, fibroblast specific protein 1; CD163, cluster of differentiation 163; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-Grabbing non-integrin; POSTN, periostin; IL-6, interlukin-6; ADAMs, a disintegrin and metalloproteinases; DR, desmoplastic reaction; PKCζ-SOX2, protein kinase Cζ-sex determining region Y-box 2; CXCR5, C-X-C chemokine receptor type 5
An overview of pathways and molecules acting on CAFs. TGF-β, transforming growth factor beta1; CaMKII, calcium/calmodulin dependent protein kinase II; CCR5, C–C motif chemokine receptor 5; PDGF, platelet- derived growth factor; FAK, focal adhesion kinase; 12(S)-HETE, 12-hydroxyeicosatetraenoic acid; CAF, cancer-associated fibroblast; CRC, colorectal cancer
Some suggested drugs against CAFs which may have a therapeutic role in colorectal tumors include: lipid nanocapsules loaded with acriflavine (LNC-ACF) or paclitaxel (LNC-PTX), NSAIDs combined with vincristine and microsatellite instability (MSI) -N1014 [32,33,34].
Cancer-associated fibroblasts (CAFs)
-
a.
Effects of microenvironment on fibroblasts
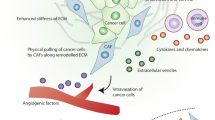
In a study performed on cultured primary human umbilical vein endothelial cells (HUVECs/ECs), it was observed that TME stiffness stimulates tumor metastasis [35]. Activin A secreted by CAFs has a major role in pro-metastatic changes in epithelial cells induced by TGF-β [36]. A study investigated the effect of TME’s stiffness on the secretion of activin A from CCD18 fibroblasts [37]. They altered CCD18 stromal cells to CAFs [37]. It was found that higher stiffness induced tumor spreading [37]. Additionally, it was observed that activin A had a significant role in the tumor metastasis because by using follistatin which is its ligand trap, tumor metastasis was halted [37]. The integrin pathway acted as a promoter for activin A secretion [37]. Arg-Gly-Asp-Ser (RGDS) peptide was beneficial in decreasing activin A secretion although not very noteworthy [37]. Follistatin also inhibited snail expression and E-cadherin reduction which highlights the role of activin A in EMT [37]. EMT is a process through which cells increase their potential to metastasis and therefore, it is considered to have an important role in tumorigenesis [38]. The serum level of Activin A was elevated in patients with stage IV tumors and when the TME stiffness was between 5.58–68 kPa [39,40,41]. Furthermore, activin A serum level was a beneficial marker for tumor metastasis [37]. So, TGF-β promotes activin A secretion by CAFs and this action is promoted by increased TME stiffness which activates TGF-β [37, 42,43,44,45,46]. Activin A secretion was the highest in the stiffness of 40 kPa [37]. Activin A signaling pathway may also be a potential therapeutic pathway [37].
In another study performed on cell lines SW620 (ATCC, CCL-227), SW480 (ATCC, CCL-228), Human colorectal fibroblast CCD-18-co (ATCC, CRL-1459), and LoVo (ATCC, CCL-229), CAFs were shown to be of importance in determining the metabolism of CRCs [47]. For instance, a study suggests that CAFs cultured with CRCs more prone to oxidative stress and autophagy produced more 1A/1B-light chain 3 (LC3) and BCL2 interacting protein 3 (BNIP3), which are the autophagy-related proteins [47]. Also, caveolin 1 (cav-1) and autophagy metabolites were decreased [47]. These events were reversed when oxidative stress inhibitors were added to the medium [47]. For example, isocitrate dehydrogenase [NADP(+)] 2 (IDH2), cav-1 and, glycolysis enzymes were increased while autophagy and proliferation were decreased [47]. Moreover, it was supported that a higher rate of autophagy was in favor of tumor cells’ survival due to increased nutrients (pyruvic acid/lactic acid) and its defensive role against oxidative harm [47]. Finally, autophagy and oxidative stress inhibitors were suggested as therapeutic options for CRC [47].
Another study, conducted on the resected primary tumors, suggested that CAFs contributed to peritoneal metastasis- colorectal cancer (PM-CRC) might consume free fatty acids (FFAs) and depend on fatty acid oxidation (FAO) [48]. Carnitine palmitoyltransferase IA (CPT1A) over-expression in CAFs enhances CRC tumor metastasis [48]. These data were confirmed by the observation that adding oleic acid to drinking water promotes tumor growth [48]. This study could not confirm the CAF inhibition but claimed that there is still room for more studies on the role of FAO inhibition on CAFs [48].
It was observed that CPT1A over-expression in CAFsPM causes altered metabolism from glycolysis to oxidative phosphorylation [48]. In addition, CPT1A over-expression leads to increased secretion of vascular endothelial growth factor-A (VEGF-A), matrix metalloproteinase2 (MMP2), and C–C motif chemokine ligand 2 (CCL2) resulting in more invasive behavior of tumor [48]. Aerobic glycolysis that plays a role in promoting tumor metastasis may be promoted by CAFsCPT1A-OE as a result of CPT1A downregulation in tumor cells [48]. It was suggested that PM-CRC patients may be appropriate to receive anti-angiogenic agents and glycolysis-inhibitors due to their dependence on glycolysis [48]. Additionally, it was observed that serum-derived carcinoembryonic antigen (CEA) stimulates fibroblasts in a paracrine manner as well as increasing the expression of a-SMA, VEGF and, fibronectin containing extra domain A (Fn-EDA) in a study carried out on the primary normal human neonatal dermal fibroblasts (ATCC PCS-201–010) and the human carcinoma cell lines HT-29 (ATCC HTB-38), LS174T (ATCC CL-188) [49]. It was also reported that serum carcinoembryonic antigen (sCEA) had a role in the switch of fibroblasts to type 2 CAFs which promote cancer metastasis through AKT serine/threonine kinase 1- the protein kinase complex mechanistic target of rapamycin complex 1/signal transducer and activator of transcription 3 (AKT1-mTORC1/STAT3) signaling pathway [49, 50]. In another study performed on the human SW620 (CCL-227, lymph node metastasis) and SW480 (CCL-228, primary tumor) colorectal adenocarcinoma cells, it was demonstrated that colon cancer cells induce malignancy by producing 12(S)-HETE which acts on CAFs [29]. This component does so by its mediatory action on CamKs and Ca2+ resulting in phosphorylation of myosin light chain 2 (MLC2) and circular chemo-repellent induced defects (CCID) formation [29]. Consistent with these data, diminished CCID development was observed as Ca2+ was chelated with 1,2-Bis(2-aminophenoxy)ethane-N, N, N', N'-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM), rhosin inhibited Rho-associated kinase (RHO/ROCK) and, MLC2 blockade by hindering myosin light chain kinase (MYLK) [29]. RHO had positive feedback on Ca2+ release and by blocking it calcium which is an essential component in tumor mobility [29]. As a result, it was suggested that drugs that act on calcium accessibility may be a good option to fight against metastasis [29].
