Abstract
Purpose
The objective of this trial was to evaluate the safety and efficacy of melatonin oral gel mouthwashes in the prevention and treatment of oral mucositis (OM) in patients treated with concurrent radiation and systemic treatment for head and neck cancer.
Methods
Randomized, phase II, double-blind, placebo-controlled trial (1:1 ratio) of 3% melatonin oral gel mouthwashes vs. placebo, during IMRT (total dose ≥ 66 Gy) plus concurrent Q3W cisplatin or cetuximab. Primary endpoint: grade 3–4 OM or Severe Oral Mucositis (SOM) incidence by RTOG, NCI, and a composite RTOG-NCI scales. Secondary endpoints: SOM duration and grade 2–4 OM or Ulcerative Oral Mucositis (UOM) incidence and duration.
Results
Eighty-four patients were included in the study. Concurrent systemic treatments were cisplatin (n = 54; 64%) or cetuximab (n = 30; 36%). Compared with the placebo arm, RTOG-defined SOM incidence was numerically lower in the 3% melatonin oral gel arm (53 vs. 64%, P = 0.36). In patients treated with cisplatin, assessed by the RTOG-NCI composite scale, both SOM incidence (44 vs. 78%; P = 0.02) and median SOM duration (0 vs. 22 days; P = 0.022) were significantly reduced in the melatonin arm. Median UOM duration assessed by the RTOG-NCI scale was also significantly shorter in the melatonin arm (49 vs. 73 days; P = 0.014). Rate of adverse events and overall response rate were similar between the two arms.
Conclusions
Treatment with melatonin oral gel showed a consistent trend to lower incidence and shorter SOM duration and shorter duration of UOM. These results warrant further investigation in phase III clinical trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck cancer (HNC) is the sixth most common cancer, with more than 800,000 new cases occurred worldwide in 2016 [1]. Locally advanced HNC commonly requires a multidisciplinary approach with radiotherapy and concurrent systemic treatment or surgery followed by radiotherapy with or without systemic therapies. Concurrent systemic treatment includes platinum-based chemotherapy [2] or the anti-EGFR monoclonal antibody cetuximab [3] for platinum unsuitable patients. About 90% of the patients treated with radiation therapy for upper aero-digestive tract tumors suffer oral mucositis (OM) [4,5,6]. When radiation is administered with concurrent systemic treatment, between 65 and 90% of the patients experience grade 3–4 OM, known as severe oral mucositis (SOM) [6, 7].
OM is the main adverse event of concurrent treatment with both cisplatin [4] and cetuximab [8]. OM is a relevant adverse event because it is troublesome for the patients, impacts their general health and nutritional status [6], quality of life, and can become a gateway to opportunistic infections [9]. Of concern, treatment breaks or delays may be forced by all these complications compromising the continuity of the antitumor treatment [6], ultimately leading to impaired outcomes [10]. In addition, OM leads to an increase in the overall costs of the treatment of the patients with HNC due to increased rates of hospital admission, opioid use, and nutritional support [9, 11,12,13].
The most frequently used scores for assessing OM are the RTOG (morphology and pain), NCI-CTCAE (function and pain), and WHO (functional and morphological) scales. Anterior (mouth, tongue) and posterior (larynx, hypopharynx) tumor sites are best captured by RTOG and NCI scales, respectively [7]. Incidence of OM is really underestimated [7].
OM related to concurrent treatment for HNC has no effective treatment options beyond symptomatic management [7]. Furthermore, no drugs have been approved by any regulatory body to prevent or treat OM as yet.
OM is triggered by oxidative damage directly caused by radiation and/or systemic antineoplastic treatments. Oxidative stress signal upregulation leads to the release of reactive oxygen species (ROS), subsequent DNA damage and increased cell death. Increased ROS levels activate inflammatory cytokines by generating NAPDH oxidase, mitochondrial generated ROS, activation of NFKB and inflammasome components (NLRP3). This stimulates the transcription of proinflammatory cytokines such as IL1b, TNF-a and IL-6. The inflammation caused subsequent ulceration and pain of the oral mucosa. After healing, the tissues may appear healthy, but the cell physiology remains significantly altered [14].
