Abstract
Purpose
To review the diagnostic and therapeutic procedures of patients diagnosed with Endometrial Stromal Sarcoma (ESS) and Undifferentiated Uterine Sarcoma (USS) at our institution and investigate their clinical outcomes and factors affecting prognosis.
Methods
We retrospectively collected demographic data, preoperative diagnostic methods and therapeutic management of patients treated for ESS and UUS between January 1995 and December 2019 at Vall d’Hebron Barcelona Hospital Campus, Spain. Overall survival and disease-free survival were calculated. Cox proportional-hazards regression models were calculated.
Results
Sixty-three patients were included in the study, of which 51(81%) had a diagnosis of ESS and 12(19%) of UUS. Twenty patients (31.7%) were diagnosed after a previous non-oncologic surgery, and 12 of them (60%) suffered from tumor disruption. Cytoreductive procedures were needed in 29 patients (46%), and optimal cytoreduction was achieved in 80.9% of the patients. The median follow-up was 7.6 years (IQR = 0.99–14.31). Five-year overall survival was 57.6% (44.2–68.8) and was significantly better for low-grade ESS (LG-ESS) patients (p < 0.01). Five-year disease-free survival was 57.1% (42.8–69.1) and was also significantly higher in LG-ESS cohort (p = 0.03). After multivariate analysis histological type, age, FIGO stage, optimal surgery and mitotic index were found significantly correlated with survival. For high-grade EES (HG-ESS) and USS patients adjuvant radiotherapy also correlated with improved survival.
Conclusion
Overall survival and disease-free survival are significantly better in patients with LG-ESS cohort. HG-ESS and UUS show similar survival outcomes. Age, FIGO stage, optimal surgery and histological type were significantly correlated with survival in the global cohort, whilst adjuvant radiotherapy correlated with improved survival in HG-ESS and UUS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial Stromal Sarcomas (ESS) and Undifferentiated Uterine Sarcoma (UUS) are the most rare uterine malignancies accounting for less than 2% of all uterine tumors and 25% of all uterine sarcomas [1]. According to the 2014 World Health Organization classification, there are four categories of endometrial stromal tumors: benign endometrial stromal nodule (ESN), low-grade endometrial stromal sarcoma (LG-ESS), high-grade endometrial stromal sarcoma (HG-ESS) and undifferentiated uterine sarcoma (UUS) [2].
LG-ESS mainly affects peri-menopausal women. The reported overall disease-specific 5-year survival rates are 80–90% for initial stages and decreases to 50% for stage III-IV [1]. However, the recurrence risk is high and characterized by late relapses, suggesting that long-term follow-up is required [3]. The most frequent site of extra-uterine pelvic extension is the ovaries, frequently in association with endometriosis [4].
HG-ESS was reintroduced in the 2014 WHO classification as a distinct entity, after the discovery of a subset of ESS with a YWHAE-FAM22 gene rearrangement [5]. Previously, the stratification between LG-ESS and HG-ESS was performed using a mitotic index over 10 figures per 10 high-power fields (HPFs), although there were controversies about its correlation with clinical outcomes. More recently, another HG-ESS subtype has been described with alterations in the gene BCOR [6]. Patients with HG-ESS have earlier and more frequent recurrences and are more likely to die of this disease than patients with LG-ESS [7], but appear to have a more favorable prognosis than UUS [5].
UUS is a high-grade sarcoma, lacking a specific line of differentiation, which constitutes a diagnosis of exclusion [6, 8]. More than 60% of patients are diagnosed with advanced stage diseases and associated with a very poor prognosis [1]. A mitotic index higher than 25 mitoses per 10HPFs has a negative prognostic impact in UUS [9].
