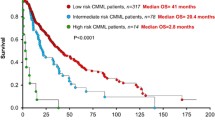
Abstract
Chronic myelomonocytic leukemia (CMML) is a hematologic malignancy that overlaps with myeloproliferative neoplasms (MPN) and myelodysplastic syndromes (MDS) and tends to transform into acute myeloid leukemia (AML). Among cases of CMML, > 90% have gene mutations, primarily involving TET2 (~ 60%), ASXL1 (~ 40%), SRSF2 (~ 50%), and the RAS pathways (~ 30%). These gene mutations are associated with both the clinical phenotypes and the prognosis of CMML, special CMML variants and pre-phases of CMML. Cytogenetic abnormalities and the size of genome are also associated with prognosis. Meanwhile, cases with ASXL1, DNMT3A, NRAS, SETBP1, CBL and RUNX1 mutations may have inferior prognoses, but only ASXL1 mutations were confirmed to be independent predictors of the patient outcome and were included in three prognostic models. Novel treatment targets related to the various gene mutations are emerging. Therefore, this review provides new insights to explore the correlations among gene mutations, clinical phenotypes, prognosis, and novel drugs in CMML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic myelomonocytic leukemia (CMML), which is caused by an abnormality of hematopoietic stem cells (HSCs), is characterized by an overlap syndrome with both myelodysplastic syndromes (MDS) and myeloproliferative neoplasms (MPN) [1], with one-third of patients transforming to acute myeloid leukemia (AML). An average of 14 ± 5 gene mutations in the coding regions are detected in CMML patients [2], and approximately 90% of patients have at least one somatic mutation. The mutations are classified into the following categories: (i) epigenetic regulator mutations (1) histone modification: ASXL1 (~ 40%), EZH2 (5–7%), UTX (~ 15%); (2) DNA methylation: TET2 (~ 60%), IDH1 and IDH2 (~ 1%, ~ 5%), DNMT3A (~ 5%); (ii) spliceosome machinery mutations: SRSF2 (~ 50%), SF3B1 (5–10%), ZRSR2 (~ 6%), U2AF1 (~ 10%), and PRPF8 (~ 1%); (iii) signaling mutations: KRAS (~ 15%), NRAS (~ 10%), JAK2 (~ 10%), CBL (~ 15%), PTPN11 (5%), FLT3 (~ 3%), and NPM1 (~ 5%); (iv) transcription factors: RUNX1 (~ 15%), CEBPA (~ 8%), and SETBP1 (~ 15%); (v) DNA damage response genes: TP53 (1%), PHF6(~ 5%) [3,4,5,6,7,8,9] (Fig. 1). In this study, we briefly summarized the mutations and their prognostic relevance in CMML.
Clinical significance of gene mutations in CMML
Epigenetic regulators mutations
Histone modification
Epigenetic regulator mutations include genes involved in histone modification (ASXL1, EZH2, UTX) and DNA methylation (TET2, IDH1/2, DNMT3A). ASXL1 contains EED (embryonic ectoderm development), EZH2 (histone 3 lysine 27 (H3K27) methyltransferase), and SUZ12 (recombinant suppressor of Zeste 12 homolog) and regulates chromatin by interacting with polycomb group repressive complex proteins (PRC) 2 and PRC1. PRC2 recruits chromatin and results in the trimethylation of the H3K27 mark (H3K27me3), which is a repressive mark of silencing gene transcription. PRC1 catalyzes ubiquitylation of histone H2A at lysine 119 and then contributes to cytosine-phosphate-guanine (CpG) transcriptional repression, which silences gene mutations and induces myeloid transformation [10]. ASXL1-mutated patients are more likely to have anemia, leukocytosis, extramedullary disease, and high cytogenetic risk according to the Spanish cytogenetic risk stratification system [11].
As a catalytic submit of PRC2, EZH2 mutations affect H3K27me3 and inhibit gene expression, and they are associated with “proliferative”-CMML (MP-CMML) phenotype. Although EZH2 mutations have no impact on OS or leukemia-free survival (LFS), codominant EZH2 and ASXL1 mutations lead to a shorter overall survival (OS) than patients with only ASXL1 mutations. ASXL1/EZH2 comutations may synergistically contribute to the activity of PRC2 and deplete the methylation of H3K27, which disturbs normal transcription [13].
UTX (KDM6A) is a H3K27 demethylase, affecting the MLL3/4 H3K4 methyltransferase complex and an X-linked protein that inhibits CMML development by regulating self-renewal and differentiation of HSCs. Gata and Zfpm1 are two important transcription factors that are regulated by UTX to promote erythroid development and inhibit myeloid differentiation by remarkably decreasing H3K27me3 and increasing H3K4me3 in the promoter region [14]. UTX mutations may lead to anemia, myelodysplasia and chromosomal aberrations [15], and comutations of UTX and TP53 accelerate CMML development.
DNA methylation
TET2 mutations occur in hematological malignancies such as MDS (5–20%), MPN (~ 15%), CMML (~ 60%), AML (8–30%) and lymph hyperplastic disorders of T and B cells [16, 17]. TET2 belongs to the dioxygenase superfamily, and it can gradually convert 5-methylcytosine (5-mC) into 5-hydroxymethylcytosine (5-hmC), 5-formylcytosine (5-fC) and 5-carboxycytosine (5-caC) with the participation of α-ketoglutaric acid (α-KG) [4, 16, 18]. And a lower level of 5-hmC showed better OS (20 vs. 4 months, P = 0.03) in high-risk patients [9]. TET2 mutations play a key role in the self-renewal of HSCs [19], which cause differentiation bias between slight granulomonocytic expansion and the inhibition of erythroid differentiation. This process may be associated with “dysplastic” CMML (MD-CMML) [20]. Patients with TET2 mutations are more likely to have normal karyotypes, advanced age [9], low cytogenetic risk [21] and a higher response rate to hypomethylating agents (HMAs) [18, 21, 22]. The mechanism of TET2 mutations in HMAs has not been clarified, and the reasons for this knowledge gap are as follows: TET2 mutations can transform the proliferative advantage and/or are inherently sensitive to HMAs; TET2 may have an effect on enzymes related to the activation and metabolism of HMAs; and heterogeneity of TET2 mutations (especially the number, type, gene location and clonal phenotype) may affect the sensitivity of HMAs [4]. The presence of TET2 mutations indicates a poor prognosis for patients with MDS [18] but a better outcome in CMML [4, 23,24,25].
Isocitrate dehydrogenase (IDH) 1 and IDH2 are cofactors of TET2 that can produce α-KG and contribute to the oxidization of 5-mC to cytosine by TET2 enzymes [16], whereas α-KG-dependent dioxygenases are involved in a variety of cellular processes, such as hypoxia, angiogenesis, extracellular matrix collagen maturation, and epigenetic regulation. When IDH1/2 mutations occur, D-2-hydroxyglutarate (D-2HG) can be generated and it has a weak competitive inhibitory effect on α-KG-dependent dioxygenases. This oncometabolite impairs some histone lysine demethylases, such as TET2 and the Jumonji-C domain containing family, which cause histone and DNA hypermethylation and inhibit cell differentiation, all of which lead to leukemogenesis. Furthermore, D-2HG is a biomarker for the detection of IDH1/2 mutations at diagnosis and the prediction of the clinical response [26].