Melatonin is an ubiquitous methoxyindole produced in the pineal gland and many other organs of vertebrates. Despite being commonly known as the hormone of darkness due to its sleep promotion effects, cytoprotection may have been the first phylogenetic function of melatonin [15]. Indeed, melatonin synthesis and release do not exhibit a daily cycle in these other organs [16]. In the extra-pineal organs, melatonin behaves as a potent radical-free scavenger that prevents mitochondrial damage [17] and plays a role as an antiinflammatory and cytoprotective agent which helps to resist oxidative stress [18, 19].
Oral administration of 3% melatonin oral gel prevented OM in rats subject to irradiation, restoring and maintaining pre-irradiation levels of NFkB and NLRP3 signaling inflammatory pathways involved in mucositis, and restoring physiological melatonin levels in irradiated tissues [20]. Based on this preclinical background, the rationale of the present study postulates that this effect can also be seen in humans. High-load 3% melatonin mucoadhesive oral gel would prevent the mucosal damage caused by chemoradiation or bioradiation. Therefore, this strategy could be used to avoid inflammation, mucosal disruption, and ulcer formation during and after irradiation. To test this hypothesis, we designed a proof of concept randomized phase II, placebo-controlled, clinical trial aiming to evaluate the safety and efficacy of 3% melatonin oral gel in the prevention and treatment of oral mucositis in patients with HNC undergoing chemo/bioradiation.
Methods
Study design
This Phase IIa, multicentric, prospective, randomized, double-blind, and placebo-controlled exploratory clinical trial was performed in 11 centers in Spain. The study protocol was registered at ClinicalTrials.gov. All centers and oncologists gained centralized quality assurance accreditation for the trial on radiation dosimetry and specific training in OM grading assessment. The investigator team included one radiation oncologist and one medical oncologist in every center. Assessment of radiation total volume and mucosa volume were standardized to ensure homogeneity between centers. Key inclusion criteria were as follows: age over 18; ECOG PS [0–1]; pathology-proven diagnosis of HN squamous cell carcinoma or nasopharyngeal undifferentiated carcinoma; stage III or IV-M0 (TNM-2010 7th edition) tumor of the oral cavity, oropharynx, or any HN site with lymph nodes at cervical level II to increase surface oral mucosa involved in the radiation field; planned total dose ≥ 66 Gy. Patients must be deemed eligible for treatment with chemo- or bio-radiation with either cisplatin or cetuximab and a radiation plan with IMRT.
Treatment
Antitumor treatment
Patients were treated with Volumetric Modulated Arc Therapy-Simultaneous Integrated Boost (VMAT-SIB) once daily, 5 days per week for 7 weeks, either 2.12 Gy/day, total dose 69.96 Gy in radical treatment or 2 Gy/day, total dose 66 Gy in postoperative treatment. The design of the volumes and dose levels was previously agreed upon by three radiation oncologists. The planned total dose was ≥ 66 Gy. Concurrent systemic treatments were either cisplatin 100 mg/m2 Q3W starting on the first day of radiation therapy (day 1) or cetuximab 400 mg/m2 loading dose (day -7), then 250 mg/m2/week from day 1 for the entire duration of radiation treatment. Up to three cycles of platin-based neoadjuvant chemotherapy were allowed.
Investigational medicinal products
Figure 1 shows the overall study design, treatment, observation, and follow-up plan. Eligible patients were randomly assigned (1:1 ratio) to receive 3% melatonin oral gel or placebo oral gel. Investigational medicinal products (IMPs) were administered as five mouthwashes a day (10 ml per mouthwash, 1,500 mg/day in the melatonin arm). Each mouthwash must be 2 min long and to be followed by swallowing the product.