Surgery is the cornerstone of treatment for ESS [10]. The surgical protocol includes hysterectomy and debulking of macroscopic disease. The role of lymphadenectomy and ovarian conservation is under debate, especially in LG-ESS [11, 12]. In large population-based studies lymph node metastases have been noted in less than 10% of patients undergoing lymphadenectomy [11]. Although lymphadenectomy contributes to FIGO staging and influences future decisions about adjuvant treatment, it does not improve ESS prognosis, therefore not being recommended in the surgical management of ESS with staging purpose [10]. Regarding ovarian conservation, although over 80% of LG-ESS tumors are estrogen receptor positive [12], ovarian conservation does not seem to affect 5-year overall survival of patients affected from LG-ESS stage I [11].
Recurrences develop in 23–59% of all patients with ESS, and 15–25% of these patients die of recurrent disease [13]. Hormonotherapies (high-dose progestins, aromatase inhibitors and gonadotropin releasing hormone (GnRH) agonist) are effective forms of hormonal treatment in patients with advanced or recurrent ESS [1]. In contrast, there is insufficient evidence concerning the efficacy of radiotherapy and chemotherapy in the recurrence setting [1, 11].
The aim of this study was to retrospectively investigate clinical outcomes of patients diagnosed with ESS and UUS in a single referral center, to deeply understand this disease and to identify factors influencing its prognosis.
Material and methods
Study design
We performed a retrospective review of the patients treated for ESS and UUS at Vall d’Hebron Barcelona Hospital Campus, a referral hospital in Barcelona, Spain. This review was approved by the Ethics Committee of the Hospital.
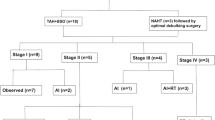
We identified all the patients diagnosed of ESS ans UUS between January 1995 and December 2019 at our institution. We included in the analysis those patients who were operated and completed the primary treatment at our center. We excluded from the analysis those patients who were not suitable for treatment with curative intention at the moment of diagnosis. The pathology reports were reviewed and patients were classified into three categories according to 2014 WHO classification: LG-ESS, HG-ESS and UUS. All the diagnostics were made by experienced gyneco-pathologists on the basis of histological and immunohistochemical features. We collected demographic data, information about previous symptoms, preoperative diagnostic methods and therapeutic management.
Patients who suffered from “tumor disruption” – non-oncological manipulation during the primary surgery because of a lack of suspicion of neoplasm were identified. The procedure was classified as “tumor disruption” in the case of specimen morcellation (laparoscopic myomectomy, subtotal hysterectomy, total hysterectomy with vaginal morcellation) or in the case of specimen fragmentation (myomectomy or subtotal hysterectomy without morcellation). In the case of patients diagnosed as a result of a previous surgery without oncological suspicion, the surgery recorded in the database was the second surgery with oncologic intention. If the first surgery was considered oncologically optimal, and no other surgical procedure was performed, this first surgery was considered for the analysis. Information about follow-up and recurrences was obtained from medical records, and progression-free survival and overall survival were calculated.
Radiotherapy was administered to patients with local pelvic and/or aortic spread of the disease (stage II, III FIGO) and/or patients with stage I FIGO and histological features of aggressive tumor (grade 3, necrosis, high mitotic index and big tumor size). Chemotherapy was administered to patients at stage III-IV FIGO, and in some patients at initial stage with bad histological features (i.e. patients with positive peritoneal cytology). The preferred regimes were doxorubicin in monotherapy or ifosfamide plus epirubicin. Hormonotherapy was indicated only to patients with LG-ESS expressing hormone receptors in the recurrence setting.
Statistical analysis
All data were anonymized, and the database was closed in March 2020. Data were subjected to a descriptive analysis of the variables distributions, based on numbers and percentages for qualitative variables, and mean (SD) or median (interquartile range) values for quantitative variables (depending on normality tests of the distributions). Survival curves were plotted using the Kaplan–Meier method and compared with the log rank test. Multivariate Cox proportional-hazards regression models were then calculated. All variables statistically significant in univariate analysis (log rank test P < 0.05) were considered in the multivariate models. Variables were not considered for analysis in the multivariable model when proportion of missing values exceeded 10% of the observations or significant collinearity was detected. No missing values imputation or prediction was performed. Overall survival was calculated based on the interval from diagnosis to patient death or last follow-up. Disease-free survival was defined as the interval between diagnosis and local relapse and/or distant metastasis. All statistical tests were two sided, and the threshold for statistical significance was set at p < 0.05. Analyses were performed with Stata 14.2 (StataCorp, TX, USA).