An overview of CAF-related pathways associated with CRC prognosis. FAP, fibroblast activation protein-α; snc-RNA, small noncoding RNA; a-SMA, alpha-smooth muscle actin; HGF, hepatocyte growth factor; FSP1, fibroblast specific protein 1; CD163, cluster of differentiation 163; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-Grabbing non-integrin; POSTN, periostin; CAF, cancer-associated fibroblast; CRC, colorectal cancer
Vitamin D or 1,25(OH)2D3 was suggested as a protective factor against CRC through its role in inhibiting CAFs in a study on the tissue samples from colon primary tumor and morphologically normal colonic mucosa [51]. Its upregulation on CAFs was shown to be a good prognostic factor which indicated a better prognosis [51].
The role of CAFs in tumorigenesis of colorectal cancer
The role of CAFs in angiogenesis was confirmed by observing VEGF-positive CAFs in the metastatic CRC [52]. These CAFs may be originated from mesenchymal-to-mesothelial transition (MMT) [52]. In another study, it was detected that colorectal tumorigenesis in mice was diminished by down-regulation of periostin [25]. Periostin is a component of non-structural matrix proteins and it is produced by CAFs [21, 53]. It was also detected that down-regulation of PN led to reduced proliferative cell number. Therefore, it was implied that it had a role in epithelial cell proliferation along with tumorigenesis [25]. In addition, Yes-associated protein 1/ WW-domain-containing transcription regulator 1 (YAP/TAZ) knockdown resulted in a major decrease in cell proliferation regardless of PN enrichment which is consistent with the idea that PN induces cell proliferation via YAP/TAZ pathway [25]. PN triggers YAP via the integrin-FAK-Src pathway [25]. Decrease in PN led to a decrease in IL6 in tumor cells and stimulation of YAP by PN led to increased levels of IL6 [25]. Also, PN production was enhanced through IL-6-STAT3 signaling [25].
In a study aimed at finding the correlation between PN and CRC, it was found that PN can induce hostile acts of the tumor via integrin (ITG) α5β1 and ITGα6β4 in an AKT-dependent manner [26]. Also, it was observed that after introducing PN to the medium the levels of pAKT and pERK raised [26]. Thus, PN was suggested as a valuable marker to determine poor prognosis [26]. Combination treatment with agents targeting autophagy and PN-ITG-AKT-dependent signaling pathway was suggested as a promising therapy against CRC [26]. Furthermore, it was supported that angiogenesis and ECM remodeling are carried out by the collaboration of snail-producing fibroblasts [12]. Consistent with this data, it was suggested that there is a correlation between snail1 expression and CD34, an indicator for angiogenesis [12]. Endothelial activation by PDGF was facilitated by snail1 [12]. Snail1 might be a good option to estimate the benefit of treatment for the patient [12]. Table 1 provides a summary of tumorigenesis-related factors.
The role of CAFs in the metastasis of colorectal cancer
CAFs are very heterogenous and they vary in expressing different factors including αSMA, vimentin, and FAP [54]. These factors are not always an indicator of stimulated fibroblasts [54]. Bone morphogenic proteins (BMPs) are secreted by epithelial cells to maintain their stability [55]. Especially it is realized that BMP2 and BMP7 have a significant tumor suppressor role by inhibiting invasion and EMT [56,57,58,59,60]. Gremlin1 (GREM1) which is expressed by CAFs and to a lesser extent by cancer cells can cause tumor metastasis [61,62,63]. Tumor budding (TB) is another factor that is important in determining metastatic potential [64]. In addition, TB plays an important role in lymph node metastasis (LNM) of submucosal invasive colorectal cancer (SiCRC) [64, 65]. Another study suggested that the utility of TB may vary among individuals [66].
It was also observed that FAP-1 overexpression, which is almost exclusively expressed by CAFs, was an essential LNM determinant [15, 66, 67]. FAP-1 might be a useful indicator to assess LNM in SiCRC through its role in inducing cancer growth [66]. It can both, directly and indirectly, play a key role in identifying ECM [66]. Directly by damaging ECM and indirectly by maintaining protease/protease inhibitor balance [15, 67, 68]. FAP-1 can modify collagen fibers and other proteins in the ECM and this process might grant treatment options for researchers [17, 69].
Another study suggested that CRC progression is monitored by CAFs through the wnt pathway and especially wnt2 which mediates CRC-CAF interaction and is secreted in a paracrine fashion [54]. Factors important in determining tumor invasion such as TNM stage, LNM, invasion to vessels, angiogenesis and, recurrence are highly associated with wnt2 expression [14, 54]. Also, wnt2 was not related to tumor location [54]. This finding was consistent with previous data suggesting that wnt2 secretion from CAFs promoted CRC metastasis and invasion through muffling dendritic cell (DC) differentiation by stimulating suppressor of cytokine signaling-3 (SOCS3) production in DC precursors, and therefore, suppressing the p-Janus Kinase 2 (JAK2)/p- STAT3 (Tyr705) pathway [13, 70]. Furthermore, wnt2 might be a therapeutic option against CRC [54]. It was observed that anti-wnt2 therapy subdued tumor progression through CD8 + T-cells and increased the effect of anti-programmed death-1 (anti-PD-1) therapy [13].