Study design. All patients were to be treated with the IMP from two or three days before the start of systemic treatment (cisplatin or cetuximab) until one to four weeks after completion of radiotherapy, when oral mucositis improved to RTOG grade 1. If oral mucositis improved to RTOG grade 1 between one to four weeks after the end of radiation therapy, patients permanently discontinued IMP. Patients with RTOG grade ≥ 2 OM at four weeks after the end of radiation discontinued IMP treatment. Thus, the duration of the treatment with IMP was between 8 and 12 week. All patients received standard symptomatic treatment for OM along the study according to the routine clinical practice of the hospital
Endpoints, populations, and procedures
OM was the adverse event of interest. The severity of the mucositis was graded according to both RTOG and NCI scales. A composite of both scales, from now on RTOG-NCI composite scale, was also used. The composite scale captured patients who had OM as assessed either by RTOG or by NCI. In other words, a patient experienced a grade 3–4 adverse event by the composite scale if he or she experienced a grade 3–4 RTOG- or a grade 3–4 NCI-adverse event or both. An investigator meeting was held before study initiation to achieve an agreement in criteria harmonization between investigators. OM was assessed two times per week by a trained investigator at every visit until improvement to lower than grade 2. Intention-to-treat (ITT) population comprised all randomized patients. Both modified ITT (mITT) and safety populations comprise all randomized patients who received at least one medication dose. Percentage of patients with RTOG grade 3–4 OM, named RTOG severe oral mucositis (SOM), in the mITT population, was the primary endpoint of the trial. Extension of SOM to grade 2, leads to the definition of grade 2–3-4 OM or ulcerative oral mucositis (UOM). Secondary endpoints were NCI-defined SOM incidence, RTOG-NCI composite scale-defined SOM incidence, SOM duration, UOM incidence, UOM duration, and safety. Adverse events were assessed by NCI-CTCAE v4.0. Efficacy analyses were performed in the mITT population. Additional endpoints were the need for opioids and the need for special nutritional support and procedures (feeding tubes). The protocol included a subgroup analysis according to the systemic antitumor treatment (cisplatin or cetuximab).
Statistics
Based on previously published data [21, 22], we assumed that 70% of patients treated with placebo would experience RTOG-defined SOM. Estimation of efficacy allowed for predicting that this percentage would be decreased to 35% in the melatonin arm. Sample size calculation showed that a total of 76 evaluable patients (38 patients per treatment arm) would provide at least 80% power to detect as significant (at 0.05 two-sided significance level) this 35-percentage points difference (from 70 to 35%). Foreseeing a 10% drop-out rate, a total of 84 patients (42 per arm) was planned to be included.
A comparison of incidence rates of SOM or UOM (2 dichotomous variables leading squared tables) was performed with the Chi-square Fisher-exact test. A comparison of duration of SOM or UOM (independent quantitative variables, less than 30 observations) was performed with the Mann–Whitney U test. Other qualitative variables were compared with the Chi-square test (or Chi-square-Fisher exact test if squared tables). Missing data were handled with the last observation carried forward method.
Ethics
The study was approved by the Institutional Review Board of all the participant centers and was conducted in accordance with the Declaration of Helsinki as adopted by the World Medical Association in the Fortaleza-2013 meeting. All patients provided written informed consent before starting any of the procedures of the study.
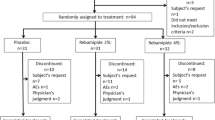
Study flow-chart. mITT modified intention to treat, PP per protocol. *Per protocol (PP) population comprise the patients of the mITT population who received at least 70% of the IMP and no were subject to major violations of the protocol. **Five patients were excluded before the administration of the first dose of the IMP: two from the melatonin arm (no fulfillment of selection criteria) and three from the placebo arm (two deaths and one withdrawal of consent)
Results
Patient disposition
Ninety patients were screened for eligibility and signed informed consent. Six of them were screening failures (all due to non-fulfillment with selection criteria). Therefore, 84 patients (ITT) were randomized to the investigational 3% melatonin oral gel arm (n = 42; 50%) or to the placebo arm in 11 Spanish centers between October 2015 and August 2017 (Fig.2). Five patients were excluded before the administration of the first dose of the IMP. Therefore, 79 patients (mITT) were randomly assigned to the melatonin arm (n = 40; 51%) or the placebo arm (n = 39; 49%).