Results
Baseline characteristics
A total of 63 patients were reviewed and included in the study, of which 31 (49.2%) had a diagnosis of LG-ESS, 20 (31.8%) of HG-ESS and 12 (19%) of UUS. Mean age at diagnosis was 59.1 years (SD: 13.5), being the patients in LG-ESS cohort significantly younger than those in HG-ESS and UUS groups (53.2y vs. 63.9y vs. 66.4y, p = 0.002), and more frequently premenopausal at diagnosis (61.3% vs. 30% vs. 8.3%, p = 0.003). Most women in both groups presented vaginal bleeding as initial symptom (57.1%), and only 14.3% of the patients were asymptomatic at diagnosis. The baseline characteristics of the patients analyzed are shown in Table 1.
Diagnostic and therapeutic management
Twenty patients (31.7%) of the global cohort were diagnosed as a result of a surgery without a suspicion of oncologic disease, and 12 of them (60%) suffered from tumor disruption. Morcellation of the specimen occurred in four cases, being three of them part of the LG-ESS group. Fragmentation of the specimen occurred in eight patients, four of them in the LG-ESS group. Of note, 45.1% of the group of patients affected by LG-ESS was diagnosed after a surgery performed without oncologic intention.
Only 25 patients (39.7%) were diagnosed preoperatively with a biopsy and underwent a primary procedure performed by a gynecologic oncologic team. We observed a 46.2% correlation between the preoperative biopsies and definitive pathologic diagnosis, being this correlation significantly higher in LG-ESS than in HG-ESS and UUS (75% vs. 25% vs. 16.7%, p = 0.033). In 18 cases (28.6%) the diagnosis was not performed preoperatively, but there existed a high suspicion of oncologic disease and, therefore, the surgery was also performed by a gynecologic oncologic team.
All patients were operated as first treatment. A laparotomic approach was used in 48 patients (76.2%), while the rest underwent a minimally invasive surgery. All patients received a hysterectomy and bilateral adnexectomy; 36 patients also underwent pelvic lymphadenectomy (57.1%) and 15 a para-aortic lymph node dissection (23%). There were no differences in the performance of lymphadenectomy between groups. Cytoreductive procedures were needed in 29 patients (46%) of the global cohort, and they were significantly more used in patients affected by HG-ESS or UUS (p = 0.02). Optimal cytoreduction was achieved in 80.9% of the patients of the cohort.
Radiotherapy was used similarly across the entire cohort, regardless the histology (36.5%). Chemotherapy was more frequently used in HG-ESS and UUS cohort (9.7% vs. 40% vs. 33.3%, p = 0.03) (Table 2).
Pathological findings
Tumor diameter in patients affected with HG-ESS and UUS was significantly higher than in patients affected with LG-ESS (115 mm vs. 100 mm vs. 36 mm, p = 0.005). Most HG-ESS and UUS tumors presented necrosis at the pathological evaluation, differently to what occurred with LG-ESS specimens (70% vs. 91.7% vs.19.3%, p = 0.01). Median mitotic index was significantly higher in HG-ESS and UUS compared to LG-ESS tumors (35 vs. 26.5 vs 1.5, p < 0.001). Curiously, lymphovascular space invasion (LVSI), lymph node involvement and FIGO stage distribution were similarly found across the three groups (Table 3).