In another study aimed at finding the correlation between miRNAs and tumor progression, it was observed that upregulation of miR-192 or miR-17 in CAFs is associated with a less invasive manner of tumor cells which was consistent with previous data suggesting that carcinomatous cells follow CAFs footprints during metastasis [71,72,73]. This study supported that upregulation of miR-192, miR-17 and, miR-200 was associated with down-regulation of ECM remodeling genes and this pathway might be a promising treatment that inquires further study [73]. Additionally, another study suggested that CAF-derived exosomal miRNAs have prognostic and diagnostic roles [31]. This study focused on miR-21 which was expressed in high amounts in CAFs and could be transferred to CRC cells via stroma [31]. High levels of miR-21 were associated with poor prognosis, extra invasion and, the proliferation of cancer cells [31].
In a study investigating various factors that correlate CAFs to CRC, it was observed that down-regulation of follistatin like 1 (FSTL1) and transgelin (TAGLN) in CCD-18Co cell line lead to proliferation stimulation in HT-29 and LoVo cell lines [74]. Secreted protein acidic and cysteine-rich (SPARC) protein might serve the same function [74]. TAGLN can inhibit tumor metastasis both in an autocrine and paracrine way [74]. In addition, FSTL1 did not play an essential role in tumor prognosis [74]. Moreover, β1-integrin activation in fibroblasts induced by Tenascin C (TNC) peptides which hold TNIIIA2 in azoxymethane/dextran sodium sulfate (AOM/DSS) mouse models was correlated with stimulated metastatic behavior of colitis-associated colorectal cancer (CAC) [10]. This pathway could be a promising therapeutic option that is blocked by a cryptic de-adhesive site within fibronectin molecule (FNIII14) [10].
Moreover, in another study investigating the role of RAB31 secreted by CAFs it was observed that not all CAFs secrete RAB31 [23]. Among growth factors and cytokines which are induced by RAB31, hepatocyte growth factor (HGF) seemed to have a more important role [23]. HGF is produced by fibroblast and stimulates epithelial cell migration [75]. RAB31 induces HGF secretion by binding to it and enhancing its secretion or by contributing to the transportation complex [23]. As a result, RAB31 augments CRC invasion via HGF/Met signaling pathway [23]. Another study suggested that c-Met phosphorylation which was stimulated by fibroblast-derived HGF played a significant role in tumor invasion and there is room for more investigation about its potential efficacy as a treatment [76]. Additionally, it was observed that CAFs promote tumor progression by stimulating 18F-fluorodeoxyglucose (FDG) uptake thus standardized uptake value (SUV) [77]. So, SUVmax might be a beneficial indicator of tumor progression [77]. Table 2 provides a summary of CAF-associated factors related to metastasis.
CAFs and the prognosis of colorectal cancer
A study supported that upregulation of FAP-1 in the cancer front might be a beneficial predictor of prognosis [15, 67]. Moreover, it was suggested that FAP caused resistance to immune checkpoint blockade in CAFs by stimulating anti-PD-1 expression. Also, it was observed that an increased level of FAP was associated with an increased level of myeloid-derived suppressor cells (MDSCs) which plays a major role in immunosuppression [18]. Also, increased FAP caused a decrease in CD3+ cells, helper T cell 1 (TH1), and natural killer T (NKT) cells, and an increase in macrophages, monocytes, and CD11b+ cells which were associated with immunosuppression and tumor progression [15, 18]. In addition, it led to augmented levels of CD25+forkhead Box P3 (FOXP3+) T lymphocytes through regulating dipeptidyl peptidase 4 (DPP4), CD73, and, Bcl-2 Homology 3 (BH3) [15]. Another mechanism of immunosuppression induced by FAP was the higher expression of CCL2 [18]. CCL2 conducted this action by increasing regulatory T cells and MDSCs in TME [78, 79]. Therefore, anti-CCL2 therapy might be an efficient option [18]. FAP/nuclear β-catenin (BCAT) upregulation in invasion to the lymph nodes was correlated with poor disease-free survival (DFS) rates among patients suffering from adenocarcinoma (AdC) [17].
It was suggested that POSTN expression plays a role in tumor growth, proliferation and, metastasis and contributes to the tumor categorization of T, N, or M [21]. The TNM system is developed for staging tumors regarding their size, lymph node involvement, and metastasis [80]. Also, it was shown that POSTN alters the activity of the HEK 293 T cell line from tumorigenic but non-metastatic to metastatic [81, 82]. It was also related to TB which is considered as an indicator of poor prognosis [21, 83]. In addition, there was a link between higher levels of POSTN and CpG island methylator phenotype (CIMP)-high and B-Raf proto-oncogene, serine/threonine kinase (BRAF) which are indicators of changes in neoplasia [21]. Furthermore, secreted frizzled-related protein1/2 (SFRP1/2), which stimulates tumor malignancy by modifying CAF phenotype, was expressed as SOX2 was stimulated through PKCζ inhibition [11]. Also, Sfrp targets the wnt signaling pathway which induces its tumorigenicity [11]. PKCζ generates immunosuppression mediated by consensus molecular subtype 4/copper chaperone for superoxide dismutase3 (CMS4/CCS3) as well [11]. Moreover, PKCζ and TGF-β maintain the link between epithelium and the stroma that subsequently, causes decreased PKCζ expression by the upregulation of TGF-β [11]. Both stromal PKCζ and SOX2 levels were the indicators of bad prognosis [11].