Demographics
The baseline characteristics of the 84 patients included in the ITT population are shown in Table 1. All demographics were well balanced between arms. Fifty patients (63%) were treated with cisplatin, and twenty-nine patients (37%) were treated with cetuximab.
Efficacy
Efficacy results for SOM and UOM incidence and duration were analyzed in the mITT population (n = 79), and are summarized in Table 2.
SOM incidence and duration: mITT population
The percentage of patients who experienced SOM was numerically lower in the melatonin arm compared with the placebo arm when assessed by the RTOG scale (53 vs. 64%; P = 0.36). The ascertainment of this result was the primary objective of the study. The percentage of patients treated with cisplatin who experienced SOM was significantly lower in the melatonin arm compared with the placebo arm, when assessed by the RTOG-NCI composite scale (44 vs. 78%; P = 0.02). The absolute reduction of SOM incidence in the melatonin group was 34%.
When SOM duration was calculated in all patients, regardless of the patient experience of SOM or not, according to the RTOG-NCI scale, the median duration of SOM was 15 days shorter in the melatonin group than in the placebo group; this clinically relevant difference was statistically significant (6 vs. 21 days; P = 0.022). In patients treated with cisplatin, the median duration of SOM according to the RTOG-NCI scale was 22 days shorter in the melatonin group; this clinically relevant difference was also statistically significant (0 vs. 22 days; P = 0.022). In the subgroup of patients treated with cetuximab, the median duration of SOM according to the RTOG-NCI scale, was numerically shorter (15 vs. 21 days; P = 0.44) in the melatonin arm.
SOM duration calculated in those patients who experienced SOM according to the RTOG-NCI scale was numerically lower in the melatonin arm in the entire population (19 vs. 30 days; P = 0.34) and the subgroup of patients treated with cetuximab (17 vs. 30 days; P = 0.22). No statistically significant differences were observed in SOM duration in patients who experienced SOM.
UOM incidence and duration: mITT population
The percentage of patients who experienced UOM, according to the RTOG-NCI scale, was not significantly different in the melatonin arm compared with the placebo arm (88 vs. 92%; P = 0.71).
UOM duration was calculated in those patients who experienced UOM (about 90%). The median duration of UOM was significantly shorter in the melatonin arm compared with the placebo arm according to the RTOG-NCI composite scale (49 vs. 73 days; P = 0.014). In the subgroup of patients treated with cisplatin, the median duration of UOM was significantly shorter in the melatonin arm compared with the placebo arm according to the RTOG-NCI scale (40 vs. 63; P = 0.026). In the cetuximab subgroup, median UOM duration in patients who experienced UOM was numerically lower in the melatonin arm (58 vs. 74 days; P = 0.35), also according to the RTOG-NCI composite scale.
Additional efficacy analysis
In the mITT population, the percentage of patients who required major opioids was numerically lower in the melatonin arm compared with the placebo arm (60 vs. 74%; P = 0.17). In the mITT population, compared with patients in the placebo arm, the percentage of patients who required nutritional support (73% vs. 95%; P = 0.01) and the rate of patients who required either an enteral feeding tube or parenteral feeding (18% vs. 36%; P = 0.06) were both numerically lower in the melatonin arm. Three (7.5%) and one patient (2.6%) required systemic antibiotics in the melatonin and placebo arm, respectively.
Patients with previous surgery, as well as those who received neoadjuvant chemotherapy (many of whom may have a complete or almost complete response before concurrent treatment), could not be evaluated for tumor response. Overall response rate was not different between the 24 evaluable patients in the melatonin group and the 31 evaluable patients in the placebo group (75% vs. 81%; P = 0.62).