DNA methyltransferase (DNMT), including DNMT1, DNMT3A (2p23) and DNMT3B, play an important role in DNA methylation [27], and DNMT3A mutations can be seen in AML (~ 22%), MDS (~ 10%), MPN (15%), CMML (~ 5%), adult systemic mastocytosis (ASM,1%) and T-cell lymphoproliferative disorders [16]. The mutational hot spot of DNMT3A is Arg882 and it mainly influences the catalytic methyltransferase domain [28], which is associated with cancer, especially hematological malignancies. Patients with DNMT3A mutations have a higher frequency of white blood cells, immature blood myeloid cells, absolute monocyte count, blasts and abnormal karyotypes [12]. DNMT3A mutations have no impact on OS or LFS in Groupe Français des Myelodysplasies (GFM), but some studies found that DNMT3A-mutations can shorten OS and LFS [3, 8]. Moreover, DNMT3A and RAS mutations can cooperate to regulate the self-renewal of hematopoietic stem/progenitor cells (HSPCs) and promote myeloid malignancies [27].
Splicing mutations
Splicing mutations are common in solid tumors and hematologic malignancies, including CMML, where they are involved with SRSF2 (~ 50%), SF3B1 (5–10%), U2AF1 (~ 10%), ZRSR2 (~ 6%), and PRPF8 (1%) [3,4,5]. SRSF2(17q25) mutations are associated with advanced age, mild anemia, normal karyotypes and exclusively EZH2 mutations [29]. Although SRSF2 mutations can shorten OS in MDS and primary myelofibrosis, they have no relationship with OS and LFS in CMML [29, 30]. However, it has also been reported that SRSF2 mutations may be associated with shorter OS but have no effect on LFS in younger patients (≤ 65 years) [31].
The main mutational hot spot of SF3B1 is K700E (90%) [31], SF3B1 mutations have a high frequency of bone marrow ring sideroblasts (RS) in MDS and CMML, and the mutations can be regarded as the only genetic predictor of high hemoglobin levels [12], while they have no impact on OS or LFS [30]. PRPF8 mutations are linked with increased myeloblasts and the RS phenotype when excluding SF3B1 mutations, and the RS phenotype suggests a similar pathogenetic mechanism [32].
U2AF1(U2AF35, U2 small nuclear RNA auxiliary factor 1) mutations can be seen in ~ 10% of CMML, MDS and secondary AML [4, 33], and the mutations have no prognostic significance in CMML [30, 34]. ZRSR2 mutations are infrequent and do not have an independent prognostic impact on OS.
Signaling pathway mutations
Signaling pathway mutations are associated with a proliferative phenotype such as MP-CMML, which mostly affects granulocyte–macrophage–colony-stimulating factor (GM-CSF) signaling (JAK2, NRAS, KRAS, CBL, FLT3, PTPN11) [35]. JAK2V617F mutations are driver mutations of proliferative phenotypes (such as high hemoglobin, high hematocrit, leukocytosis and splenomegaly) with less thrombocytopenia, normal karyotypes, and frequently co-occurrence with TET2 [36]. In addition, JAK2V617F mutations are associated with an increased thrombotic risk in patients with MPN but not in CMML [37]. In summary, JAK2V617F mutations have no impact on OS, LFS or thrombosis-free survival in CMML [36]; however, they are common in CMML with myelofibrosis (CMML-F) and these patients have a worse OS [38].
RAS pathway mutations are frequent in CMML (~ 30%) [3] and are associated with cell signaling and proliferation with increasing blasts of bone marrow [9] and they stimulate self-renewal of HSCs. These mutations are more likely to present as MP-CMML and contribute to CMML/juvenile myelomonocytic leukemia (JMML) development [27].
Casitas B-cell lymphoma gene (CBL) mutations occur in ~ 15% of CMML [3] and are target receptors of several tyrosine kinases (RTKs), such as FLT3 and PDGFR. CBL could activate the RAS pathway and pSTAT5 via activation of RTKs [9], which all have potential to accelerate the formation of leukemia. Moreover, CBL family E3 ubiquitin ligases down-regulate JAK2 stability and signaling via the adaptor protein LNK/SH2B3, while CBL mutations can prolong the JAK2 half-life and extend JAK signaling and elevated JAK protein levels and signaling [39]. Furthermore, CBL mutations can lead to uniparental disomy of 11q in myeloid neoplasms, which may increase the rates of hereditary disease. CBL mutations are associated with advanced age, a high frequency of splenomegaly [9], monosomy 7, comutations with RUNX1 or TET2 [25, 40], and rarely with comutations with JAK-STAT [40], and they are associated with poor prognosis [9, 21, 41, 42].
FLT3, type III of RTKs, observed in 8 (62%) FLT3 ITD and 5 (38%) FLT3 TKD mutations in a cohort of 466 patients with CMML. FLT3 mutations can lead to the proliferation of leukemia cells. However, there were no differences in clinical phenotypes and OS in CMML between FLT3-mutated and FLT3-nonmutated patients, which may be due to a limited number of FLT3-mutated patients in CMML [43, 44].
PTPN11, SRC homology domain 2, is a ubiquitous protein tyrosine phosphatase that regulates many signaling pathways. Mutations of PTPN11 can activate MAPK pathways, including many target proteins, such as RAS and CBL [44]. But PTPN11 mutations are seen in 5% of CMML and do not predict prognosis [3].
NPM1 (nucleophosmin 1) protein is responsible for adjusting and controlling genomic stability, ribosome biogenesis, inhibiting pathways and the dependent stress response of p53/MDM2 (mouse double minute 2 homolog). Compared to the mutational frequency of AML (20–30%) [45], NPM1 mutations are rare in CMML (~ 5%) and have a high frequency in MD-CMML and a high risk of blast transformation [6, 46].
Transcription and nucleosome assembly
RUNX1 mutations occur in 10–15% of CMML patients and are associated with a high frequency of thrombocytopenia [3, 35] and an unfavorable prognosis [21, 41]. Conversely, Meggendorfer, M et al. reported RUNX1 mutations have no effect on OS in CMML [29]. SETBP1 mutations are rare in CMML and neither affect OS nor predict leukemia transformation of CMML [11, 31]. However, some studies, including the CMML-specific prognostic model (CPSS-MOL), found that RUNX1 and SETBP1 are associated with a poor OS [7, 12].
CEBPA mutations are more common in AML with a better OS, and the mutational frequency is ~ 8% in CMML, which may be secondary mutations [3], the clinical and prognostic significance are not clear, although many studies have tested for CEBPA mutations in CMML.
DNA damage
TP53 mutations are suppressor genes with an ~ 1% frequency in CMML [3], and they are associated with complex cytogenetics [47] and a higher response to decitabine [48]. TP53 and PTPN11 mutations are involved in an unfavorable OS after HMAs treatment but are not associated with the drug response [18]. PHF6 is a chromatin adaptor, and its mutational frequency is 5% in CMML [3] with unclarified significance for the prognosis.
Mutations in prephase CMML and special CMML variants
CMML can be divided into primary CMML and secondary CMML; the latter includes therapy-related CMML (t-CMML) [49], which accounts for 9–11% of all cohorts of CMML [50,51,52]. Most t-CMML patients have history of hematologic malignancies, prostate or colon cancer and receive radiotherapy or chemotherapy [51], which are linked with high rates of cytogenetic risk, high LDH levels, a lower platelet count, and a worse OS and LFS [51, 52]. However, the frequency of gene mutations was similar to de novo CMML [50, 52].