In another study, α-SMA, AE binding protein 1 (AEBP1) and, zinc finger E-box binding homeobox 1 (ZEB1) were correlated to invasion to the submucosa while suggesting more investigations about the benefits of CD10 as a marker [84]. Additionally, podoplanin (PDPN) − αSMAhigh CAFs indicated the malignancy of the tumor while PDPN/S100A4high CAFs indicated TB and invasion to the lymphovascular [85]. Consistent with this finding, another study supported that fibrosis and αSMA in metastatic lymph nodes (MLN) are associated with poor prognosis, increased recurrence rates and, lower survival rates [86]. In another study investigating the prognostic value of CAF-related markers including a-SMA, FSP1, and FAP in the colon and rectal cancer, it was observed that their potential role is less significant in rectal cancer compared to colon cancer [20]. Table 3 provides a summary of the factors discussed in this section. Also, Fig. 3 illustrates some of the well-known CAF-related prognostic factors.
The role of CAFs in the treatment of colorectal cancer
In a study comparing the effectiveness of different therapeutic options against CAFs in CRC including chemotherapy with oxaliplatin, alternative chemotherapy containing SN38 (NK012) and PTX, fibroblast growth factor targeting (sunitinib, nintedanib) from multiple kinase inhibitors family, hypoxia-inducible factor (HIF), and EMT inhibitors, p53 activators, and apoptosis and autophagy stimulators i.e. ACF, it was found that anti-proliferative agents and chemotherapies were not efficient against CAFs [32]. Meanwhile, PTX sensitivity was higher when added to CAFs when compared to the previously mentioned agents but it was lower when administered to the CRC cell line [32]. However, sunitinib and ACF sensitivity were similar in both CRC cells and CAFs [32]. IC50 against CAFs was lowest for ACF and PTX. In addition, it was observed that combination therapy with PTX/ACF albeit well-tolerated, was not more effective than each treatment alone [32]. PTX monotherapy was more cytotoxic and CAFs were more sensitive to ACF monotherapy [32]. Both PTX and ACF were covered by lipid nanocapsules (LNC) [32].
In another study aimed at investigating the regulatory effects of matrine on the CRC tumorigenesis, it led to the inhibition of CRC tumorigenesis via reducing circular SLC7A6 (circSLC7A6) [24]. CircSL7A6 is an exosomal secretion of CAFs which has a regulatory effect on CXCR5 via tumor-associated miRNAs (such as miRNA-21 and miRNA-200 family) [24].
In a study investigating the potential targets for CRC treatment, it was observed that WNT2 and WNT54 knockdown is beneficial in inhibiting tumor formation [87]. Similarly, knockdown of collagen type III alpha 1 chain (COL3A1), junctional adhesion molecule 3 (JAM3), AEBP1 and, CAF-derived TGFB3 was efficient [87]. COL3A1 encodes a component of collagen and its expression by fibroblasts is enhanced by TGF-β [88]. JAM3 (JAM-C) encodes an adhesion molecule that enables endothelial cell adhesion [89]. AEBP1 (ACLP) is also encoded by fibroblasts and acts in ECM remodeling [90, 91]. This study also stated that the combination therapy by targeting ovarian cancer G-protein-coupled-receptor-1 (OGR1) protein with chemotherapy might have a promising outcome [87]. PRO-C6, a biomarker of collagen VI formations was proved to be beneficial in determining the response to bevacizumab treatment [5]. In addition, collagen formation was associated with poor prognosis in metastatic colorectal cancer (mCRC) [5]. Also, desmoplasia was associated with unhinged collagen turn-over which indicated a poor prognosis [5]. The patients with lower levels of all four types of collagen had a longer survival rate. From the biomarkers studied, PRO-C3 was the best in predicting prognosis [5].
Maraviroc, a CCR5 antagonist was found to be a beneficial treatment against CAF-enriched cancers like colon cancer due to the role of CCR5 in CAF gathering [28]. Vincristine monotherapy was suggested to be ineffective because of its role in inducing the release of TGF-βs and IL-6 from CAFs and subsequently, metastasis [33]. On the contrary, combining vincristine with anti-inflammatory treatments eliminated the complications of monotherapy [33]. CAF induces 5-FU resistance by increasing its IC50 twice naïve colorectal cells [34]. Moreover, CAF increases migratory properties of CRC cells by upregulating clusters of differentiation 44 positive (CD44+) cells and side-population (SP) cells [34]. Another mechanism of resistance-induction by CAFs is through the increased level of Thr68 phosphorylation of checkpoint kinase 2 (CHK2) in CRC cells [92]. CHK2 is a protein that promotes tumor cell survival via maintaining genome integrity, providing a survival signal while cells are exposed to agents that might damage DNA, and mitosis progression [92]. A study aimed at finding the therapeutic potency of MSI-N1014 in CRC demonstrated that this small-molecule agent did so by reducing CAF formation and diminishing 5-FU resistance by eliminating IL-6, leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5), mTOR, and β-catenin [34]. Also, the levels of miR-142-3p which acts as a tumor suppressor increased after introducing MSI-N1014 [34]. This study also stated that miR-142-3p could be a helpful therapeutic agent besides being a useful marker in determining prognosis [34]. In a study investigating the benefits of NADPH oxidase 4 (NOX4) as a therapeutic target, it was observed that this factor mediates CAF differentiation and accumulation [93]. It does so by producing reactive oxygen species (ROS) unrelated to TGF-β [93]. Also, inhibiting this enzyme was effective in repealing the tumor-promoting functions of CAFs and backsliding the a-SMA-positive CAFs into fibroblasts [93]. The latter data were suggestive of the revertible nature of CAFs [93]. The NOX1/4-inhibitor used in this study was setanaxib (GKT137831) [93]. In summary, NOX-inhibitors seem to be beneficial agents, since CAFs have an essential role in tumor resistance to chemotherapy [93].
Also, there are some ongoing studies like aflibercept that are conducting in phase two [94]. There is a list of mentioned treatment options in Table 4.