Compliance: mITT population
Tolerability was the most frequent reason for ending the study treatment (total 26 patients, 17/40 melatonin vs. 9/39 placebo), lack of compliance (total 8 patients, 5 vs. 3), and patient decision (total 7 patients, 3 vs. 4). Median treatment duration was shorter in the melatonin arm compared with the placebo arm (28 vs. 57 days). The duration of the IMP treatment (melatonin or placebo) was shorter in the group of patients treated with concurrent cisplatin compared with patients with concurrent cetuximab in both the melatonin arm (21 vs. 65 days; P = 0.0022), and the placebo arm (48 vs. 72 days; P < 0.01).
Tolerability: safety population
Table 3 summarizes safety results. The rate of adverse events (AEs) was calculated in 79 patients (40 from the melatonin arm and 39 from the placebo arm). Overall, seventy-seven patients (98%) experienced AEs, and 28 patients (35%) presented serious AE (SAEs), 14 in the melatonin arm (35%), and 14 in the placebo arm (36%). The most frequent SAEs were gastrointestinal AEs, which were observed in 15 patients (19%), 5 in the melatonin arm (13%), and 10 in the placebo arm (26%).
Most of these AEs were related to systemic oncologic treatment (cisplatin or cetuximab) and with radiotherapy. The rate of patients with grade 3–4 RTOG AEs was lower in the melatonin arm compared with the placebo arm, 12 patients (30%) vs. 19 patients (49%). Nineteen patients experienced AEs deemed to be related to the IMP, 11 in the melatonin group (28%) and 8 in the placebo group (21%). There were 3 deaths in the safety population (3.7%), none of them related to the IMP: two patients in the melatonin arm (one tumor hemorrhage and one hip fracture and post-surgery infection) and one patient in the placebo arm (hematemesis). The rate of AEs of particular interest that could match with the safety profile of melatonin, such as somnolence or increased liver function tests was not significantly higher in the melatonin arm. The percentage of patients that required hospital admission was 33% in the melatonin arm and 38% in the placebo arm.
Discussion
The results of this exploratory study indicate that 3% melatonin oral gel can prevent and shorten SOM and UOM in patients with HNC undergoing concurrent chemoradiation. A strong trend in favor of the melatonin arm is observed for multiple endpoints of incidence and duration of SOM and UOM, with some endpoints reaching statistical significance, mostly in the subgroup of patients treated with concurrent cisplatin-radiation treatment. However, the exploratory design makes statistical significance less relevant than it is in other trial designs. Radiation-cetuximab treatment leads to extensive and highly symptomatic OM even at a lower dose of radiation therapy [23]. In these patients, the antiinflammatory attributes of melatonin shorten the duration of UOM despite having no impact on the incidence of UOM.
High-dose melatonin oral gel was well tolerated. The dose we investigated to treat OM was high compared with the low doses used to treat sleep disorders (Circadin™, melatonin tablets 2 mg). This was necessary to enable melatonin to reach the mitochondria, where the molecule works as a scavenger of reactive oxygen species leading to an antioxidant effect [24].
Moreover, the tumor response rate was not different between arms, indicating no impact of 3% melatonin oral gel on concurrent chemoradiation treatment efficacy. This observation is in line with the expected lack of interaction of melatonin with cytotoxic treatments. Evaluation of any possible beneficial antitumor effect of melatonin [25] was outside the scope of this study.
While most published studies on OM have used the WHO scale [7], which is based on the appearance of oral cavity ulcers and diet tolerance, this scale fails to take into account relevant symptoms associated with OM such as pain. We think that using a composite scale RTOG-NCI with dual morphologic-functional assessment resulted in an improved ability to capture all SOM episodes. The antitumor regimen of the study is consistent with widely used regimens with cisplatin at 100 mg/m2 Q3W, and high median radiation dose, with 82% of the patients (those who had no previous radical resective surgery) treated with 69.9 Gy. This similarity to real life is one of the strengths of the study.