In addition, there are prephase CMML and special CMML variants (except t-CMML). It is worth noting that prephase CMML has an inherent risk of evolving into CMML, e.g., clonal hematopoiesis of indeterminate potential (CHIP), idiopathic cytopenias of unknown significance (ICUS) and oligomonocytic CMML (O-CMML) [49]. The clinical phenotype and gene mutations of O-CMML are similar to those of classical CMML, and some O-CMML patients evolve into CMML (38%, median: 12 months) and AML (26%, median: 10 months) [53]. Patients with pre-CMML conditions need to be dynamically monitored to prevent disease progression with prompt treatment. Special CMML variants means that patients fulfilled the CMML criteria but had some specific characteristics of other neoplasms or did not fulfill all criteria of CMML, e.g., mastocytosis with CMML(SM-CMML) [49], CMML-F, and O-CMML.
Gene mutations may be an indication for diagnoses. For example, CHIP-like mutations or related lymphoid/myeloid neoplasms can be detected in some prephase CMML. CMML-F is rare in cohort of CMML (3.1%, 20/651), and it is significantly associated with JAK2V617F and less dysregulation of p53, which has a worse OS than CMML without fibrosis [38]. Patients with SM-CMML commonly presented with mast cell mediator symptoms, splenomegaly, hepatomegaly, lymphadenopathy and are associated with KITD816V mutations (p < 0.0001). The median OS is not significantly different between classical CMML and SM-CMML; fortunately, no patients were observed to develop AML from SM-CMML [54]. In addition, missense mutations of ethanolamine kinase 1 (ETNK1) were found to be frequent in SM-CMML and they may cause a potential pathogenetic mechanism [55]. However, most mutations, such as ASXL1, SRSF2, and TET2, are not disease-specific and are common in myeloid neoplasms.
In addition, CMML patients and healthy individuals can be differentiated from both the variant allele frequency (VAF) or clone size of the mutations (median 39.2% vs 9–10%)[56]. In prephase CMML, the rates of VAF were 2–12% in CHIP, 30–40% in clonal cytopenia of unknown significance (CCUS), 30–50% in MDS, and none in ICUS [57]. The VAF rates were 29–59% in CMML patients with DNMT3A mutations [8]. Monitoring VAF can help us diagnose CMML and realize the allele burden in classical CMML, prephase CMML and special CMML variants (e.g., JAK2V617F in CMML-F, KIT D816V in SM-CMML). Of note, studies found that the VAF of most patients (70.8%) was not significantly different at diagnosis and follow-up [58].
Cytogenetic abnormalities and copy number variants in CMML
Cytogenetic abnormalities occur in ~ 30% of CMML, including 64–72% single, 16% two, and 11–20% complex abnormalities [59, 60]. The most frequent cytogenetic abnormalities are trisomy 8, -Y, abnormalities of chromosome 7, 20q-, trisomy 21 and del (3q) [60]. There are mainly two cytogenetic risk stratifications in CMML, including the Spanish [61] and the Mayo-French cytogenetic risk stratification system [60].
Fifty-five percent of patients with del 3(q) patients had co-occurrence SF3B1 mutations, and these abnormalities were related to the RS phenotype and had a better OS than other abnormal karyotypes. ASXL1 mutations are associated with abnormal karyotypes and a low incidence of co-occurrence with −Y (P = 0.04) and del (3q) (P = 0.03), U2AF1 mutations with monosomy karyotypes (P = 0.03), comutations of SRSF2 (P = 0.02) and TET2 with normal karyotypes [60, 62].
Cases with normal karyotypes have a better OS and a low incidence of AML transformation than those with abnormal karyotypes [59]. However, over 67% of patients with low risk karyotypes or no metaphases had copy number variants/alterations (CNVs/CNAs) and copy number neutral loss of heterozygosity (CNN-LOH), which is associated with a shorter OS and progression-free survival if the size of genome (including CNVs/CNAs and CNN-LOH) is larger than 11 Mb or ≥ 1 CNAs. In addition, the presence of interstitial CNN-LOH is associated with a poor OS. These results indicate that some low-risk karyotypes may be stratified into intermediate-risk groups by single nucleotide polymorphism (SNP) arrays [63]. The result of genome-wide CNVs analysis by next generation sequencing was consistent with the SNP analysis [64].
Novel therapies
Current treatment includes supporting treatment, HMAs, HSCT and novel therapies. In this study, we only focused on novel drugs because other treatments have been extensively studied during past decades (Table 1).
Epigenetics inhibitors
Epigenetic modifiers can be found in various hematological tumors and have been shown to induce cell developmental arrest, apoptosis and macrophage death, as well as tumor cell differentiation [16]. These effects are seen in both solid tumors and hematological tumors [69].
Histone modifying inhibitors
The first-generation hypomethylation agents include azacitidine, decitabine and histone deacetylase inhibitors (HDACi), which have been tested in clinical trials [16, 70]. In MPN patients, HDACi inhibited proliferation and induced JAK2 cell death, which normalized spleen and blood cell counts in mice. However, patients with MDS, AML and CMML did not have improved clinical outcomes either with HDACi alone or in combination with azacitidine/decitabine [16]. Recently, a phase 1b/2b trial for oral panobinostat plus azacitidine showed that it could improve complete remission (CR) rates compared with monotherapy with azacitidine (27.5% vs. 14.3%) but it did not improve 1-year OS or disease progression [70]. Furthermore, effective biomarkers to assess the efficiency of HMAs or HDACi are lacking, and more studies are needed to verify this treatment approach in the future.
DNA methylation inhibitors
Guadecitabine (GDAC), a novel HMA, may be effective in CMML patients, extending decitabine exposure and prolonging OS in a small case study of patients with high-risk MDS and low blast count AML (blast percent, 20–30%) who failed treatment with azacitidine [71].
IDH inhibitors [26, 72] can selectively inhibit mutant IDH proteins and induce cell differentiation; the objective response rate was ~ 40%, and a lasting (> 1 year) durable response was observed in phase I trials for advanced hematological malignancies [72].
Signaling pathway inhibitors
In MP-CMML, conventional treatment including hydroxyurea or cytarabine can improve leukocytosis and splenomegaly, but they may aggravate cytopenia and not improve clinical symptoms. HMAs can improve proliferative symptoms as well as myeloid infiltration but which are more suitable for MD-CMML and may be less toxic, including neutropenia, in MD-CMML. The European Hematology Association/European LeukemiaNet (ELN/EHA) recommends considering HMAs treatment when MD-CMML patients with excess 10% blasts are ineligible for HSCT. Therefore, novel targets for MP-CMML-like signaling pathway inhibitors are necessary.