Interactions between CAFs and immune system
It was observed that FAP expressed by CAFs has a critical role in anti-tumor immune response regulation by induction of tumor-promoting inflammation [95]. Moreover, genes with a role in immune cell responses were expressed more in FAP-enriched environments, accentuating its role as an inflammation promoter [15]. A link between FAP overexpression and immune therapy resistance was suggested [79]. In a study utilizing the xCell algorithm, it was found that in a FAP-rich environment, the function of NKT and TH1 cells were inhibited; however, the operation of the Tregs, macrophages, and monocytes was promoted [15]. Another study suggested a mechanism for immunosuppression by FAP via CCL2 [18].
In the CMS1 subtype of CAFs, upregulation of proteins with critical roles in immune response pathways was demonstrated [96]. These proteins are correlated with a dispersed immune infiltrate containing cytotoxic T cells and TH1 [96]. For more instance, BRAF (v-raf murine sarcoma viral oncogene homolog B1) mutations were highly occurring in CMS1 cell-line [96]. In another study, it was found that the levels of stromal CD14+ cells, stromal and tumoral IL-2Rα, stromal and tumoral CD33, and tumoral PD-L1 were higher in a FAP-α-enriched environment [97].
-
a.
CAF and immunotherapy
As for immunotherapy, different approaches have been suggested related to CAFs. One of the mechanisms can be fibroblast recruitment and activation blockage. To achieve this goal different targets such as TGF- β, FAK, fibroblast growth factor receptor (FGFR), connective tissue growth factor (CTGF), and sonic hedgehog (SHH) are suggested. Another mechanism might be shifting the fibroblast sub-population balance via targeting chimeric antigen receptor (CAR)-T cells and antibody–drug conjugates (ADCs). One other mechanism can be blocking immunosuppressive and tumor-promoting signals from fibroblasts by the inhibition of vitamin D receptor, angiotensin, CXCL12/CXCR4, and TGF- β [98]. Moreover, it was observed that CAFs produce IL-6 which causes immunosuppression in the murine-derived Colon26 cancer cells [99]. One possible mechanism for this phenomenon might be conducted by the regulation of CD8+ or FoxP3+ lymphocytes [99]. Therefore, targeting the IL-6 production pathway or the pathway by which it exercises its effects or even targeting IL-6 itself might be beneficial in treating CAF-related cancers including CRC.
Conclusion
The importance of CAFs in prognosis, diagnosis, tumorigenesis, metastasis, and treatment of CRC was discussed in this article. Several studies are suggesting the role of different CAF-related markers in tumorigenesis i.e. VEGF, periostin, and PDGF. These markers might be beneficial as therapeutic targets. They can also serve as tumor markers for early detection of the disease. Moreover, some studies have reported the pathways through which these markers promote tumor progression. These pathways might be potential targets for developing new drugs and treatments. Some other studies have investigated the role of CAF-related markers in tumor metastasis including vimentin, FAP-1, GREM-1, WNT, αSMA, miRs (miR-17, miR-192, and miR-200), β1-integrin, and RAB31. These markers can be used to determine the stage of the disease and its prognosis. Additionally, some of these studies have illustrated the pathways through which these actions are conducted like SOCS3, p-JAK2/p-STAT3 (Tyr705), and HGF/Met. Furthermore, some factors including miRNA-21, FAP-1 (through Anti-PD-1 and MDSCs, alterations in cell lines, DPP4, CD73 and, BH3, etc.), POSTN, SFRP1/2, PKCζ, AEBP1, ZEB1, α-SMA, CD10, FSP1, Collagen formation, desmoplasia, and PRO-C3 were discussed along with this review. These pathways and factors are potential therapeutic targets that need to be more investigated. In addition, some potential therapeutic targets are discussed in this study. Some of them are as follows: wnt, β1-integrin, c-Met, CCL2, SLC7A6, COL3A1, JAM3, AEBP1 and, CAF-derived TGFB3, OGR1, CCR5, IL-6, LGR5, mTOR, β-catenin, miR-142-3p, and NOX4. Some agents discussed are as follows: FNIII14, PTX, ACF, matrine, the combination of vincristine and anti-inflammatory agents, MSI-N1014, and GKT137831. The role of these agents and therapeutics is not clearly elucidated yet. Therefore, there is room for more study in this field.
Future perspectives
The current treatment of CRC contains chemotherapy, immunotherapy, and monoclonal antibodies [100]. However, the emergence of resistance to these agents requires more investigations to find new therapies [100]. Cholesterol metabolism is a potential therapeutic for cancer treatment owing to its role in tumor growth and formation. Besides, NPC intracellular cholesterol transporter 1 (NPC1), sterol O-acyltransferase 1 (SOAT1), and squalene epoxidase (SQLE) are metabolites of the cholesterol metabolism pathway are suggested to be effective for cancer treatment and their effectiveness in treating CRC should be investigated [101]. Moreover, metabolic needs of the cancer cells vary during the different stages of tumor progression, and by understanding more about these differences we can specify the suitable therapeutic option for every stage along with the common therapeutic modalities, and subsequently increase the efficacy of the treatment [102]. Mitochondrial metabolism, plasma-derived circulating tumor DNA (ctDNA), the combination of telomere-targeting agents and immunotherapy, T-cell-receptor engineered cells, gene therapy along with current conventional therapy, and CD73 are other potential treatments or drug targets [102,103,104,105,106,107].
The use of artificial intelligence (AI) in detecting tumors is a novel area. In a study, it was reported that incorporating AI, enhanced the detection of small and non-advanced adenomas; whereas, detection of advanced adenomas did not change significantly [108].
Some suggested potential CAF-related therapeutic targets to treat pancreatic cancer are as follows: depletion of CAF-derived annexin, Sonic hedgehog inhibitor (cyclopamine, CPA), PD1 checkpoint blockade, sonic hedgehog inhibitor (vismodegib), depletion of Big-h3, treatment with Nutilin-3a that induces p53 activation, anti-SPARC with Nab-paclitaxel, miRNA therapies that target ZEB and its downstream pathway, PEGPH20 (digests hyaluronic acid), galunisertib (TGFB-1 inhibition), depletion of ANXA6 extracellular vesicles in CAFs, AMD3100 (a CXCL12 chemokine receptor inhibitor), and DKK3 blocking monoclonal antibody [109]. All these data might be applicable to CRC and more studies in this field are needed. Communication network factor 2 CCN2 is expressed by CAFs and it has an essential role in metastasis and neovascularization. It acts so by interacting with integrin and regulating mechanotransduction (hippo/YAP/TAZ, MRTFA, and integrin/FAK signaling) [110]. FG-3019 which is a humanized monoclonal antibody and is one of the anti-cellular CCN2 strategies is has been shown to improve the pancreatic tumor’s responses to chemotherapy [110]. Therefore, it might be of benefit to investigate the effectiveness of other anti-CCN2 strategies [110].