Several other medicinal products have been investigated with the same objectives of OM prevention and treatment, and have data vs. placebo from randomized phase II trials. GC4419, a superoxide dismutase mimetic, has been shown to reduce the incidence of SOM from 65% (placebo) to 43% with GC4419 (P = 0.009) [26]. GC4419 is administered daily, the intravenous route over 60 min, before each radiation fraction. A few oral agents have also been compared with placebo and recently reviewed in a comprehensive article [27]. Results with rebamipide, an agent orally administered as a liquid that stimulates prostaglandin synthesis in gastric mucosa, were published in 2017 and showed a non-significant reduction of the incidence of SOM (25% vs. 39%; P = 0.17) [28]. In turn, results with clonidine, an agonist of the alpha-2-adrenergic receptors, administered as a mucoadhesive buccal tablet, were published in 2020 showing a non-significant reduction of the incidence of SOM (45 vs. 60%; P = 0.06) [29]. Finally, a small study with oral melatonin capsules enrolled only 39 patients out of 80 planned, and showed a reduction of SOM in these patients and was also published in 2020 [30]. The favorable safety profile of 3% melatonin oral gel, the convenience of the oral form of administration, which is more comfortable for patients, as well as the mechanical cleaning effect on oral mucosa derived from 5 mouthwashes a day, are considered benefits of this approach compared with parenteral or other oral treatments.
Our study has limitations such as multiple testing and suboptimal compliance, particularly in the cisplatin arm, despite the higher efficacy observed in this subgroup of patients. Compliance is a known challenge in studies dealing with supportive measures. In contrast, our study has strengths too. The homogeneity of the results in several scales and endpoints, mostly in the subgroup of patients treated with cisplatin, supports the validity of the trial. Moreover, the reproducible results in the RTOG-NCI composite scale further support the robustness of the data.
In conclusion, treatment with 3% melatonin oral gel demonstrated a consistent trend to lower the incidence and shorten SOM duration, and lower the duration of UOM. In the subgroup of patients treated with cisplatin, the reduction of duration and incidence of SOM reached statistical significance. These results deserve further investigation in a large phase III clinical trial.
References
Fitzmaurice C, Akinyemiju TF, Lami FH, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016; a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–68.
Pignon JP, Le Maître A, Maillard E, Bourhis J, MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14.
Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78.
Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–62.
Bourhis J, Lapeyre M, Tortochaux J, et al. Accelerated radiotherapy and concomitant high dose chemotherapy in non resectable stage IV locally advanced HNSCC: results of a GORTEC randomized trial. Radiother Oncol. 2011;100:56–61.
Elting LS, Keefe DM, Sonis ST, et al. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy. Cancer. 2008;113:2704–13.
Peterson DE, Bensadoun RJ, Roila F. Management of oral and gastrointestinal mucositis: ESMO clinical practice guidelines. Ann Oncol. 2011;22(Suppl 6):vi78–84.
Yokota T, Onoe T, Ogawa H, et al. Distinctive mucositis and feeding-tube dependency in cetuximab plus radiotherapy for head and neck cancer. Jpn J Clin Oncol. 2015;45:183–8.
Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68:1110–20.
Russo G, Haddad R, Posner M, Machtay M. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist. 2008;13:886–98.
Murphy BA. Clinical and economic consequences of mucositis induced by chemotherapy and/or radiation therapy. J Support Oncol. 2007;5(9 Suppl 4):13–21.
Nonzee NJ, Dandade NA, Markossian T, et al. Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis: results from a Northwestern University Costs of Cancer Program pilot study with head and neck and nonsmall cell lung cancer patients who received care at a county hospital, a Veterans Administration hospital, or a comprehensive cancer care center. Cancer. 2008;113:1146–52.
Elting LS, Chang YC. Costs of oral complications of cancer therapies: estimates and a blueprint for future study. J Natl Cancer Inst Monogr. 2019;53:Igz010.
Yuan A, Sonis S. Emerging therapies for the prevention and treatment of oral mucositis. Expert Opin Emerg Drugs. 2014;19:343–51.
Cardinali DP, Hardeland R. Inflammaging, metabolic syndrome and melatonin: a call for treatment studies. Neuroendocrinology. 2017;104:382–97.
Acuña-Castroviejo D, Escames G, Venegas C, et al. Extrapineal melatonin sources regulation, and potential functions. Cell Mol Life Sci. 2014;71:2997–3025.
Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253–78.
Sánchez A, Calpena AC, Clares B. Evaluating the oxidative stress in inflammation: role of melatonin. Int J Mol Sci. 2015;16:16981–7004.
Tan DX, Manchester LC, Estebanubero E, Zhou Z, Reiter RJ. Melatonin as a potent and inducible endogenous antioxidant synthesis and metabolism. Molecules. 2015;20:18886–906.
Ortiz F, Acuña-Castroviejo D, Doerrier C, et al. Melatonin blunts the mitochondrial/NLRP3 connection and protects against radiation-induced oral mucositis. J Pineal Res. 2015;58:34–49.
Le QT, Kim HE, Schneider CJ, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo-controlled study. J Clin Oncol. 2011;29:2808–14.
Henke M, Alfonsi M, Foa P, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2011;29:2815–20.
Walsh L, Gillham C, Dunne M, et al. Toxicity of cetuximab versus cisplatin concurrent with radiotherapy in locally advanced head and neck squamous cell cancer (LAHNSCC). Radiother Oncol. 2011;98:38–41.
Reiter RJ, Tan DX, Rosales-Corral SA, Galano A, Zhou XJ, Xu B. Mitochondira: central organelles for melatonin`s antioxidant and anti-aging actions. Molecules. 2018;23:509.
Reiter RJ, Rosales-Corral SA, Tan DX, et al. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci. 2017;18:843.
Anderson CM, Lee CM, Saunders DP, et al. Phase IIb, randomized, double-blind trial of GC4419 versus placebo to reduce severe oral mucositis due to concurrent radiotherapy and cisplatin for head and neck cancer. J Clin Oncol. 2019;37:3256–65.
Sonis ST, Villa A. Phase II investigational oral drugs for the treatment of radio/chemotherapy induced oral mucositis. Expert Opin Investig Drugs. 2018;27:147–54.
Yokota T, Ogawa T, Takahashi S, et al. Efficacy and safety of rebamipide liquid for chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: a multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II study. BMC Cancer. 2017;17:314.
Giralt J, Tao Y, Kortmann RD, et al. Mucoadhesive buccal tablet for the amelioration of oral mucositis in patients treated with concomitant chemoradiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2020;106:320–8.
Elsabagh HH, Moussa E, Mahmoud SA, et al. Efficacy of melatonin in prevention of radiation-induced oral mucositis: a randomized clinical trial. Oral Dis. 2020;26:566–72.
Funding
Spherium Biomed, S.L.U. (Ferrer). Spain.
Author information
Authors and Affiliations
Contributions
AL, RM and GE conceived the study. AL, RM, CT, PG, RB and VV contributed to study design. AL, RM, JM, JR, NF, JGM, RM, IP, ML, MGVM, LC and JG recruited patients, treated patients and collected the data. AL, RM, CT, PG, RB and VV analyzed and interpret the data. AL, RM and VV wrote the first draft of the manuscript. All authors read and contributed to the final version.
Corresponding author
Ethics declarations
Conflict of interest
Pedro Grima Ph.D., Ramón Bosser Ph.D., Cristina Tarragó Ph.D. were workers at Spherium Biomed, S.L.U. (Ferrer) when this trial was designed, the patients were enrolled, and the data was analyzed. The other authors declared no conflict of interest.
Ethical approval
The study was approved by the Institutional Review Board of all the participant centers and was conducted in accordance with the Declaration of Helsinki as adopted by the World Medical Association in the Fortaleza-2013 meeting.
Informed consent
All patients provided written informed consent before starting any of the procedures of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lozano, A., Marruecos, J., Rubió, J. et al. Randomized placebo-controlled phase II trial of high-dose melatonin mucoadhesive oral gel for the prevention and treatment of oral mucositis in patients with head and neck cancer undergoing radiation therapy concurrent with systemic treatment. Clin Transl Oncol 23, 1801–1810 (2021). https://doi.org/10.1007/s12094-021-02586-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02586-w