Ruxolitinib, a JAK1/2 inhibitor, was approved for treating myelofibrosis in 2011 and for treating polycythemia vera in 2014 by the Food and Drug Administration (FDA), but its efficacy in MDS/MPN, including CMML, still needs to be evaluated in clinical trials. A phase I trial of ruxolitinib showed that it could reduce splenomegaly, decrease the hyperactivation of GM-CSF in CMML-1 (peripheral blood: 2–4% blasts including promonocytes; bone marrow: 5–9% blasts), especially those with proliferative phenotypes, and achieve hematological improvement regardless of previous therapies. Furthermore, the incidence of anemia and thrombocytopenia were 51 and 54%, respectively, and they were safe, with mild hematological toxicity and fewer grade 3/4 adverse events [73, 74]. The combination of ruxolitinib and azacytidine was particularly effective in improving proliferative features, including reductions in splenomegaly (64%), and they showed improvements in the total symptom score by the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF, 78%) [73]. Pacritinib (a JAK/FLT3 inhibitor) and momelotinib (a JAK2 inhibitor) had less hematological toxicity in myelofibrosis, but they have not been studied in CMML [75]. In addition, patients with CMML-F benefit from JAK2 inhibitors and other drugs targeting the JAK-STAT pathway [38].
Antineoplastics show an insufficient effect in patients with some gene mutations, such as B-Raf and K-Ras mutations. K-Ras mutations are seen in pancreatic, colon, lung cancers, AML and CMML (< 1%). Tipifarnib, a farnesyl transferase inhibitor, was approved for treating AML by the FDA and tested in clinical trials for CMML [3]. Meanwhile, some bisubstrate inhibitors and peptidomimetics, such as FTI 276, FTI 277, B1086, L731, B956, L735, L739, L750, BMS-214662, L778123 and L778123, are being tested in clinical trials [76]. GDC-0941 (pictilisib), a PI3K inhibitor and Ras-regulated effector, was identified to not only improve leukocytosis, anemia, and splenomegaly but also prolong OS. The Akt inhibitor MK-2206, achieved favorable hematological responses in K-Ras and Nf1 mice with MPN but had no effect on Raf/MEK/ERK signaling [77].
GM-CSF hypersensitivity was detected in approximately 90% of patients, while myeloid and monocytic progenitors were sensitive to GM-CSF inhibitors. KB003 (lenzilumab), a novel GM-CSF inhibitor, is in development [78].
Patients with SM-CMML respond to midostaurin, a KIT inhibitor, which can decrease monocyte counts and improve clinical symptoms [79].
Transcription modulators
Trabectedin is a DNA minor groove binder that regulates transcription, disturbs DNA repair mechanisms and selectively depletes the myelomonocytic lineage. Furthermore, trabectedin decreases the expression of RAS super family and it plays an important role in cell growth and cytoskeletal dynamics [80].
Other drugs
Thrombocytopenia affects approximately 40% of CMML patients. Eltrombopag is a trombopoietin receptor agonist that activates the JAK/STAT5 pathway and leads to macrophage proliferation and differentiation; it was approved for immune thrombocytopenia and tested against MDS and AML. Some successful cases have been reported for CMML patients with thrombocytopenia [81, 82]. Recently, eltrombopag was used in a small number of CMML patients after HMAs failure, but the results have generally been unfavorable. Compared with MDS patients who failed HMAs treatment, the hematological response rate was lower in CMML (23 vs. 14%). Side effects were more frequent than in MDS patients, who mainly presented with leukocytosis, myelofibrosis progression, the presence of blasts in peripheral blood and higher rates of AML transformation [83].
SL-401, a selective IL-3 receptor and CD123 inhibitor, was approved by the FDA for treating blastic plasmacytoid dendritic cell neoplasms (BPDCN) and was tested in hematological malignancies such as CMML [84].
Leinalidomide is a novel immunoregulatory drug approved for multiple myeloma and MDS, especially for patients with 5q-, and there have also been related studies in CMML. Studies were conducted with azacitidine alone or in combination with lenalidomide or vorinostat, and researchers found that azacitidine plus lenalidomide achieved a higher overall response rates (ORR) than monotherapy with azacitidine or lenalidomide. In addition, the ORR duration was associated with a low number of gene mutations, and side effects were not significantly increased compared with azacitidine monotherapy [85].
Discussion
In hematological malignancies, many gene mutations, such as DNMT3A, TET2, ASXL1, SRSF2, TP53, JAK2, CBL and SF3B1, are associated with advanced age, which is also known as CHIP/age-related clonal hematopoiesis (ARCH) [29, 40, 65]. These mutations can exist in healthy individuals [66], but continuous accumulation can cause clonal expansion during CMML formation, so ARCH/CHIP are regard as precancerous lesions with an increased risk of hematological cancer and all-cause mortality [65]. TET2, ASXL1, and SRSF2 mutations may be detected in early stage for most CMML patients and can be regarded as driver mutations, while KRAS, NRAS, RUNX1, U2AF1 and CBL mutations are secondary subclonal hierarchy that may cause disease progression [5]. Interestingly, TET2, ASXL1, and SRSF2 mutations commonly co-occur in association with other gene mutations, which may suggest that two distinct hits at HSCs cause convergence of the downstream pathway, but this hypothesis needs further verification by single colony sequencing [5].
Gene mutations are beneficial to diagnose CMML; for example, myeloid neoplasms with myelodysplasia and monocytosis, such as CMML, should be seriously considered when co-mutations of TET2 and SRSF2 occur [89]. In addition, aberrant expression of CD56 may predict the presence of TET2 mutations (P < 0.0001), and the combination of aberrant CD56 expression and > 94% M1 monocytes can produce a strong prediction for final diagnosis of CMML [56]. Single-cell sequencing identified the mutational clonality of CMML in monocytes, lymphocytes and neutrophils, and it plays an important role in diagnosis of CMML [58]. Moreover, mutational analysis has high concordance between peripheral blood and bone marrow, and the results from peripheral blood can predict the results from bone marrow, while the negative predictive value of peripheral blood screening was 100%. Therefore, bone marrow assessment is not recommended for patients without mutations in peripheral blood, but more samples are needed for verification [56]. In addition, gene mutations may be associated with clinical phenotypes; for example, ASXL1, JAK2, NRAS, SETBP1, SRSF2, and EZH2 are involved in MP-CMML, while TET2, U2AF1, SF3B1, and NPM1 are involved in MD-CMML [5, 12, 13, 27, 36, 46], while CBL, NRAS, KRAS and ASXL1 are more likely to present as extramedullary disease [9, 27, 41].
Gene mutations are linked to the prognosis. Some studies found that ASXL1 [12, 23, 31, 35, 42], RUNX1 [12, 35], SETBP1 [5, 12, 35], NRAS [12], CBL [42], and DNMT3A [5, 23] mutations are correlated with a poor OS, but only ASXL1 mutations were identified to independently predict an inferior OS and have been incorporated into prognostic models [12, 68, 90]. The number of gene mutations is associated with a shorter OS [9, 56], and advanced disease is associated with higher mutational burdens in CMML and MDS [5]. However, some studies found that the accumulation of gene mutations did not affect OS [21, 67] or AML-free survival, even after receiving HSCT [21].