Outcomes and prognostic factors
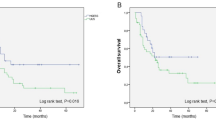
The median follow-up of the global cohort was 7.6 years (IQR = 0.99–14.31). Five-year overall survival (OS) for the entire cohort was 57.6% (44.2–68.8) and was significantly better for LG-ESS patients (85.9% vs. 35% vs. 30%, p < 0.01). Five-year disease-free survival (DFS) for the entire cohort was 57.1% (42.8–69.1) and was also significantly higher in LG-ESS cohort (78.8%) than in HG-ESS (33.3%) and UUS cohort (30%) (p = 0.03).
In univariate analysis, HG-ESS or UUS tumors, age, FIGO stage III-IV, higher mitotic index and not receiving an optimal surgery or receiving chemotherapy as adjuvant treatment were associated to a worse survival. Tumor disruption was not found to impact on overall survival. After multivariant analysis, presenting HG-ESS or UUS tumors, age, FIGO stage and optimal surgery remained as independent prognostic factors for the global cohort (Table 4; Fig. 1). In the subgroup analysis of the total HG-ESS and UUS patients, adjuvant radiotherapy and presenting an advanced FIGO stage at diagnosis seemed to be correlated with survival (Table 5).
Discussion
Endometrial Stromal Tumors are rare entities and scarcely reported in literature. The optimal treatment for this condition is still a matter of debate. We report a single-institution series of 63 patients with low-grade, high-grade stromal sarcoma and undifferentiated uterine sarcoma, based on the experience of a gynecologic oncology referral center.
With regard to the diagnostic procedures performed in these patients, we observed that the majority of patients with LG-ESS were symptomatic at diagnosis (74.2%), in accordance with previous studies [14]. Notwithstanding this fact, only 39.7% of the patients of the total cohort were diagnosed preoperatively by an in-office biopsy, what is also in accordance with previous reported studies [15, 16]. LG-ESS are often misdiagnosed as leiomyomas before tumor resection [12], and this fact supposed that in our series 45.2% of LG-ESS patients were diagnosed after a previous surgery without oncological intention. Only 35.5% of LG-ESS patients were diagnosed by a previous endometrial biopsy, even when the reported sensitivity of diagnostic curettage in identifying LG-ESS is as high as 71.4% [14], although not all these sarcomas involve the uterine mucosa. These results make evident that endometrial preoperative biopsy should be mandatory before a surgery for a presumed fibroid, especially in symptomatic patients. Nevertheless, we did not observe worse outcomes in patients with tumor disruption in the first surgery, probably due to the limited number of cases. Higher mortality rates of uterine sarcoma have been reported after power morcellation in different series [17, 18]. In order to overcome this concern, several investigators proposed alternative minimally invasive techniques to traditional power morcellation, as the use of large, insufflated bags [19]. Growing evidence supports the safety of transvaginal specimen retrieval through posterior colpotomy [20] or transvaginal scalp morcellation after hysterectomy [21].
The role of lymphadenectomy in ESS surgical management is not conclusive. In our cohort, only 57.1% of the patients received a pelvic lymphadenectomy and 23% a para-aortic lymph node dissection. Lymph node involvement among these patients was uncommon (17.6% in LG-ESS, 20% in HG-ESS and 33.3% in UUS) according to previous reported studies [3, 14, 15, 22]. In a large cohort of patients with LG-ESS, Wang et al. reported no differences neither in recurrence-free survival nor in overall survival after performing lymphadenectomy [11]. Seagle et al. found that in women with HG-ESS, not performing lymphadenectomy and pathologically positive surgical margins were negative prognostic factors [23]. Other series and clinical guidelines report a lack of prognostic impact of pelvic or aortic lymphadenectomy when nodes are not macroscopically involved [10, 15, 24]. Accordingly, our findings do not support routine lymphadenectomy at the time of initial treatment in ESS, as it does not seem to impact on patient’s survival. Although prospective studies are lacking, it seems that lymphadenectomy should be considered only as part of a cytoreductive procedure [10].