References
Kuipers EJ, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065.
Cheng L, et al. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am J Clin Oncol. 2011;34(6):573–80.
Raftery L, Goldberg RM. Optimal delivery of cytotoxic chemotherapy for colon cancer. Cancer J. 2010;16(3):214–9.
Vodenkova S, et al. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol Ther. 2020;206:107447.
Nissen NI, et al. Prognostic value of blood-based fibrosis biomarkers in patients with metastatic colorectal cancer receiving chemotherapy and bevacizumab. Sci Rep. 2021;11(1):865.
Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–22.
Quante M, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19(2):257–72.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–98.
Wu X, et al. Repurposing vitamin D for treatment of human malignancies via targeting tumor microenvironment. Acta Pharm Sin B. 2019;9(2):203–19.
Fujita M, et al. Peptide TNIIIA2 derived from Tenascin-C contributes to malignant progression in colitis-associated colorectal cancer via β1-Integrin activation in fibroblasts. Int J Mol Sci. 2019;20(11):2752.
Kasashima H, et al. Stromal SOX2 upregulation promotes tumorigenesis through the generation of a SFRP1/2-expressing cancer-associated fibroblast population. Dev Cell. 2021;56(1):95-110.e10.
Herrera A, et al. Endothelial cell activation on 3D-matrices derived from PDGF-BB-stimulated fibroblasts is mediated by Snail1. Oncogenesis. 2018;7(9):76.
Huang, T.X., et al. Targeting cancer-associated fibroblast-secreted WNT2 restores dendritic cell-mediated antitumour immunity. Gut 2021:322924.
Unterleuthner D, et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis. 2020;23(2):159–77.
Coto-Llerena M, et al. High expression of FAP in colorectal cancer is associated with angiogenesis and immunoregulation processes. Front Oncol. 2020;10:979.
Nishishita R, et al. Expression of cancer-associated fibroblast markers in advanced colorectal cancer. Oncol Lett. 2018;15(5):6195–202.
Solano-Iturri JD, et al. Altered expression of fibroblast activation protein-α (FAP) in colorectal adenoma-carcinoma sequence and in lymph node and liver metastases. Aging (Albany NY). 2020;12(11):10337–58.
Chen L, et al. FAP positive fibroblasts induce immune checkpoint blockade resistance in colorectal cancer via promoting immunosuppression. Biochem Biophys Res Commun. 2017;487(1):8–14.
Wanandi SI, et al. Cancer-associated fibroblast (CAF) secretomes-induced epithelial-mesenchymal transition on HT-29 colorectal carcinoma cells associated with hepatocyte growth factor (HGF) signalling. J Pak Med Assoc. 2021;71(2):S18–24 (Suppl 2).
Herrera M, et al. Cancer-associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Cancer Sci. 2013;104(4):437–44.
Oh HJ, et al. Overexpression of POSTN in tumor stroma is a poor prognostic indicator of colorectal cancer. J Pathol Transl Med. 2017;51(3):306–13.
Mochizuki S, et al. Expression and function of a disintegrin and metalloproteinases in cancer-associated fibroblasts of colorectal cancer. Digestion. 2020;101(1):18–24.
Yang T, et al. Increased RAB31 expression in cancer-associated fibroblasts promotes colon cancer progression through HGF-MET signaling. Front Oncol. 2020;10:1747.
Gu C, Lu H, Qian Z. Matrine reduces the secretion of exosomal circSLC7A6 from cancer-associated fibroblast to inhibit tumorigenesis of colorectal cancer by regulating CXCR5. Biochem Biophys Res Commun. 2020;527(3):638–45.
Ma H, et al. Periostin promotes colorectal tumorigenesis through integrin-FAK-Src pathway-mediated YAP/TAZ activation. Cell Rep. 2020;30(3):793-806.e6.
Thongchot S, et al. Periostin regulates autophagy through integrin α5β1 or α6β4 and an AKT-dependent pathway in colorectal cancer cell migration. J Cell Mol Med. 2020;24(21):12421–32.
Chen, W., et al. CaMKII mediates TGFβ1-induced fibroblasts activation and its cross talk with colon cancer cells. Dig Dis Sci. 2021.
Tanabe Y, et al. Blockade of the chemokine receptor, CCR5, reduces the growth of orthotopically injected colon cancer cells via limiting cancer-associated fibroblast accumulation. Oncotarget. 2016;7(30):48335–45.
Stadler S, et al. Colon cancer cell-derived 12(S)-HETE induces the retraction of cancer-associated fibroblast via MLC2, RHO/ROCK and Ca(2+) signalling. Cell Mol Life Sci. 2017;74(10):1907–21.
Herrera M, et al. Differential distribution and enrichment of non-coding RNAs in exosomes from normal and cancer-associated fibroblasts in colorectal cancer. Mol Cancer. 2018;17(1):114.
Bhome R, et al. Exosomal microRNAs derived from colorectal cancer-associated fibroblasts: role in driving cancer progression. Aging (Albany NY). 2017;9(12):2666–94.
Fourniols T, et al. Inhibition of colorectal cancer-associated fibroblasts by lipid nanocapsules loaded with acriflavine or paclitaxel. Int J Pharm. 2020;584:119337.
Wawro ME, et al. Nonsteroidal anti-inflammatory drugs prevent vincristine-dependent cancer-associated fibroblasts formation. Int J Mol Sci. 2019;20(8):1941.