Patients with CMML were stratified into five groups who received different treatments, including active monitoring, supporting care, HMAs, HSCT, and novel drugs, according to prognosis groups. Supporting treatment is only suitable for patients who are stable, low risk or without major clinical symptoms such as cytopenias or myeloproliferation disease. HMAs were recommended for treating severe CMML, especially MD-CMML. However, the ORR and CR rates were 41 and ~ 20%, respectively [24]. The median OS was 17 months, and no responder had a poor OS [91]. Moreover, patients who failed HMAs therapy had a median OS of ~ 7 months, and half of patients had transformed to AML [92]. Thus far, HSCT is the only curative option for eradicating clone burden of hematopoietic neoplasms, especially fails to HMAs; however, very few individuals can receive HSCT treatment due to advanced age, comorbidities, donor status, economic status, etc. It has been reported that OS, rates of relapse-free and acute graft-versus-host disease after HSCT are 33, 27, and 33% at 4 years, respectively [93]. In addition, mutations involving ASXL1, JAK2, and RUNX1, especially TP53, indicated a poor outcome after allo-HSCT [94]. Thus, studies to identify novel drugs are essential.
Although current studies have provided a new view of gene mutations and their relevance, they do not appear to encourage therapeutic potential over HMAs, and we suspect that epigenetic and splicing mutations are more frequent in CMML, so epigenetic drugs are still dominant in CMML. In addition, some CMML patients with proliferative symptoms such as splenomegaly, extramedullary infiltration and high white blood cells are considered to have poor prognoses, in which the splenomegaly affects the therapeutic effect of HMAs, and these symptoms can be effectively improved by signaling pathway inhibitors, so these inhibitors have a good application prospect in MP-CMML. Therefore, we hold the opinion that CMML patients may benefit from HMA combined with novel drugs or novel drug monotherapy when they are unsuitable for HSCT or fail treatment with HMAs. The development of next generation sequencing, genome-wide CNV analysis, SNPs, and VAF analysis would help us identify these patients, and novel drugs may revolutionize the treatment options, allow for the development of personalized accuracy treatments, predict drug susceptibility or the risk of progression of disease in individuals and, as far as possible, decrease treatment toxicity.
References
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405. https://doi.org/10.1182/blood-2016-03-643544.
Merlevede J, Droin N, Qin T, Meldi K, Yoshida K, Morabito M, et al. Mutation allele burden remains unchanged in chronic myelomonocytic leukaemia responding to hypomethylating agents. Nat Commun. 2016;7:10767. https://doi.org/10.1038/ncomms10767.
Patnaik MM, Tefferi A. Chronic Myelomonocytic leukemia: 2020 update on diagnosis, risk stratification and management. Am J Hematol. 2020;95(1):97–115. https://doi.org/10.1002/ajh.25684.
Coltro G, Mangaonkar AA, Lasho TL, Finke CM, Pophali P, Carr R, et al. Clinical, molecular, and prognostic correlates of number, type, and functional localization of TET2 mutations in chronic myelomonocytic leukemia (CMML)-a study of 1084 patients. Leukemia. 2020;34(5):1407-21. https://doi.org/10.1038/s41375-019-0690-7.
Patel BJ, Przychodzen B, Thota S, Radivoyevitch T, Visconte V, Kuzmanovic T, et al. Genomic determinants of chronic myelomonocytic leukemia. Leukemia. 2017;31(12):2815–23. https://doi.org/10.1038/leu.2017.164.
Peng J, Zuo Z, Fu B, Oki Y, Tang G, Goswami M, et al. Chronic myelomonocytic leukemia with nucleophosmin (NPM1) mutation. Eur J Haematol. 2016;96(1):65–71. https://doi.org/10.1111/ejh.12549.
Itzykson R, Fenaux P, Bowen D, Cross NCP, Cortes J, De Witte T, et al. Diagnosis and treatment of chronic myelomonocytic leukemias in adults: recommendations from the European hematology association and the European leukemianet. Hemasphere. 2018;2(6):e150. https://doi.org/10.1097/HS9.0000000000000150.
Patnaik MM, Barraco D, Lasho TL, Finke CM, Hanson CA, Ketterling RP, et al. DNMT3A mutations are associated with inferior overall and leukemia-free survival in chronic myelomonocytic leukemia. Am J Hematol. 2017;92(1):56–61. https://doi.org/10.1002/ajh.24581.
Jankowska AM, Makishima H, Tiu RV, Szpurka H, Huang Y, Traina F, et al. Mutational spectrum analysis of chronic myelomonocytic leukemia includes genes associated with epigenetic regulation: UTX, EZH2, and DNMT3A. Blood. 2011;118(14):3932–41. https://doi.org/10.1182/blood-2010-10-311019.
Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128(4):735–45. https://doi.org/10.1016/j.cell.2007.02.009.
Patnaik MM, Itzykson R, Lasho TL, Kosmider O, Finke CM, Hanson CA, et al. ASXL1 and SETBP1 mutations and their prognostic contribution in chronic myelomonocytic leukemia: a two-center study of 466 patients. Leukemia. 2014;28(11):2206–12. https://doi.org/10.1038/leu.2014.125.
Elena C, Galli A, Such E, Meggendorfer M, Germing U, Rizzo E, et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood. 2016;128(10):1408–17. https://doi.org/10.1182/blood-2016-05-714030.
Patnaik MM, Vallapureddy R, Lasho TL, Hoversten KP, Finke CM, Ketterling R, et al. EZH2 mutations in chronic myelomonocytic leukemia cluster with ASXL1 mutations and their co-occurrence is prognostically detrimental. Blood Cancer J. 2018;8(1):12. https://doi.org/10.1038/s41408-017-0045-4.
Zheng L, Xu L, Xu Q, Yu L, Zhao D, Chen P, et al. Utx loss causes myeloid transformation. Leukemia. 2018;32(6):1458–65. https://doi.org/10.1038/s41375-018-0011-6.
Thieme S, Gyarfas T, Richter C, Ozhan G, Fu J, Alexopoulou D, et al. The histone demethylase UTX regulates stem cell migration and hematopoiesis. Blood. 2013;121(13):2462–73. https://doi.org/10.1182/blood-2012-08-452003.
Ungerstedt JS. Epigenetic modifiers in myeloid malignancies: the role of histone deacetylase inhibitors. Int J Mol Sci. 2018;19(10). https://doi.org/10.3390/ijms19103091.
Lasho TL, Vallapureddy R, Finke CM, Mangaonkar A, Gangat N, Ketterling R, et al. Infrequent occurrence of TET1, TET3, and ASXL2 mutations in myelodysplastic/myeloproliferative neoplasms. Blood Cancer J. 2018;8(3):32. https://doi.org/10.1038/s41408-018-0057-8.
Bejar R, Lord A, Stevenson K, Bar-Natan M, Perez-Ladaga A, Zaneveld J, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014;124(17):2705–12. https://doi.org/10.1182/blood-2014-06-582809.
Itzykson R, Kosmider O, Renneville A, Morabito M, Preudhomme C, Berthon C, et al. Clonal architecture of chronic myelomonocytic leukemias. Blood. 2013;121(12):2186–98. https://doi.org/10.1182/blood-2012-06-440347.
Itzykson R, Solary E. An evolutionary perspective on chronic myelomonocytic leukemia. Leukemia. 2013;27(7):1441–50. https://doi.org/10.1038/leu.2013.100.
Duchmann M, Yalniz FF, Sanna A, Sallman D, Coombs CC, Renneville A, et al. Prognostic role of gene mutations in chronic myelomonocytic leukemia patients treated with hypomethylating agents. EBioMedicine. 2018;31:174–81. https://doi.org/10.1016/j.ebiom.2018.04.018.