Chemotherapy, radiotherapy and hormone therapy are important adjuvant treatments for ESS and UUS patients. Nevertheless, clinical trials have failed to show a definitive survival benefit of adjuvant radiotherapy or chemotherapy, surely influenced by the rarity and heterogeneity of this disease [25]. In our study we observed a benefit in overall survival after administering radiotherapy to the HG-ESS and UUS cohort as previously reported in other series [15, 23]. Based on retrospective and scarce cohorts, adjuvant chemotherapy has shown no benefit on survival of patients with LG-ESS [23, 26]. However, in HG-ESS and UUS patients the use of adjuvant chemotherapy has been correlated with improved disease-free survival in early-stages [15, 25], although we did not observe this survival benefit in our patients. Hormonal treatments have been considered effective as adjuvant treatment of LG-ESS and in recurrent tumors [24, 27]. A recent meta-analysis on hormone therapy including 315 LG-ESS patients concluded that it could reduce the risk of recurrence only in patients with FIGO stage I-II, without having a benefit on overall survival [28]. However, the optimal dose, regimen and duration of hormonal treatment are not well established.
Regarding prognostic factors, many studies intended to identify those with higher impact on patient’s survival. In our work, we found that older age, advanced FIGO stage and higher mitotic index had a negative impact on overall survival, while optimal surgery was associated with a better prognosis in the global cohort. In the study published by Kyriazoglou et al. analyzing 61 patients with uterine sarcoma of different histologies, increased mitotic index was described as the only recognized independent significant prognostic factor in the multivariate analysis [29]. A recent report by Hardell et al. demonstrated the prognostic value of mitotic index cut-off (of 25 mitoses/10 high power fields) in UUS in an independent cohort of 40 patients [9]. They concluded that the subgroup of patients with lower mitotic index can achieve long-term survival.
The main limitation of the present study is its retrospective design and the reduced number of cases included in the cohort, even though we are a referral center and reported the cases of the past 25 years. This fact prevented us from obtaining information that could also impact on patient’s survival, as the performance status or other analytical parameters. The long period of inclusion implies that the criteria for the use of adjuvant therapies were probably different along the cohort. Furthermore, patients were diagnosed and treated according to morphologic and immunohistochemical features of the tumors, but without molecular studies. The recently described genotype classification to diagnose HG-ESS was used only in few, recent cases. In the same way, UUS represents a heterogeneous subset of sarcomas in which molecular analysis could define diagnostic categories that also could benefit from specific targeted treatments. As a strength of our work, we must take into account that all the diagnoses were performed by the same experienced team of expert gyneco-pathologists. What is more, our study throws some light on clinical and prognostic characteristics of UUS affected patients, as they have been scarcely reported across the literature.
As a conclusion, overall survival and disease-free survival were significantly better in patients with LG-ESS, while HG-ESS and UUS showed similar survival outcomes in our cohort. Age, FIGO stage, optimal surgery and mitotic index were significantly correlated with survival in the global cohort, and adjuvant radiotherapy correlated with improved survival in HG-ESS and UUS patients. We did not observe worse outcomes in patients with tumor disruption in the initial surgery, probably due to the limited number of cases. The present cohort represents one of the largest retrospective series of a single center reported in literature. Given that endometrial stromal tumors are rare entities, the evidence regarding their diagnostic and therapeutic management is mainly based on retrospective, scarce series, as prospective trials are difficult to launch. In this specific scenario, we consider that our experience could provide limited but significant evidence to improve the quality of the assistance of these patients.
References
Horng H-C, et al. Uterine sarcoma Part II—Uterine endometrial stromal sarcoma: The TAG systematic review. Taiwan J Obstet Gynecol. 2016;55(4):472–9. https://doi.org/10.1016/j.tjog.2016.04.034.
Conklin CMJ, Longacre TA. Endometrial stromal tumors: the new WHO classification. Adv Anat Pathol. 2014;21(6):383–93. https://doi.org/10.1097/PAP.0000000000000046.
Chan JK, et al. Endometrial stromal sarcoma: a population-based analysis. Br J Cancer. 2008;99(8):1210–5. https://doi.org/10.1038/sj.bjc.6604527.