Yadav VK, et al. Preclinical evaluation of the novel small-molecule MSI-N1014 for treating drug-resistant colon cancer via the LGR5/β-catenin/miR-142-3p network and reducing cancer-associated fibroblast transformation. Cancers (Basel). 2020;12(6):1590.
Reid SE, Zanivan S. Tumor stiffness extends its grip on the metastatic microenvironment. Mol Cell Oncol. 2017;4(6):e1372866.
David CJ, Massagué J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat Rev Mol Cell Biol. 2018;19:419–35.
Bauer J, et al. Increased stiffness of the tumor microenvironment in colon cancer stimulates cancer associated fibroblast-mediated prometastatic activin A signaling. Sci Rep. 2020;10(1):50.
Zhou D, et al. RBP2 induces stem-like cancer cells by promoting EMT and is a prognostic marker for renal cell carcinoma. Exp Mol Med. 2016;48(6):e238.
Wildi S, et al. Overexpression of activin A in stage IV colorectal cancer. Gut. 2001;49(3):409–17.
Kawano S, et al. Assessment of elasticity of colorectal cancer tissue, clinical utility, pathological and phenotypical relevance. Cancer Sci. 2015;106(9):1232–9.
Emon B, et al. Biophysics of tumor microenvironment and cancer metastasis—a mini review. Comput Struct Biotechnol J. 2018;16:279–87.
Wipff PJ, et al. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179(6):1311–23.
Olsen AL, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301(1):G110–8.
Perepelyuk M, et al. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am J Physiol Gastrointest Liver Physiol. 2013;304(6):G605–14.
Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17(3):320–9.
Shibahara K, et al. Production of gastrointestinal tumors in mice by modulating latent TGF-β1 activation. Cancer Res. 2013;73(1):459–68.
Zhou W, et al. Oxidative stress induced autophagy in cancer associated fibroblast enhances proliferation and metabolism of colorectal cancer cells. Cell Cycle. 2017;16(1):73–81.
Peng S, et al. Enhancing cancer-associated fibroblast fatty acid catabolism within a metabolically challenging tumor microenvironment drives colon cancer peritoneal metastasis. Mol Oncol. 2021;15:1391.
Abdul-Wahid A, et al. Serum-derived carcinoembryonic antigen (CEA) activates fibroblasts to induce a local re-modeling of the extracellular matrix that favors the engraftment of CEA-expressing tumor cells. Int J Cancer. 2018;143(8):1963–77.
Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol. 2014;4:62.
Ferrer-Mayorga G, et al. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2017;66(8):1449–62.
Demuytere J, et al. The role of the peritoneal microenvironment in the pathogenesis of colorectal peritoneal carcinomatosis. Exp Mol Pathol. 2020;115:104442.
González-González L, Alonso J. Periostin: a matricellular protein with multiple functions in cancer development and progression. Front Oncol. 2018;8:225.
Aizawa T, et al. Cancer-associated fibroblasts secrete Wnt2 to promote cancer progression in colorectal cancer. Cancer Med. 2019;8(14):6370–82.
Hardwick JC, et al. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology. 2004;126(1):111–21.
Beck SE, et al. BMP-induced growth suppression in colon cancer cells is mediated by p21WAF1 stabilization and modulated by RAS/ERK. Cell Signal. 2007;19(7):1465–72.
Beck SE, et al. Bone morphogenetic protein signaling and growth suppression in colon cancer. Am J Physiol Gastrointest Liver Physiol. 2006;291(1):G135–45.
Flier SN, et al. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010;285(26):20202–12.
Wu WK, et al. Bone morphogenetic protein signalling is required for the anti-mitogenic effect of the proteasome inhibitor MG-132 on colon cancer cells. Br J Pharmacol. 2008;154(3):632–8.
Zeisberg M, Shah AA, Kalluri R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem. 2005;280(9):8094–100.
Kosinski C, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci USA. 2007;104(39):15418–23.
Namkoong H, et al. The bone morphogenetic protein antagonist gremlin 1 is overexpressed in human cancers and interacts with YWHAH protein. BMC Cancer. 2006;6:74.
Karagiannis GS, et al. Enrichment map profiling of the cancer invasion front suggests regulation of colorectal cancer progression by the bone morphogenetic protein antagonist, gremlin-1. Mol Oncol. 2013;7(4):826–39.
Lugli A, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30(9):1299–311.
Mitrovic B, et al. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol. 2012;25(10):1315–25.
Sugai T, et al. Microenvironmental markers are correlated with lymph node metastasis in invasive submucosal colorectal cancer. Histopathology. 2021;79:584.
Lee HO, et al. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer. 2011;11:245.
Kelly T, et al. Fibroblast activation protein-α: a key modulator of the microenvironment in multiple pathologies. Int Rev Cell Mol Biol. 2012;297:83–116.
Liu R, et al. Fibroblast activation protein: a potential therapeutic target in cancer. Cancer Biol Ther. 2012;13(3):123–9.
Kramer N, et al. Autocrine WNT2 signaling in fibroblasts promotes colorectal cancer progression. Oncogene. 2017;36(39):5460–72.
Li J, et al. Carcinoma-associated fibroblasts lead the invasion of salivary gland adenoid cystic carcinoma cells by creating an invasive track. PLoS One. 2016;11(3):e0150247.
Gaggioli C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9(12):1392–400.
Ast V, et al. MiR-192, miR-200c and miR-17 are fibroblast-mediated inhibitors of colorectal cancer invasion. Oncotarget. 2018;9(85):35559–80.
Chen SX, et al. Identification of colonic fibroblast secretomes reveals secretory factors regulating colon cancer cell proliferation. J Proteomics. 2014;110:155–71.
Owusu BY, et al. Hepatocyte growth factor, a key tumor-promoting factor in the tumor microenvironment. Cancers (Basel). 2017;9(4):35.
Satoh K, et al. Tumor budding in colorectal carcinoma assessed by cytokeratin immunostaining and budding areas: possible involvement of c-Met. Cancer Sci. 2014;105(11):1487–95.