Zeidan AM, Hu X, Long JB, Wang R, Ma X, Podoltsev NA, et al. Hypomethylating agent therapy use and survival in older patients with chronic myelomonocytic leukemia in the United States: a large population-based study. Cancer. 2017;123(19):3754–62. https://doi.org/10.1002/cncr.30814.
Patnaik MM, Lasho TL, Vijayvargiya P, Finke CM, Hanson CA, Ketterling RP, et al. Prognostic interaction between ASXL1 and TET2 mutations in chronic myelomonocytic leukemia. Blood Cancer J. 2016;6(1):e385-e. https://doi.org/10.1038/bcj.2015.113.
Coston T, Pophali P, Vallapureddy R, Lasho TL, Finke CM, Ketterling RP, et al. Suboptimal response rates to hypomethylating agent therapy in chronic myelomonocytic leukemia; a single institutional study of 121 patients. Am J Hematol. 2019;94(7):767–79. https://doi.org/10.1002/ajh.25488.
Kohlmann A, Grossmann V, Klein HU, Schindela S, Weiss T, Kazak B, et al. Next-generation sequencing technology reveals a characteristic pattern of molecular mutations in 72.8% of chronic myelomonocytic leukemia by detecting frequent alterations in TET2, CBL, RAS, and RUNX1. J Clin Oncol. 2010;28(24):3858–65. https://doi.org/10.1200/JCO.2009.27.1361.
Mondesir J, Willekens C, Touat M, de Botton S. IDH1 and IDH2 mutations as novel therapeutic targets: current perspectives. J Blood Med. 2016;7:171–80. https://doi.org/10.2147/JBM.S70716.
Chang YI, You X, Kong G, Ranheim EA, Wang J, Du J, et al. Loss of Dnmt3a and endogenous Kras(G12D/+) cooperate to regulate hematopoietic stem and progenitor cell functions in leukemogenesis. Leukemia. 2015;29(9):1847–56. https://doi.org/10.1038/leu.2015.85.
Muller J, Verrijzer P. Biochemical mechanisms of gene regulation by polycomb group protein complexes. Curr Opin Genet Dev. 2009;19(2):150–8. https://doi.org/10.1016/j.gde.2009.03.001.
Meggendorfer M, Roller A, Haferlach T, Eder C, Dicker F, Grossmann V, et al. SRSF2 mutations in 275 cases with chronic myelomonocytic leukemia (CMML). Blood. 2012;120(15):3080–8. https://doi.org/10.1182/blood-2012-01-404863.
Patnaik MM, Lasho TL, Finke CM, Hanson CA, Hodnefield JM, Knudson RA, et al. Spliceosome mutations involving SRSF2, SF3B1, and U2AF35 in chronic myelomonocytic leukemia: prevalence, clinical correlates, and prognostic relevance. Am J Hematol. 2013;88(3):201–6. https://doi.org/10.1002/ajh.23373.
Patnaik MM, Wassie EA, Padron E, Onida F, Itzykson R, Lasho TL, et al. Chronic myelomonocytic leukemia in younger patients: molecular and cytogenetic predictors of survival and treatment outcome. Blood Cancer J. 2015;5:e270. https://doi.org/10.1038/bcj.2014.90.
Kurtovic-Kozaric A, Przychodzen B, Singh J, Konarska MM, Clemente MJ, Otrock ZK, et al. PRPF8 defects cause missplicing in myeloid malignancies. Leukemia. 2015;29(1):126–36. https://doi.org/10.1038/leu.2014.144.
Kar SA, Jankowska A, Makishima H, Visconte V, Jerez A, Sugimoto Y, et al. Spliceosomal gene mutations are frequent events in the diverse mutational spectrum of chronic myelomonocytic leukemia but largely absent in juvenile myelomonocytic leukemia. Haematologica. 2013;98(1):107–13. https://doi.org/10.3324/haematol.2012.064048.
Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, Yamamoto R, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478(7367):64–9. https://doi.org/10.1038/nature10496.
Itzykson R, Duchmann M, Lucas N, Solary E. CMML: clinical and molecular aspects. Int J Hematol. 2017;105(6):711–9. https://doi.org/10.1007/s12185-017-2243-z.
Patnaik MM, Pophali PA, Lasho TL, Finke CM, Horna P, Ketterling RP, et al. Clinical correlates, prognostic impact and survival outcomes in chronic myelomonocytic leukemia patients with the JAK2V617F mutation. Haematologica. 2019;104(6):e236-e9. https://doi.org/10.3324/haematol.2018.208082.
Tefferi A, Nicolosi M, Mudireddy M, Szuber N, Finke CM, Lasho TL, et al. Driver mutations and prognosis in primary myelofibrosis: Mayo-Careggi MPN alliance study of 1,095 patients. Am J Hematol. 2018;93(3):348–55. https://doi.org/10.1002/ajh.24978.
Gur HD, Loghavi S, Garcia-Manero G, Routbort M, Kanagal-Shamanna R, Quesada A, et al. Chronic myelomonocytic leukemia with fibrosis is a distinct disease subset with myeloproliferative features and frequent JAK2 pV617F mutations. Am J Surg Pathol. 2018;42(6):799–806. https://doi.org/10.1097/pas.0000000000001058.
Lv K, Jiang J, Donaghy R, Riling CR, Cheng Y, Chandra V, et al. CBL family E3 ubiquitin ligases control JAK2 ubiquitination and stability in hematopoietic stem cells and myeloid malignancies. Genes Dev. 2017;31(10):1007–23. https://doi.org/10.1101/gad.297135.117.
Schnittger S, Bacher U, Alpermann T, Reiter A, Ulke M, Dicker F, et al. Use of CBL exon 8 and 9 mutations in diagnosis of myeloproliferative neoplasms and myelodysplastic/myeloproliferative disorders: an analysis of 636 cases. Haematologica. 2012;97(12):1890–4. https://doi.org/10.3324/haematol.2012.065375.
Damm F, Itzykson R, Kosmider O, Droin N, Renneville A, Chesnais V, et al. SETBP1 mutations in 658 patients with myelodysplastic syndromes, chronic myelomonocytic leukemia and secondary acute myeloid leukemias. Leukemia. 2013;27(6):1401–3. https://doi.org/10.1038/leu.2013.35.
Padron E, Garcia-Manero G, Patnaik MM, Itzykson R, Lasho T, Nazha A, et al. An international data set for CMML validates prognostic scoring systems and demonstrates a need for novel prognostication strategies. Blood Cancer J. 2015;5:e333. https://doi.org/10.1038/bcj.2015.53.
Daver N, Strati P, Jabbour E, Kadia T, Luthra R, Wang S, et al. FLT3 mutations in myelodysplastic syndrome and chronic myelomonocytic leukemia. Am J Hematol. 2013;88(1):56–9. https://doi.org/10.1002/ajh.23345.
Jenkins C, Luty SB, Maxson JE, Eide CA, Abel ML, Togiai C, et al. Synthetic lethality of TNK2 inhibition in PTPN11-mutant leukemia. Sci Signal. 2018;11(539):eaao5617. https://doi.org/10.1126/scisignal.aao5617.
Heath EM, Chan SM, Minden MD, Murphy T, Shlush LI, Schimmer AD. Biological and clinical consequences of NPM1 mutations in AML. Leukemia. 2017;31(4):798–807. https://doi.org/10.1038/leu.2017.30.