Prat J, Mbatani N. Uterine sarcomas. Int J Gynecol Obstet. 2015;131:S105–10. https://doi.org/10.1016/j.ijgo.2015.06.006.
Lee C-H, et al. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 2012;36(5):641–53. https://doi.org/10.1097/PAS.0b013e31824a7b1a.
Parra-Herran C, Howitt BE. Uterine Mesenchymal Tumors: Update on Classification, Staging, and Molecular Features. Surg Pathol Clin. 2019;12(2):363–96. https://doi.org/10.1016/j.path.2019.01.004.
Cuppens T, Tuyaerts S, Amant F. Potential therapeutic targets in uterine sarcomas. Sarcoma. 2015;2015:243298. https://doi.org/10.1155/2015/243298.
Cotzia P, et al. Undifferentiated uterine sarcomas represent under-recognized high-grade endometrial stromal sarcomas. Am J Surg Pathol. 2019;43(5):662–9. https://doi.org/10.1097/PAS.0000000000001215.
Hardell E, Westbom-Fremer S, Schoolmeester JK, Ma A, Carlson JW. Validation of a mitotic index cutoff as a prognostic marker in undifferentiated uterine sarcomas. Am J Surg Pathol. 2017;41(9):7.
Amant F, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for endometrial stromal sarcoma. J Int Gynecol Cancer Soc. 2014. https://doi.org/10.1097/IGC.0000000000000205.
Wang M, Meng S-H, Li B, He Y, Wu Y-M. Survival outcomes of different treatment modalities in patients with low-grade endometrial stromal sarcoma. Chin Med J (Engl). 2019;132(9):1128–32. https://doi.org/10.1097/CM9.0000000000000259.
Bai H, et al. Ovary and uterus-sparing procedures for low-grade endometrial stromal sarcoma: a retrospective study of 153 cases. Gynecol Oncol. 2014;132(3):654–60. https://doi.org/10.1016/j.ygyno.2013.12.032.
Ryu H, et al. Long-term treatment of residual or recurrent low-grade endometrial stromal sarcoma with aromatase inhibitors: A report of two cases and a review of the literature. Oncol Lett. 2015;10(5):3310–4. https://doi.org/10.3892/ol.2015.3674.
Cui R, Yuan F, Wang Y, Li X, Zhang Z, Bai H. Clinicopathological characteristics and treatment strategies for patients with low-grade endometrial stromal sarcoma. Medicine (Baltimore). 2017;96(15):e6584. https://doi.org/10.1097/MD.0000000000006584.
Meurer M, et al. Localized high grade endometrial stromal sarcoma and localized undifferentiated uterine sarcoma: a retrospective series of the French Sarcoma Group. Int J Gynecol Cancer. 2019;29(4):691–8. https://doi.org/10.1136/ijgc-2018-000064.
Wais M, Tepperman E, Bernardini MQ, Gien LT, Jimenez W, Murji A. A multicentre retrospective review of clinical characteristics of uterine sarcoma. J Obstet Gynaecol Can. 2017;39(8):652–8. https://doi.org/10.1016/j.jogc.2017.03.090.
Graebe K, et al. Incidental power morcellation of malignancy: a retrospective cohort study. Gynecol Oncol. 2015;136(2):274–7. https://doi.org/10.1016/j.ygyno.2014.11.018.
Bretthauer M, et al. Uterine morcellation and survival in uterine sarcomas. Eur J Cancer Oxf Engl. 2018;101:62–8. https://doi.org/10.1016/j.ejca.2018.06.007.
Bogani G, et al. Morcellation of undiagnosed uterine sarcoma: A critical review. Crit Rev Oncol Hematol. 2016;98:302–8. https://doi.org/10.1016/j.critrevonc.2015.11.015.
Bogani G, Dowdy SC, Cliby WA, Ghezzi F, Rossetti D, Mariani A. Role of pelvic and para-aortic lymphadenectomy in endometrial cancer: Current evidence. J Obstet Gynaecol Res. 2014;40(2):301–11. https://doi.org/10.1111/jog.12344.