Shangguan C, et al. Cancer-associated fibroblasts enhance tumor (18)F-FDG uptake and contribute to the intratumor heterogeneity of PET-CT. Theranostics. 2018;8(5):1376–88.
Chang AL, et al. CCL2 Produced by the glioma microenvironment is essential for the recruitment of regulatory T Cells and myeloid-derived suppressor cells. Cancer Res. 2016;76(19):5671–82.
Yang X, et al. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res. 2016;76(14):4124–35.
Hortobagyi, G.N., Edge S.B., Giuliano A. New and important changes in the TNM staging system for breast cancer. Am Soc Clin Oncol Educ Book. 2018;38:457–67.
Yan W, Shao R. Transduction of a mesenchyme-specific gene periostin into 293T cells induces cell invasive activity through epithelial-mesenchymal transformation. J Biol Chem. 2006;281(28):19700–8.
Xu X, et al. Periostin expression in intra-tumoral stromal cells is prognostic and predictive for colorectal carcinoma via creating a cancer-supportive niche. Oncotarget. 2016;7(1):798–813.
Zheng X, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–30.
Sugai T, et al. Analysis of the expression of cancer-associated fibroblast- and EMT-related proteins in submucosal invasive colorectal cancer. J Cancer. 2018;9(15):2702–12.
Choi SY, et al. Podoplanin, α-smooth muscle actin or S100A4 expressing cancer-associated fibroblasts are associated with different prognosis in colorectal cancers. J Korean Med Sci. 2013;28(9):1293–301.
Ikuta D, et al. Fibrosis in metastatic lymph nodes is clinically correlated to poor prognosis in colorectal cancer. Oncotarget. 2018;9(51):29574–86.
Horman SR, et al. Functional profiling of microtumors to identify cancer associated fibroblast-derived drug targets. Oncotarget. 2017;8(59):99913–30.
Zanotti S, et al. Opposing roles of miR-21 and miR-29 in the progression of fibrosis in Duchenne muscular dystrophy. Biochim Biophys Acta. 2015;1852(7):1451–64.
Langer HF, et al. A novel function of junctional adhesion molecule-C in mediating melanoma cell metastasis. Cancer Res. 2011;71(12):4096–105.
Layne MD, et al. Impaired abdominal wall development and deficient wound healing in mice lacking aortic carboxypeptidase-like protein. Mol Cell Biol. 2001;21(15):5256–61.
Tumelty KE, et al. Aortic carboxypeptidase-like protein (ACLP) enhances lung myofibroblast differentiation through transforming growth factor β receptor-dependent and -independent pathways. J Biol Chem. 2014;289(5):2526–36.
Gonçalves-Ribeiro S, et al. Carcinoma-associated fibroblasts affect sensitivity to oxaliplatin and 5FU in colorectal cancer cells. Oncotarget. 2016;7(37):59766–80.
Hanley CJ, et al. Targeting the myofibroblastic cancer-associated fibroblast phenotype through inhibition of NOX4. J Natl Cancer Inst. 2018;110(1):109–20.
PERMAD. Personalized marker-driven early switch to aflibercept in patients with metastatic colorectal cancer (PERMAD). In: ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02331927. Accessed 6 Jan 2015.
Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res. 2014;2(3):187–93.
Guinney J, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–6.
Zadka Ł, et al. Interplay of stromal tumor-infiltrating lymphocytes, normal colonic mucosa, cancer-associated fibroblasts, clinicopathological data and the immunoregulatory molecules of patients diagnosed with colorectal cancer. Cancer Immunol Immunother. 2021;70(9):2681–700.
Barrett RL, Puré E. Cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy. Elife. 2020;9:e57243.
Kato T, et al. Cancer-associated fibroblasts affect intratumoral CD8(+) and FoxP3(+) T cells via IL6 in the tumor microenvironment. Clin Cancer Res. 2018;24(19):4820–33.
Patsalias A, Kozovska Z. Personalized medicine: stem cells in colorectal cancer treatment. Biomed Pharmacother. 2021;141:111821.
Xu H, et al. Cholesterol metabolism: new functions and therapeutic approaches in cancer. Biochim Biophys Acta Rev Cancer. 2020;1874(1):188394.
Vasan K, Werner M, Chandel NS. Mitochondrial metabolism as a target for cancer therapy. Cell Metab. 2020;32(3):341–52.
Boonstra PA, et al. Clinical utility of circulating tumor DNA as a response and follow-up marker in cancer therapy. Cancer Metastasis Rev. 2020;39(3):999–1013.
Mender I, et al. Telomere stress potentiates STING-dependent anti-tumor immunity. Cancer Cell. 2020;38(3):400-411.e6.
Gaissmaier L, Elshiaty M, Christopoulos P. Breaking bottlenecks for the TCR therapy of cancer. Cells. 2020;9(9):2095.
Montaño-Samaniego M, et al. Strategies for targeting gene therapy in cancer cells with tumor-specific promoters. Front Oncol. 2020;10:605380.
Roh M, et al. Targeting CD73 to augment cancer immunotherapy. Curr Opin Pharmacol. 2020;53:66–76.
Barua I, et al. Artificial intelligence for polyp detection during colonoscopy: a systematic review and meta-analysis. Endoscopy. 2021;53(3):277–84.
Norton J, et al. Pancreatic cancer associated fibroblasts (CAF): under-explored target for pancreatic cancer treatment. Cancers (Basel). 2020;12(5):1347.
Leask A. A centralized communication network: recent insights into the role of the cancer associated fibroblast in the development of drug resistance in tumors. Semin Cell Dev Biol. 2020;101:111–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
Not applicable.
Informed consent
Informed consent was obtained from the participant included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamali Zonouzi, S., Pezeshki, P.S., Razi, S. et al. Cancer-associated fibroblasts in colorectal cancer. Clin Transl Oncol 24, 757–769 (2022). https://doi.org/10.1007/s12094-021-02734-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02734-2