Vallapureddy R, Lasho TL, Hoversten K, Finke CM, Ketterling R, Hanson C, et al. Nucleophosmin 1 (NPM1) mutations in chronic myelomonocytic leukemia and their prognostic relevance. Am J Hematol. 2017;92(10):E614–8. https://doi.org/10.1002/ajh.24861.
Quesada AE, Routbort MJ, DiNardo CD, Bueso-Ramos CE, Kanagal-Shamanna R, Khoury JD, et al. DDX41 mutations in myeloid neoplasms are associated with male gender, TP53 mutations and high-risk disease. Am J Hematol. 2019;94(7):757–66. https://doi.org/10.1002/ajh.25486.
Welch JS, Petti AA, Miller CA, Fronick CC, O’Laughlin M, Fulton RS, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375(21):2023–36. https://doi.org/10.1056/NEJMoa1605949.
Valent P, Orazi A, Savona MR, Patnaik MM, Onida F, van de Loosdrecht AA, et al. Proposed diagnostic criteria for classical chronic myelomonocytic leukemia (CMML), CMML variants and pre-CMML conditions. Haematologica. 2019;104(10):1935–49. https://doi.org/10.3324/haematol.2019.222059.
Takahashi K, Pemmaraju N, Strati P, Nogueras-Gonzalez G, Ning J, Bueso-Ramos C, et al. Clinical characteristics and outcomes of therapy-related chronic myelomonocytic leukemia. Blood. 2013;122(16):2807–11. https://doi.org/10.1182/blood-2013-03-491399.
Subari S, Patnaik M, Alfakara D, Gangat N, Elliott M, Hogan W, et al. Patients with therapy-related CMML have shorter median overall survival than those with de novo CMML: Mayo clinic long-term follow-up experience. Clin Lymphoma Myeloma Leuk. 2015;15(9):546–9. https://doi.org/10.1016/j.clml.2015.06.002.
Patnaik MM, Vallapureddy R, Yalniz FF, Hanson CA, Ketterling RP, Lasho TL, et al. Therapy related-chronic myelomonocytic leukemia (CMML): molecular, cytogenetic, and clinical distinctions from de novo CMML. Am J Hematol. 2018;93(1):65–73. https://doi.org/10.1002/ajh.24939.
Geyer JT, Tam W, Liu Y-C, Chen Z, Wang SA, Bueso-Ramos C, et al. Oligomonocytic chronic myelomonocytic leukemia (chronic myelomonocytic leukemia without absolute monocytosis) displays a similar clinicopathologic and mutational profile to classical chronic myelomonocytic leukemia. Mod Pathol. 2017;30(9):1213–22. https://doi.org/10.1038/modpathol.2017.45.
Patnaik MM, Rangit V, Lasho TL, Hoversten KP, Finke CM, Ketterling RP, et al. A comparison of clinical and molecular characteristics of patients with systemic mastocytosis with chronic myelomonocytic leukemia to CMML alone. Leukemia. 2018;32(8):1850–6. https://doi.org/10.1038/s41375-018-0121-1.
Lasho TL, Finke CM, Zblewski D, Patnaik M, Ketterling RP, Chen D, et al. Novel recurrent mutations in ethanolamine kinase 1 (ETNK1) gene in systemic mastocytosis with eosinophilia and chronic myelomonocytic leukemia. Blood Cancer J. 2015;5:e275. https://doi.org/10.1038/bcj.2014.94.
Cargo C, Cullen M, Taylor J, Short M, Glover P, Van Hoppe S, et al. The use of targeted sequencing and flow cytometry to identify patients with a clinically significant monocytosis. Blood. 2019;133(12):1325–34. https://doi.org/10.1182/blood-2018-08-867333.
Palomo L, Ibanez M, Abaigar M, Vazquez I, Alvarez S, Cabezon M, et al. Spanish guidelines for the use of targeted deep sequencing in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br J Haematol. 2020;188(5):605–22. https://doi.org/10.1111/bjh.16175.
Hwang SM, Kim SM, Nam Y, Kim J, Kim S, Ahn YO, et al. Targeted sequencing aids in identifying clonality in chronic myelomonocytic leukemia. Leuk Res. 2019;84:106190. https://doi.org/10.1016/j.leukres.2019.106190.
Tang G, Zhang L, Fu B, Hu J, Lu X, Hu S, et al. Cytogenetic risk stratification of 417 patients with chronic myelomonocytic leukemia from a single institution. Am J Hematol. 2014;89(8):813–8. https://doi.org/10.1002/ajh.23751.
Wassie EA, Itzykson R, Lasho TL, Kosmider O, Finke CM, Hanson CA, et al. Molecular and prognostic correlates of cytogenetic abnormalities in chronic myelomonocytic leukemia: a Mayo Clinic-French Consortium Study. Am J Hematol. 2014;89(12):1111–5. https://doi.org/10.1002/ajh.23846.
Such E, Cervera J, Costa D, Sole F, Vallespi T, Luno E, et al. Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica. 2011;96(3):375–83. https://doi.org/10.3324/haematol.2010.030957.
Patnaik MM, Tefferi A. Cytogenetic and molecular abnormalities in chronic myelomonocytic leukemia. Blood Cancer J. 2016;6(2):e393. https://doi.org/10.1038/bcj.2016.5.
Palmo L, Xicoy B, Garcia O, et al. Impact of SNP array karyotyping on the diagnosis and the outcome of chronic myelomonocytic leukemia with low risk cytogenetic features or no metaphases. Am J Hematol. 2016;91(2):185-92. https://doi.org/10.1002/ajh.24227.
Shen W, Paxton CN, Szankasi P, Longhurst M, Schumacher JA, Frizzell KA, et al. Detection of genome-wide copy number variants in myeloid malignancies using next-generation sequencing. J Clin Pathol. 2018;71(4):372–8. https://doi.org/10.1136/jclinpath-2017-204823.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98. https://doi.org/10.1056/NEJMoa1408617.
Ganguly BB, Kadam NN. Mutations of myelodysplastic syndromes (MDS): an update. Mutat Res Rev Mutat Res. 2016;769:47–62. https://doi.org/10.1016/j.mrrev.2016.04.009.
Itzykson R, Kosmider O, Renneville A, Gelsi-Boyer V, Meggendorfer M, Morabito M, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol. 2013;31(19):2428–36. https://doi.org/10.1200/jco.2012.47.3314.
Patnaik MM, Itzykson R, Lasho TL, Kosmider O, Finke CM, Hanson CA, et al. ASXL1 and SETBP1 mutations and their prognostic contribution in chronic myelomonocytic leukemia: an international study of 466 patients. Leukemia. 2015;15:S234. https://doi.org/10.1016/j.clml.2015.04.122.
Feinberg AP, Koldobskiy MA, Gondor A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat Rev Genet. 2016;17(5):284–99. https://doi.org/10.1038/nrg.2016.13.
Garcia-Manero G, Sekeres MA, Egyed M, Breccia M, Graux C, Cavenagh JD, et al. A phase 1b/2b multicenter study of oral panobinostat plus azacitidine in adults with MDS, CMML, or AML with p 30% blasts. Leukemia. 2017;31(12):2799–806. https://doi.org/10.1038/leu.2017.159.