Zhang J, Li T, Zhang J, Zhu L, Lang J, Leng J. ‘Clinical characteristics and prognosis of unexpected uterine sarcoma after hysterectomy for presumed myoma with and without transvaginal scalpel morcellation. Int J Gynecol Cancer Soc. 2016;26(3):456–63. https://doi.org/10.1097/IGC.0000000000000638.
Shah JP, Bryant CS, Kumar S, Ali-Fehmi R, Malone JM, Morris RT. Lymphadenectomy and ovarian preservation in low-grade endometrial stromal sarcoma. Obstet Gynecol. 2008;112(5):1102–8. https://doi.org/10.1097/AOG.0b013e31818aa89a.
Seagle B-LL, Shilpi A, Buchanan S, Goodman C, Shahabi S. Low-grade and high-grade endometrial stromal sarcoma: A National Cancer Database study. Gynecol Oncol. 2017;146(2):254–62. https://doi.org/10.1016/j.ygyno.2017.05.036.
Yamazaki H, et al. Long-term survival of patients with recurrent endometrial stromal sarcoma: a multicenter, observational study. J Gynecol Oncol. 2015;26(3):214–21. https://doi.org/10.3802/jgo.2015.26.3.214.
Zhang Y-Y, Li Y, Qin M, Cai Y, Jin Y, Pan L-Y. High-grade endometrial stromal sarcoma: a retrospective study of factors influencing prognosis. Cancer Manag Res. 2019;11:831–7. https://doi.org/10.2147/CMAR.S187849.
Kim WY, et al. Low-grade endometrial stromal sarcoma: a single center’s experience with 22 cases. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2008;18(5):1084–9. https://doi.org/10.1111/j.1525-1438.2007.01159.x.
Chu MC, Mor G, Lim C, Zheng W, Parkash V, Schwartz PE. Low-grade endometrial stromal sarcoma: hormonal aspects. Gynecol Oncol. 2003;90(1):170–6. https://doi.org/10.1016/s0090-8258(03)00258-0.
Cui R, Cao G, Bai H, Zhang Z. The clinical benefits of hormonal treatment for LG-ESS: a meta-analysis. Arch Gynecol Obstet. 2019. https://doi.org/10.1007/s00404-019-05308-4.
Kyriazoglou A, et al. Management of uterine sarcomas and prognostic indicators: real world data from a single-institution. BMC Cancer. 2018;18(1):1247. https://doi.org/10.1186/s12885-018-5156-1.
Funding
The present work was not supported by any grant or organization.
Author information
Authors and Affiliations
Contributions
S. Cabrera is the principal investigator, designed the study, reviewed the literature, collected and analyzed data, and wrote the paper. V. Bebia, S. Franco-Camps, L. Mañalich and U. Acosta contributed to the collection of data, critical revision of the manuscript, and read and approved the final draft here uploaded. V. Bebia performed the statistical analyses. A. Gil-Moreno and A. García-Jimenez reviewed the manuscript for intellectual and scientific content, and approved the final draft.
Corresponding author
Ethics declarations
Availability of data and material
Data of this work are available under justified request to the corresponding author.
Conflicts of interest
The authors state that they have neither financial nor personal relationships with any organization that could inappropriately influence the present work.
Ethics approval
The present research was submitted and approved by Vall d’Hebron Ethics Committee (Project Code PR(AMI)526/2019) on date Dec 11th, 2019.
Consent to participate
Not applicable regarding the decision made by Ethics Committee.
Consent for publication
All of the authors are in agreement with the final draft and consent for its publication.
Informed consent
For this type of study, no informed consent is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cabrera, S., Bebia, V., Acosta, U. et al. Survival outcomes and prognostic factors of endometrial stromal sarcoma and undifferentiated uterine sarcoma. Clin Transl Oncol 23, 1210–1219 (2021). https://doi.org/10.1007/s12094-020-02512-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02512-6