Sebert M, Renneville A, Bally C, Peterlin P, Beyne-Rauzy O, Legros L, et al. A phase II study of guadecitabine in higher-risk myelodysplastic syndrome and low blast count acute myeloid leukemia after azacitidine failure. Haematologica. 2019;104(8):1565–71. https://doi.org/10.3324/haematol.2018.207118.
Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016;21(117):373–80.
Assi R, Kantarjian HM, Garcia-Manero G, Cortes JE, Pemmaraju N, Wang X, et al. A phase II trial of ruxolitinib in combination with azacytidine in myelodysplastic syndrome/myeloproliferative neoplasms. Am J Hematol. 2018;93(2):277–85. https://doi.org/10.1002/ajh.24972.
Padron E, Dezern A, Andrade-Campos M, Vaddi K, Scherle P, Zhang Q, et al. A multi-institution phase I trial of Ruxolitinib in patients with chronic myelomonocytic leukemia (CMML). Clin Cancer Res. 2016;22(15):3746–54. https://doi.org/10.1158/1078-0432.CCR-15-2781.
Yuan T, Qi B, Jiang Z, Dong W, Zhong L, Bai L, et al. Dual FLT3 inhibitors: against the drug resistance of acute myeloid leukemia in recent decade. Eur J Med Chem. 2019;178:468–83. https://doi.org/10.1016/j.ejmech.2019.06.002.
Asati V, Mahapatra DK, Bharti SK. K-Ras and its inhibitors towards personalized cancer treatment: pharmacological and structural perspectives. Eur J Med Chem. 2017;125:299–314. https://doi.org/10.1016/j.ejmech.2016.09.049.
Akutagawa J, Huang TQ, Epstein I, Chang T, Quirindongo-Crespo M, Cottonham CL, et al. Targeting the PI3K/Akt pathway in murine MDS/MPN driven by hyperactive Ras. Leukemia. 2016;30(6):1335–43. https://doi.org/10.1038/leu.2016.14.
Padron E, Painter JS, Kunigal S, Mailloux AW, McGraw K, McDaniel JM, et al. GM-CSF-dependent pSTAT5 sensitivity is a feature with therapeutic potential in chronic myelomonocytic leukemia. Blood. 2013;121(25):5068–77. https://doi.org/10.1182/blood-2012-10-460170.
Radia DH, Green A, Oni C, Moonim M. The clinical and pathological panoply of systemic mastocytosis. Br J Haematol. 2020;188(5):623–40. https://doi.org/10.1111/bjh.16288.
Romano M, Della Porta MG, Galli A, Panini N, Licandro SA, Bello E, et al. Antitumour activity of trabectedin in myelodysplastic/myeloproliferative neoplasms. Br J Cancer. 2017;116(3):335–43. https://doi.org/10.1038/bjc.2016.424.
Modi Y, Shaaban H, Gauchan D, Maroules M. Successful treatment of severe thrombocytopenia with the use of thrombopoeitin receptor agonist eltrombopag in a patient with chronic myelomonocytic leukemia. J Oncol Pharm Pract. 2015;21(1):74–5. https://doi.org/10.1177/1078155214544076.
Gao Y, Gong M, Zhang C, Kong X, Ma Y. Successful eltrombopag treatment of severe refractory thrombocytopenia in chronic myelomonocytic leukemia. Medicine (Baltimore). 2017;96(43):e8337. https://doi.org/10.1097/MD.0000000000008337.
Ramadan H, Duong VH, Al Ali N, Padron E, Zhang L, Lancet JE, et al. Eltrombopag use in patients with chronic myelomonocytic leukemia (CMML): a cautionary tale. Clin Lymphoma Myeloma Leuk. 2016;16(Suppl):S64–6. https://doi.org/10.1016/j.clml.2016.02.009.
Hunter AM, Zhang L, Padron E. Current management and recent advances in the treatment of chronic myelomonocytic leukemia. Curr Treat Options Oncol. 2018;19(12):67. https://doi.org/10.1007/s11864-018-0581-6.
Mikkael A, Sekeres MO, Alan F. List, Olatoyosi Odenike, randomized phase II study of Azacitidine alone or in combination with lenalidomide or with vorinostat in higher-risk myelodysplastic syndromes and chronic myelomonocytic leukemia: North American Intergroup Study SWOG S1117. J Clin Oncol. 2017;35(24):2745-53. https://doi.org/10.1200/JCO10.1200/JCO.2015.
Sanada M, Suzuki T, Shih LY, Otsu M, Kato M, Yamazaki S, et al. Gain-of-function of mutated C-CBL tumour suppressor in myeloid neoplasms. Nature. 2009;460(7257):904–8. https://doi.org/10.1038/nature08240.
Dunbar AJ, Gondek LP, O’Keefe CL, Makishima H, Rataul MS, Szpurka H, et al. 250K single nucleotide polymorphism array karyotyping identifies acquired uniparental disomy and homozygous mutations, including novel missense substitutions of c-Cbl, in myeloid malignancies. Cancer Res. 2008;68(24):10349–57. https://doi.org/10.1158/0008-5472.CAN-08-2754.
Reindl C, Quentmeier H, Petropoulos K, Greif PA, Benthaus T, Argiropoulos B, et al. CBL exon 8/9 mutants activate the FLT3 pathway and cluster in core binding factor/11q deletion acute myeloid leukemia/myelodysplastic syndrome subtypes. Clin Cancer Res. 2009;15(7):2238–47. https://doi.org/10.1158/1078-0432.CCR-08-1325.
Malcovati L, Papaemmanuil E, Ambaglio I, Elena C, Galli A, Della Porta MG, et al. Driver somatic mutations identify distinct disease entities within myeloid neoplasms with myelodysplasia. Blood. 2014;124(9):1513–21. https://doi.org/10.1182/blood-2014-03-560227.
Itzykson R, Kosmider O, Renneville A, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol. 2013;31(19):2428–36.
Santini V, Allione B, Zini G, Gioia D, Lunghi M, Poloni A, et al. A phase II, multicentre trial of decitabine in higher-risk chronic myelomonocytic leukemia. Leukemia. 2018;32(2):413–8. https://doi.org/10.1038/leu.2017.186.
Alfonso A, Montalban-Bravo G, Takahashi K, Jabbour EJ, Kadia T, Ravandi F, et al. Natural history of chronic myelomonocytic leukemia treated with hypomethylating agents. Am J Hematol. 2017;92(7):599–606. https://doi.org/10.1002/ajh.24735.
Symeonidis A, van Biezen A, de Wreede L, Piciocchi A, Finke J, Beelen D, et al. Achievement of complete remission predicts outcome of allogeneic haematopoietic stem cell transplantation in patients with chronic myelomonocytic leukaemia. A study of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Br J Haematol. 2015;171(2):239–46. https://doi.org/10.1111/bjh.13576.
de Witte T, Bowen D, Robin M, Malcovati L, Niederwieser D, Yakoub-Agha I, et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood. 2017;129(13):1753–62. https://doi.org/10.1182/blood-2016-06-724500.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The manuscript does not contain clinical studies or patient data.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jian, J., Qiao, Y., Li, Y. et al. Mutations in chronic myelomonocytic leukemia and their prognostic relevance. Clin Transl Oncol 23, 1731–1742 (2021). https://doi.org/10.1007/s12094-021-02585-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02585-x